Abstract
Background/Aims
We investigated the clinical practice patterns of post-polypectomy colonoscopic surveillance among Korean endoscopists.
Methods
In a web-based survey conducted between September and November 2021, participants were asked about their preferred surveillance intervals and the patient age at which surveillance was discontinued. Adherence to the recent guidelines of the U.S. Multi-Society Task Force on Colorectal Cancer (USMSTF) was also analyzed.
Results
In total, 196 endoscopists completed the survey. The most preferred first surveillance intervals were: a 5-year interval after the removal of 1–2 tubular adenomas < 10 mm; a 3-year interval after the removal of 3–10 tubular adenomas < 10 mm, adenomas ≥ 10 mm, tubulovillous or villous adenomas, ≤ 20 hyperplastic polyps < 10 mm, 1–4 sessile serrated lesions (SSLs) < 10 mm, hyperplastic polyps or SSLs ≥ 10 mm, and traditional serrated adenomas; and a 1-year interval after the removal of adenomas with highgrade dysplasia, >10 adenomas, 5–10 SSLs, and SSLs with dysplasia. In piecemeal resections of large polyps ( > 20 mm), surveillance colonoscopy was mostly preferred after 1 year for adenomas and 6 months for SSLs. The mean USMSTF guideline adherence rate was 30.7%. The largest proportion of respondents (40.8%–55.1%) discontinued the surveillance at the patient age of 80–84 years.
Colorectal cancer (CRC) is one of the leading malignancies in terms of incidence and mortality in Korea. In 2022, the agestandardized incidence and mortality rates were 33.8 and 8.8 per 100,000 men and 19.8 and 4.7 per 100,000 women, respectively [1]. With the rapid increase in the incidence of CRC in Korea [2], the national burden of colonoscopy and polypectomy has increased markedly [3].
Surveillance colonoscopy accounts for approximately 20% of all colonoscopies performed in the United States and the United Kingdom [4], and determining the appropriate surveillance interval is important for endoscopists. Although clarity on the long-term risks of CRC after polypectomy and the population that may benefit from surveillance colonoscopy is lacking [5], appropriate surveillance may reduce such risks, especially in high-risk patients [4,6]. Therefore, referring to the guidelines regarding post-polypectomy surveillance intervals is recommended [7-9].
The Korean multi-society post-polypectomy surveillance guidelines were published in 2012 [7]. Before developing the guidelines, the Multi-Society Task Force for Guidelines for Colorectal Polyp Screening, Surveillance, and Management surveyed the status of the post-polypectomy surveillance strategy in 2011 [10-12]. After the publication of the guidelines in 2012 [7], a few studies investigated the clinical practice patterns of Korean endoscopists and their adherence to the guidelines in various clinical scenarios [13,14]. Understanding these patterns will help prepare strategies to increase adherence to the newly revised Korean guidelines [15]. However, currently available surveys are less representative as they included a relatively small number of respondents (40 gastroenterologists in one study and 138 in the other) [13,14]. In addition, a report describing the patient age at which endoscopists prefer to stop further surveillance in older adults is lacking. Therefore, in this study, we aimed to investigate the preferred surveillance intervals after colonoscopic polypectomy and the timing of surveillance discontinuation in older patients.
The Intestinal Tumor Research Group of the Korean Association for the Study of Intestinal Diseases (KASID) prospectively conducted a web-based nationwide survey on post-polypectomy surveillance from September 23, 2021 to November 12, 2021 (Supplementary Material 1). Using a web-based electronic survey tool (SurveyMonkey, San Mateo, CA, USA), the invitation e-mail including a survey link was sent to the members of the KASID (n=1,452). The individual responses were saved in a password-protected database and converted to a Microsoft Excel (Microsoft, Redmond, WA, USA) file for analysis. The study protocol was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (IRB number 2019-1510). Informed consents were obtained from all participants.
Baseline characteristics of respondents, such as sex, age, specialty, type of practice hospital, and the number of performed colonoscopies and polypectomies, were obtained. Practice hospitals were classified into: primary facilities, including primary outpatient clinics; secondary facilities, including hospitals and general hospitals; and tertiary facilities, including specialized general hospitals and academic hospitals. The survey included questions regarding the timing of performing the first and second surveillance colonoscopy based on the U.S. Multi-Society Task Force on Colorectal Cancer (USMSTF) guidelines [8]. Exceptionally, we used a 5- to 10-year interval instead of a 7- to 10-year interval to simplify the survey content. In summary of the USMSTF guidelines, the recommended intervals between baseline and the first surveillance colonoscopies are as follows depending on the baseline findings; 10 years for a normal colonoscopy and ≤ 20 hyperplastic polyps (HPs) <10 mm, and 7 to 10 years for 1–2 tubular adenomas (TAs) <10 mm, 5 to 10 years for 1–2 sessile serrated lesions (SSLs) <10 mm, and 3 to 5 years for 3–4 TAs or SSLs <10 mm, and HP ≥ 10 mm, and 3 years for 5–10 TAs or SSLs <10 mm, TA or SSL ≥10 mm, advanced adenoma, and traditional serrated adenoma (TSA), 1 year for >10 TAs, and 6 months for piecemeal resection of adenoma or SSL >20 mm [8]. If the first surveillance colonoscopy shows normal colonoscopy findings, the second surveillance colonoscopy is recommended 10 years later after the removal of 1–2 or 3–4 TAs <10 mm, 5 years after removal of 5–10 adenomas <10 mm and advanced adenomas [8]. We assumed that an adequate colonoscopy was performed in the asymptomatic average-risk population aged 50 years or more, with complete resection of all polyps by polypectomy. To investigate the interval between the first and second surveillance colonoscopies, no polyp detection during the first surveillance colonoscopy was assumed. Advanced adenomas were defined as adenomas with tubulovillous or villous histology, with high-grade dysplasia (HGD), or of ≥10 mm in diameter [16]. High-risk adenomas were defined as either ≥ 3 adenomas or any advanced adenoma, and low-risk adenomas were defined as 1–2 non-advanced adenomas <10 mm in diameter [17]. We investigated the patient age for surveillance cessation and its determining factors, in addition to the factors determining surveillance intervals with reference to the previously reported 2012 survey [11].
All responses were demonstrated using descriptive statistics. The adherence rate for the surveillance intervals was calculated based on the recent USMSTF [8] and previous Korean [7] guidelines. The relationship between adherence to the guidelines and endoscopists’ demographics was assessed through univariate logistic regression analyses for selected lesions. A two-proportion z-test was utilized to compare adherence before and after the distribution of the 2012 Korean guidelines [7], as well as to compare the proportions of influential factors determining the surveillance intervals between the present and previous study populations [11]. A two-sided P-value <0.05 was considered statistically significant. All statistical analyses were performed using R Statistical Software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Among 1,452 physicians, 295 (20.3%) agreed to participate in this survey, and 196 (13.5%) completed the questionnaire on the surveillance intervals in various clinical polyp scenarios. The male-to-female ratio of all respondents was about 3:1, and 83.7% (n=164) were aged less than 50 years. Most respondents (96.9%, n=190) were gastroenterology specialists. The proportions of those working in primary, secondary, and tertiary healthcare facilities were 20.9% (n=41), 21.4% (n=42), and 57.7% (n=113), respectively. Regarding the clinical practice experience of the respondents, 75.0% (n=147) had 4 or more years of experience in performing colonoscopy, and 70.4% (n=138) and 58.2% (n=114) performed more than 50 colonoscopies and 50 polypectomies every month, respectively. The preferred surveillance tests after colonic polyp resection were as follows: colonoscopy (98.5%, n=193), sigmoidoscopy (5.1%, n=10), fecal occult blood test (3.1%, n=6), and computed tomographic colonography (0.5%, n=1). Among the respondents, 13 selected two or more tests; colonoscopy+ sigmoidoscopy (n=6), colonoscopy+fecal occult blood test (n=4), sigmoidoscopy+fecal occult blood test (n=1), colonoscopy+ sigmoidoscopy+fecal occult blood test (n=1), and colonoscopy+ computed tomographic colonography (n=1). The Korean guidelines published in 2012 [7] were the most influential in determining the surveillance intervals (n=160, 81.6%). Table 1 shows the detailed characteristics of the respondents.
Figs. 1 and 2 show the first and second surveillance colonoscopy intervals preferred by respondents when polyps were completely removed during baseline colonoscopy. When 1–2 TAs with low-grade dysplasia (LGD) of diameter <10 mm were removed, 35.7% (n=70) of respondents preferred to perform the first surveillance colonoscopy after 5 years. A 3-year surveillance interval was the most preferred in the following scenarios: 3–4 (48.0%, n=94) or 5–10 TAs (35.2%, n=69) with LGD of diameter <10 mm, an adenoma with tubulovillous or villous histology (49.5%, n=97), an adenoma (50.0%, n=98) or HP (36.2%, n=71) ≥10 mm, ≤20 HPs in the rectum or sigmoid colon of diameter <10 mm (30.1%, n=59), ≤20 HPs proximal to the sigmoid colon <10 mm (33.7%, n=66), 1–2 (37.2%, n=73) or 3–4 SSLs (40.3%, n=79) <10 mm, an SSL ≥10 mm (41.3%, n=81), and a TSA (41.8%, n=82). A 1-year surveillance interval was the most preferred in cases of an adenoma and HGD (48.0%, n=94), >10 adenomas (69.9%, n=137), 5–10 SSLs <10 mm (35.2%, n=69), an SSL with dysplasia (34.7%, n=68), and piecemeal resection of an adenoma ≥20 mm (43.4%, n=85). A 6-month interval was the most preferred in cases of piecemeal resection of an SSL ≥20 mm (44.9%, n=88).
The 5-year interval was mostly preferred between the first and second surveillance colonoscopy in cases where either of the following were removed during baseline colonoscopy: 1–2 (45.9%, n=90) or 3–4 (38.8%, n=76) TAs with LGD <10 mm, ≤20 HPs in the rectum or sigmoid colon <10 mm (41.3%, n=81), an HP ≥ 10 mm (46.4%, n=91), or 1–2 SSLs <10 mm (34.2%, n=67). Performing the second surveillance colonoscopy after 3 years was mostly preferred in cases of: 5–10 TAs with LGD <10 mm (41.8%, n=82), an adenoma ≥10 mm (36.7%, n=72), an adenoma with tubulovillous or villous histology (34.2%, n=67), an adenoma with HGD (32.1%, n=63), >10 adenomas (31.6%, n=62), ≤20 HPs proximal to the sigmoid colon <10 mm (35.7%, n=70), 3–4 (35.7%, n=70) or 5–10 SSLs (41.3%, n=81) <10 mm, an SSL ≥10 mm (37.2%, n=73), an SSL with dysplasia (36.2%, n=71), a TSA (34.7%, n=68), and piecemeal resection of an adenoma (34.2%, n=67) or SSL (37.2%, n=73) ≥ 20 mm.
Compared to the USMSTF guidelines,8 the mean adherence rate of the respondents was 30.7%, and the respondents generally preferred shorter surveillance intervals (Fig. 3). The adherence rates to the guidelines were relatively high for cases with >10 adenomas (69.9%), TAs with a diameter ≥10 mm (50.0%), or significant villous features (49.5%), while later surveillance was more preferred in cases of piecemeal resection of large ( ≥20 mm) TAs (52.0%) or SSLs (48.5%).
When the 2012 Korean guidelines were used as a reference [7], the mean adherence rate of the respondents was 40.2%. The adherence shows an improving tendency in the following clinical scenarios compared with the results of the survey performed before the distribution of the guidelines: 5 years later for 1–2 TAs <10 mm (adherence rates: 35.7% vs. 10.3%, P<0.001); and 3 years later for 3–10 TAs <10 mm (48.0% [3–4 TAs, P<0.001] or 35.2% [5–10 TAs, P<0.001] vs. 20.3% [3–10 TAs]), adenomas ≥ 10 mm (50.0% vs. 16.8%, P<0.001), adenomas with HGD (25.5% vs. 9.9%, P<0.001), and adenomas with significant villous components (49.5% vs. 14.9%, P<0.001) [7,11].
In univariable analyses, no significant variables were associated with adherence to the USMSTF guidelines after resecting 1–2 or 3–4 TAs <10 mm (data not shown). The variables significant for adherence to the guidelines were as follows: working at a tertiary facility (odds ratio [OR], 12.89; 95% confidence interval [CI], 3.76–44.19; P<0.001) and having 4 to 9 years of colonoscopic experience (OR, 0.32; 95% CI, 0.15–0.71; P=0.005) for 5–10 TAs <10 mm; working at a secondary facility (OR, 3.97; 95% CI, 1.37–11.48; P=0.011) or a tertiary facility (OR, 11.51; 95% CI, 4.45–29.77; P<0.001) and performing 50–99 polypectomies per month (OR, 11.38; 95% CI, 1.28–101.22; P=0.029) for a TA ≥10 mm; working at a secondary facility (OR, 4.43; 95% CI, 1.44–13.63; P=0.009) or a tertiary facility (OR, 14.79; 95% CI, 5.36–40.79; P<0.001), and having 4 to 9 years (OR, 0.28; 95% CI, 0.13–0.61; P=0.001) or ≥10 years (OR, 0.47; 95% CI, 0.23–0.99; P=0.048) of colonoscopic practice for a tubulovillous adenoma or TA; age ≥50 years (OR, 2.37; 95% CI, 1.00– 5.62; P=0.049), working at a tertiary facility (OR, 25.51; 95% CI, 3.38–192.29; P=0.002), and performing ≥100 colonoscopies per month (OR, 2.46; 95% CI, 1.07–5.65; P=0.034) for an adenoma with HGD (Supplementary Table 1). For 1–2 or 3–4 SSLs <10 mm, no significant factors were identified in univariate analyses (data not shown). Factors significant for adherence to the guidelines included working at a tertiary facility (OR, 12.43; 95% CI, 2.86–54.11; P<0.001) and having 4 to 9 years of colonoscopic experience (OR, 0.37; 95% CI, 0.16–0.86; P=0.021) for 5–10 SSLs <10 mm (Supplementary Table 2). Working at a secondary facility (OR, 4.15; 95% CI, 1.22–14.07; P=0.022) or a tertiary facility (OR, 12.08; 95% CI, 4.04–36.17; P<0.001) were significant factors for SSLs ≥10 mm, while working at a tertiary facility (OR, 10.05; 95% CI, 2.93–34.48; P<0.001) and performing 50–99 colonoscopies per month (OR, 2.28; 95% CI, 1.04– 5.03; P=0.040) were significant for an SSL with dysplasia.
In various clinical scenarios, the largest proportion of respondents (range, 40.8%–55.1%) answered that they no longer perform further surveillance colonoscopy when the patient is 80 to 84 years of age at the time of baseline colonoscopy. Fig. 4 shows the detailed proportion of the respondents.
Fig. 5 shows the factors determining the surveillance intervals. Factors with a response rate of more than 80% were dysplasia (98.5%), number (93.4%), and size (92.9%) of adenoma, achievement of histologic complete resection (92.3%), quality of bowel preparation (88.3%), and significant villous component (81.6%). Compared with the survey results published in 2012 [11], significant differences were observed in the following factors: bowel preparation quality (88.3% vs. 73.8%, P<0.001), patient age (70.4% vs. 47.9%, P<0.001), successful cecal intubation (69.4% vs. 40.3%, P<0.001), adenoma detection rate (ADR; 33.2% vs. 21.7%, P=0.004), withdrawal time (30.6% vs. 12.2%, P<0.001), and adenoma location (21.4% vs. 13.3%, P=0.015).
Our study showed that Korean endoscopists preferred early surveillance colonoscopy in various clinical scenarios, with a mean USMSTF guideline adherence rate of 30.7%. Low adherence was more pronounced when the polyps belonged to lowrisk adenomas or were serrated. We think that the preference for early surveillance in most polyp scenarios may be related to the relatively lower cost of colonoscopy in Korea compared to other countries, making it more accessible. The observed low adherence in the case of low-risk adenomas can be attributed to the difference in the recommendation of surveillance intervals between the Korean guidelines published in 2012 and the recent USMSTF guidelines published in 2020 [7,8]. In this study, 81.6% of respondents tended to refer to the Korean guidelines, which recommend performing surveillance colonoscopy 5 and 3 years after polyp resections of 1–2 and 3–4 TAs, respectively, whereas recent USMSTF guidelines recommend colonoscopy after 7–10 and 3–5 years, respectively [7,8]. Low adherence in the case of serrated lesions may also be related to the lack of detailed coverage of serrated lesions in previous Korean guidelines [7]. While our results did not consistently show significant factors across various clinical scenarios, they suggest a trend of lower adherence among endoscopists working at primary facilities in the cases of high-risk adenomas, 5–10 SSLs <10 mm, SSL ≥ 10 mm, and an SSL with dysplasia. Although the current survey was conducted 9 years after the publication of the Korean multi-society guidelines [7], the adherence rates for each scenario improved by only 15% to 35%. This suggests that educational activities for the dissemination of the newly revised guidelines should be continued to achieve wide application.
In cases of adenomas with HGD, only 25.5% of respondents preferred performing a colonoscopy after 3 years, whereas 64.3% preferred it after 1 year. Although the exact reason for this finding cannot be confirmed through our investigation, it is presumed to be related to the endoscopists’ concerns regarding the histologically incomplete resection of HGD and possibility of missed polyps. Interestingly, similar results were demonstrated in a recent international survey of physicians (n=123) from 7 Asian countries (Korea, Mongolia, Thailand, Malaysia, Indonesia, Vietnam, and Myanmar) [13]. The rates of adherence (3 years) and early surveillance ( <3 years) were 35.0% and 61.8%, respectively, after the removal of a 12-mm TA with focal HGD, and it was relatively higher in the high-volume group (≥20 colonoscopies per month) than in the low-volume group (<20 colonoscopies per month) (44.9% vs. 17.8%) [13]. Further studies are required to understand the preference for a shorter surveillance interval for HGD in high-risk cases observed by index colonoscopy. Further evidence-based education is needed to dispel excessive concerns regarding HGD.
The largest proportion of the respondents preferred to perform a surveillance colonoscopy 3 years after the removal of 20 or less HPs <10 mm located in the rectum or sigmoid colon, or proximal to the sigmoid colon. In the Polyp Prevention Trial, the location and size ( ≥ 6 mm) of HPs were not significantly associated with the recurrence of any adenomas or advanced adenomas, but a study on whether isolated proximal HPs <10 mm are related to metachronous advanced neoplasia or large serrated polyps is lacking [8,18]. A surveillance colonoscopy in patients with an HP ≥10 mm or SSL <10 mm tends to be performed earlier than recommended by the guidelines; however, the quality of evidence supporting the guidelines is very low [8]. After the removal of an HP ≥10 mm, the European Society of Gastrointestinal Endoscopy (ESGE) and USMSTF guidelines recommend performing the first surveillance colonoscopy in 3 years and 3 to 5 years, respectively, and in the USMSTF guidelines, changing the surveillance interval is favored based on the concerns regarding the consistency of pathologists in distinguishing between SSLs and HPs [8,9].
Regarding the second surveillance colonoscopy, the risk of metachronous advanced neoplasia may be associated with high-risk findings at the index and first surveillance colonoscopy, but the evidence to determine an optimal interval is still lacking [19-23]. If polyps are not detected on the first surveillance colonoscopy, the ESGE guidelines recommend performing a second one after 5 years [9]. On the other hand, the USMSTF guidelines recommend the second surveillance colonoscopy depending on both baseline and the first surveillance colonoscopy findings [8]. For example, if the first surveillance colonoscopy results are normal, the recommended intervals for the second surveillance colonoscopy are 10 years later for patients with 1–2 or 3–4 TAs <10 mm at baseline, and 5 years later for those with an advanced adenoma or 5–10 adenomas <10 mm at baseline.8 In this study, most respondents preferred a second surveillance colonoscopy after 3 years in most clinical scenarios except for 1–2 TAs or SSLs <10 mm, 3–4 TAs <10 mm, 20 or less HPs <10 mm in the rectum or sigmoid colon, or an HP ≥10 mm at baseline colonoscopy. Further studies are needed on the optimal interval between the first and second surveillance colonoscopies based on the baseline and first surveillance colonoscopy findings.
The major determinants of future surveillance intervals were not only complete histologic resection but also high-risk features of adenomas in the current study. In a meta-analysis, the pooled 5-year cumulative incidence of advanced adenomas was relatively high in patients with high-risk (17.1%; 95% CI, 12.0%–23.0%) and with lower-risk (4.9%; 95% CI, 3.2%– 7.0%) adenomas at baseline colonoscopy compared with those with normal findings (3.3%; 95% CI, 1.9%–5.1%) [24]. Recurrence of adenoma was also significantly associated with age ≥60 years (pooled relative risk [RR], 1.65; 95% CI, 1.38– 1.93), adenomas in the proximal colon (pooled RR, 1.43; 95% CI, 1.30–1.57), and male sex (pooled RR, 1.22; 95% CI, 1.12– 1.32) [25]. In the USMSTF and revised Korean guidelines, an adenoma with a significant villous component is still classified as a high-risk finding, and surveillance colonoscopy is recommended after 3 years of polypectomy [8,15]. However, it was not significantly associated with recurrence (pooled RR, 1.21; 95% CI, 0.97–1.45) in a systemic review and pooled analyses [25], and long-term CRC incidence or mortality (hazard ratio, 1.16; 95% CI, 0.71–1.91) in a recent multicenter cohort study conducted in the United Kingdom [20]. Based on these findings, patients with villous histology were classified as a non-surveillance group in the ESGE guidelines [9]. Quality indicators of colonoscopy also have a significant impact on the detection of colorectal neoplasia [20,26,27]. In this study, bowel preparation quality (88.3%) and successful cecal intubation (69.4%) were considered as major determinants, but ADR (33.2%) and withdrawal time (30.6%) were not. Although ADR varies widely among endoscopists, it is a crucial quality indicator associated with long-term CRC incidence and mortality [27,28]. Longer withdrawal time can also increase the probability of polyp or adenoma detection and reduce the incidence of interval CRC [29,30].
The decision to discontinue surveillance colonoscopy in older patients is complex, with few guidelines and low quality of evidence [19,31]. Several guidelines generally recommend screening for CRC until 75 years of age for average-risk individuals [32-34], and surveillance is suggested to be discontinued at the age of 75 or 80 years unless other comorbidities affect life expectancy [9,19]. In this study, the largest proportion of respondents answered that further surveillance was no longer performed at the ages of 80–84 years in various clinical scenarios. However, a non-negligible proportion of the respondents chose 85–89 years of age, especially in cases of adenoma with high-risk features. Considering the relatively low incidence of CRC and high incidence of procedure-related adverse events in older patients [35], the decision to discontinue surveillance colonoscopy should be individualized according to the patient’s comorbidities and life expectancy [9,36].
Our study has several limitations. First, considering that this survey was conducted for members of the KASID and more than half of the respondents worked at a tertiary facility, selection bias might have occurred. Second, investigating the preferred second surveillance interval according to the types of polyps observed in the first surveillance colonoscopy was not possible as our survey assumed that the first showed no polyps. Third, although a survey on the major influential factors of surveillance was conducted, we could not investigate their actual effects on the determination of the surveillance interval in each polyp scenario. Despite these limitations, the strength of this study lies in investigating the clinical practice patterns of Korean endoscopists in various polyp scenarios according to recent international guidelines [8]. Furthermore, our findings will provide important clinical implications for the establishment of strategies to increase adherence to the revised Korean guidelines.
In conclusion, the post-polypectomy surveillance intervals preferred by Korean endoscopists varied according to the characteristics of colorectal polyps, and a tendency for early surveillance compared with the recent USMSTF guidelines was observed [8]. Discontinuation of surveillance colonoscopy was usually done when the patient was over 80 years of age. The decision of the surveillance interval is influenced not only by the characteristics of the colorectal polyp but also by the patient and operator factors. Thus, individualized measures are required to increase adherence to the guidelines.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author contributions
Conceptualization: Kim J, Gweon TG, Kim ER, Hong SN, Jung Y, Yang DH. Data curation: all authors. Formal analysis: Kim J, Yang DH. Investigation: Kim J. Methodology: Kim J, Gweon TG, Kim ER, Hong SN, Jung Y, Yang DH. Project administration: Kim J. Supervision: Kim HG, Moon CM, Jung Y, Yang DH. Visualization: Kim J. Writing - original draft: Kim J. Writing - review & editing: Yang DH. Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Material 1.
A web-based nationwide survey on post-polypectomy surveillance
Supplementary Table 1.
Factors Related to Adherence to the USMSTF Guidelines in Univariable Analyses within Adenoma Cases
Supplementary Table 2.
Factors Related to Adherence to the USMSTF Guidelines in Univariable Analyses within SSL Cases
REFERENCES
1. Jung KW, Won YJ, Kang MJ, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2022. Cancer Res Treat. 2022; 54:345–351.

2. Khil H, Kim SM, Hong S, et al. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci Rep. 2021; 11:2413.

3. Cha JM, Kwak MS, Kim HS, et al. Real-world national colonoscopy volume in Korea: a nationwide population-based study over 12 years. Gut Liver. 2020; 14:338–346.

4. Cross AJ, Robbins EC, Pack K, et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut. 2020; 69:1645–1658.

5. Robbins EC, Wooldrage K, Cross AJ. Is surveillance colonoscopy necessary for all patients with bowel polyps? BMJ. 2020; m1706.

6. Pinsky PF, Schoen RE. Contribution of surveillance colonoscopy to colorectal cancer prevention. Clin Gastroenterol Hepatol. 2020; 18:2937–2944.

7. Hong SN, Yang DH, Kim YH, et al. Korean guidelines for postpolypectomy colonoscopic surveillance. Intest Res. 2012; 10:89–109.

8. Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020; 91:463–485.

9. Hassan C, Antonelli G, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline: update 2020. Endoscopy. 2020; 52:687–700.

10. Kim SE, Hong SP, Kim HS, et al. A Korean national survey for colorectal cancer screening and polyp diagnosis methods using web-based survey. Korean J Gastroenterol. 2012; 60:26–35.

11. Hong SN, Yang DH, Kim YH, et al. Surveillance and management. a survey for post-polypectomy surveillance. Intest Res. 2011; 9:118–128.
12. Shin SJ, Lee SH, Park DI, et al. A Korean national survey for treatment modality in colon polypectomy. Intest Res. 2011; 9:196–205.

13. Oh CK, Aniwan S, Piyachaturawat P, et al. Adherence to surveillance guidelines after the removal of colorectal polyps: a multinational, multicenter, prospective survey. Gut Liver. 2021; 15:878–886.

14. Hong S, Suh M, Choi KS, et al. Guideline adherence to colonoscopic surveillance intervals after polypectomy in Korea: results from a nationwide survey. Gut Liver. 2018; 12:426–432.

15. Kim SY, Kwak MS, Yoon SM, et al. Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition. Clin Endosc. 2022; 55:703–725.

16. Lam YF, Seto WK, Tong T, et al. Rates of metachronous adenoma after curative resection for left-sided or right-sided colon cancer. Intest Res. 2018; 16:619–627.

17. Lee JK, Jensen CD, Levin TR, et al. Long-term risk of colorectal cancer and related death after adenoma removal in a large, community-based population. Gastroenterology. 2020; 158:884–894.

18. Laiyemo AO, Murphy G, Sansbury LB, et al. Hyperplastic polyps and the risk of adenoma recurrence in the polyp prevention trial. Clin Gastroenterol Hepatol. 2009; 7:192–197.

19. Rutter MD, East J, Rees CJ, et al. British Society of Gastroenterology/ Association of Coloproctology of Great Britain and Ireland/ Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020; 69:201–223.

20. Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017; 18:823–834.

21. Atkin W, Brenner A, Martin J, et al. The clinical effectiveness of different surveillance strategies to prevent colorectal cancer in people with intermediate-grade colorectal adenomas: a retrospective cohort analysis, and psychological and economic evaluations. Health Technol Assess. 2017; 21:1–536.

22. Morelli MS, Glowinski EA, Juluri R, Johnson CS, Imperiale TF. Yield of the second surveillance colonoscopy based on the results of the index and first surveillance colonoscopies. Endoscopy. 2013; 45:821–826.

23. Imperiale TF, Juluri R, Sherer EA, Glowinski EA, Johnson CS, Morelli MS. A risk index for advanced neoplasia on the second surveillance colonoscopy in patients with previous adenomatous polyps. Gastrointest Endosc. 2014; 80:471–478.

24. Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017; 112:1790–1801.

25. de Jonge V, Sint Nicolaas J, van Leerdam ME, Kuipers EJ, Veldhuyzen van Zanten SJ. Systematic literature review and pooled analyses of risk factors for finding adenomas at surveillance colonoscopy. Endoscopy. 2011; 43:560–572.

26. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014; 147:903–924.

27. May FP, Shaukat A. State of the science on quality indicators for colonoscopy and how to achieve them. Am J Gastroenterol. 2020; 115:1183–1190.

28. Cross AJ, Robbins EC, Saunders BP, Duffy SW, Wooldrage K. Higher adenoma detection rates at screening associated with lower long-term colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2022; 20:e148–e167.

29. Jung Y, Joo YE, Kim HG, et al. Relationship between the endoscopic withdrawal time and adenoma/polyp detection rate in individual colonic segments: a KASID multicenter study. Gastrointest Endosc. 2019; 89:523–530.

30. Shaukat A, Rector TS, Church TR, et al. Longer withdrawal time is associated with a reduced incidence of interval cancer after screening colonoscopy. Gastroenterology. 2015; 149:952–957.

31. Maratt JK, Calderwood AH. Colorectal cancer screening and surveillance colonoscopy in older adults. Curr Treat Options Gastroenterol. 2019; 17:292–302.

32. Săftoiu A, Hassan C, Areia M, et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020; 52:293–304.

33. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021; 325:1965–1977.
34. Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022; 162:285–299.

35. Tran AH, Man Ngor EW, Wu BU. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA Intern Med. 2014; 174:1675–1682.
Fig. 1.
Preferred first and second post-polypectomy surveillance intervals of respondents when the following conventional adenomas were removed by an adequate index colonoscopy in the asymptomatic average-risk population: (A) 1–2 TAs <10 mm, (B) 3–4 TAs <10 mm, (C) 5–10 TAs <10 mm, (D) an adenoma ≥10 mm, (E) an adenoma with tubulovillous or villous histology, (F) an adenoma with HGD, (G) >10 adenomas on single examination, and (H) piecemeal resection of an adenoma ≥20 mm. TA, tubular adenoma; HGD, high-grade dysplasia.
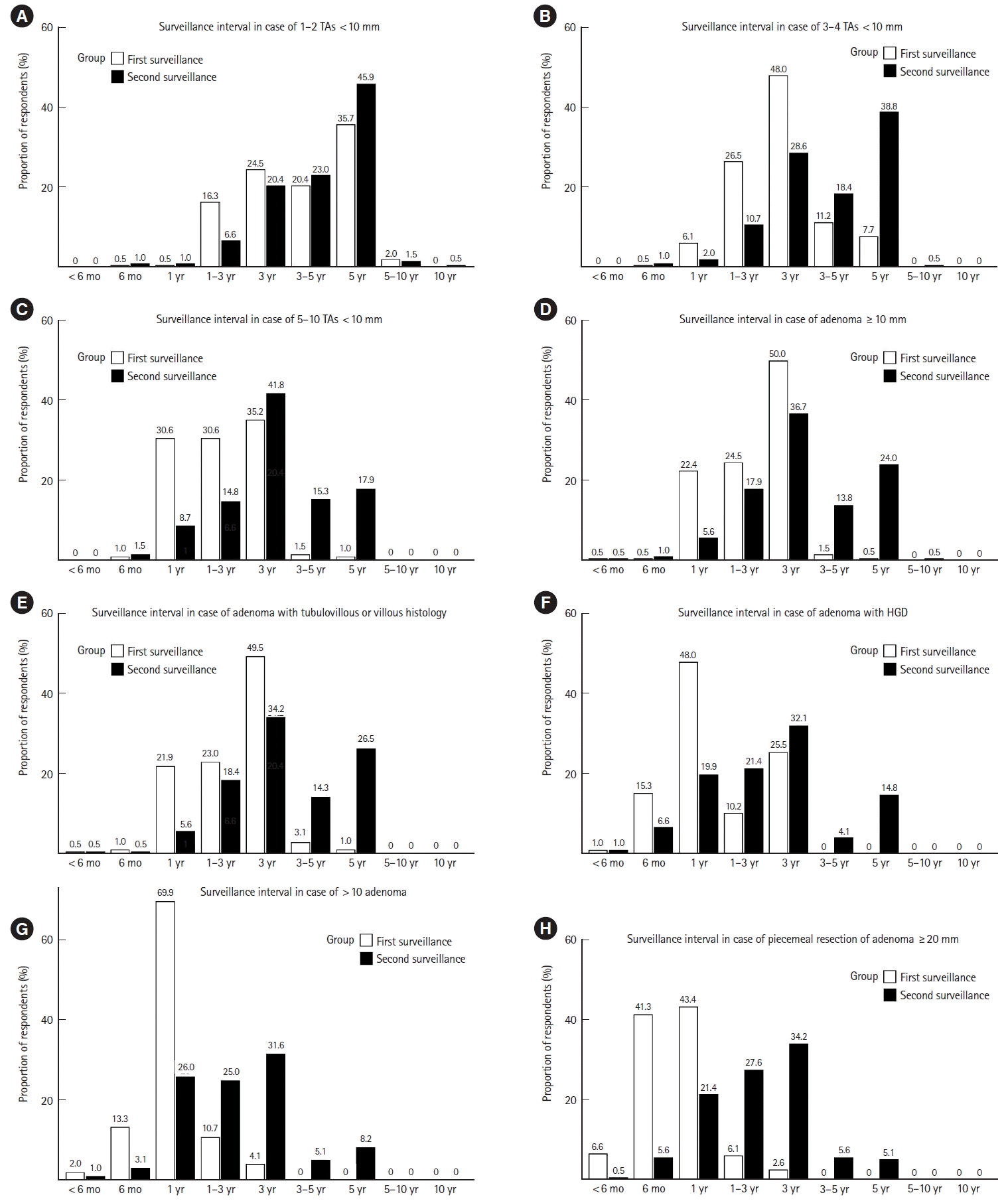
Fig. 2.
Preferred first and second post-polypectomy surveillance intervals of respondents when the following serrated polyps were removed by an adequate index colonoscopy in the asymptomatic average-risk population: (A) ≤20 HPs in the rectum or sigmoid colon <10 mm, (B) ≤20 HPs proximal to the sigmoid colon <10 mm, (C) 1–2 SSLs <10 mm, (D) 3–4 SSLs <10 mm, (E) 5–10 SSLs <10 mm, (F) an SSL ≥10 mm, (G) an SSL with dysplasia, (H) an HP ≥10 mm, (I) a TSA, and (J) piecemeal resection of an SSL ≥20 mm. HP, hyperplastic polyp; SSL, sessile serrated lesion; TSA, traditional serrated adenoma.
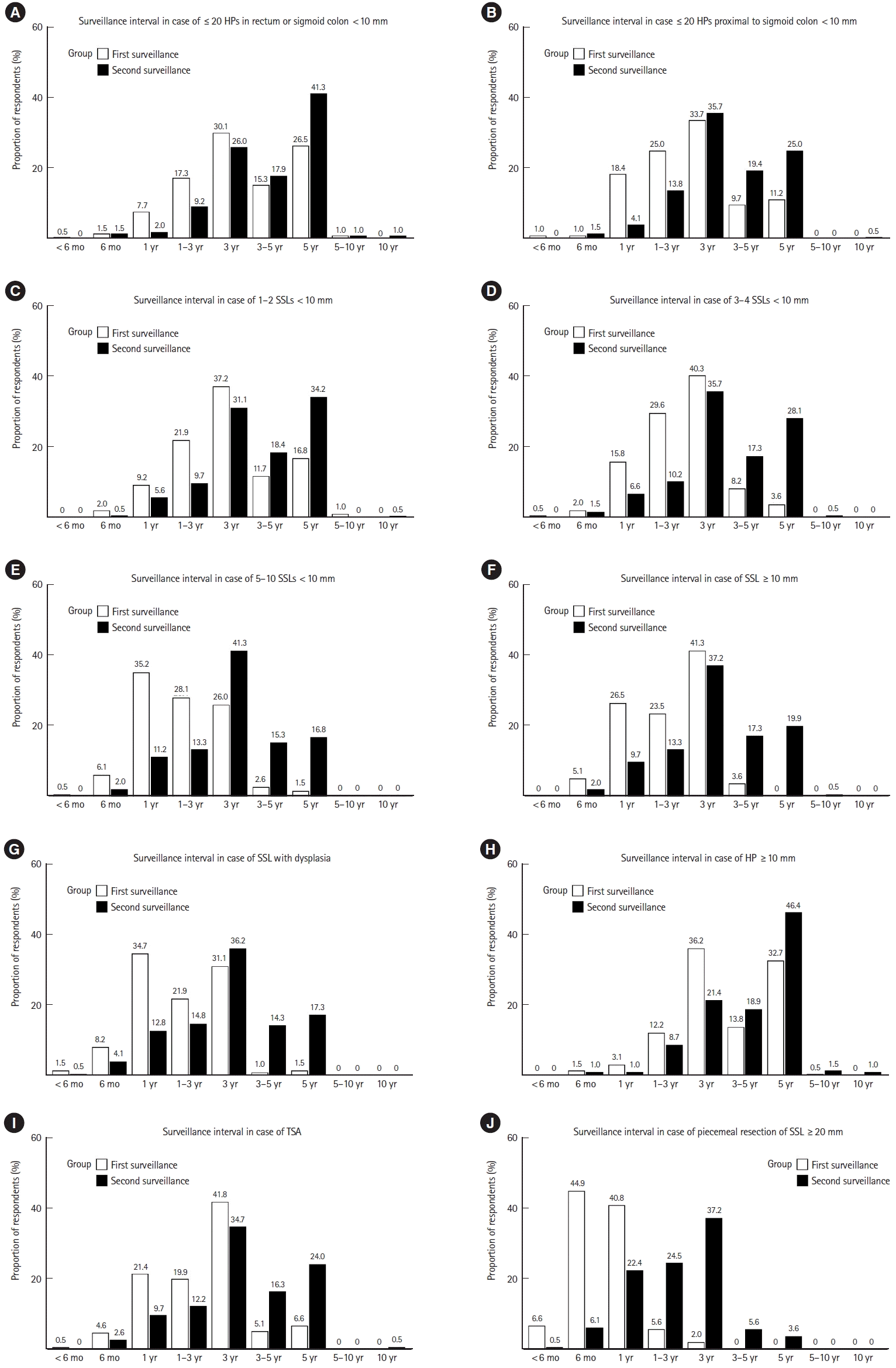
Fig. 3.
Adherence to post-polypectomy surveillance guidelines in various clinical scenarios [8]. TA, tubular adenoma; TVA, tubulovillous adenoma; HGD, high-grade dysplasia; HP, hyperplastic polyp; SSL, sessile serrated lesion; TSA, traditional serrated adenoma.
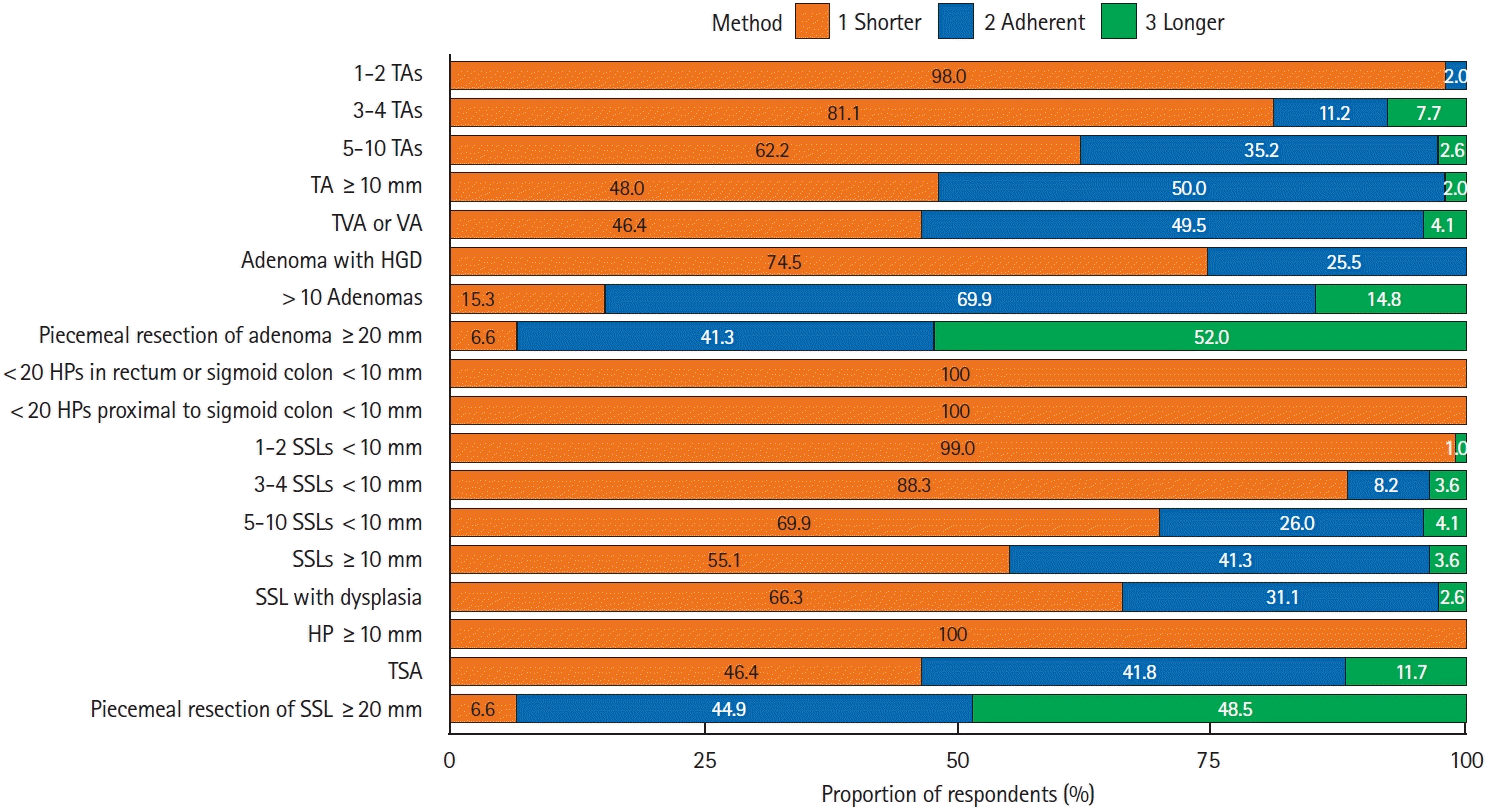
Fig. 4.
Patient age at which post-polypectomy surveillance colonoscopy was discontinued (A, B). HP, hyperplastic polyp; TA, tubular adenoma; TVA, tubulovillous adenoma; HGD, high-grade dysplasia.
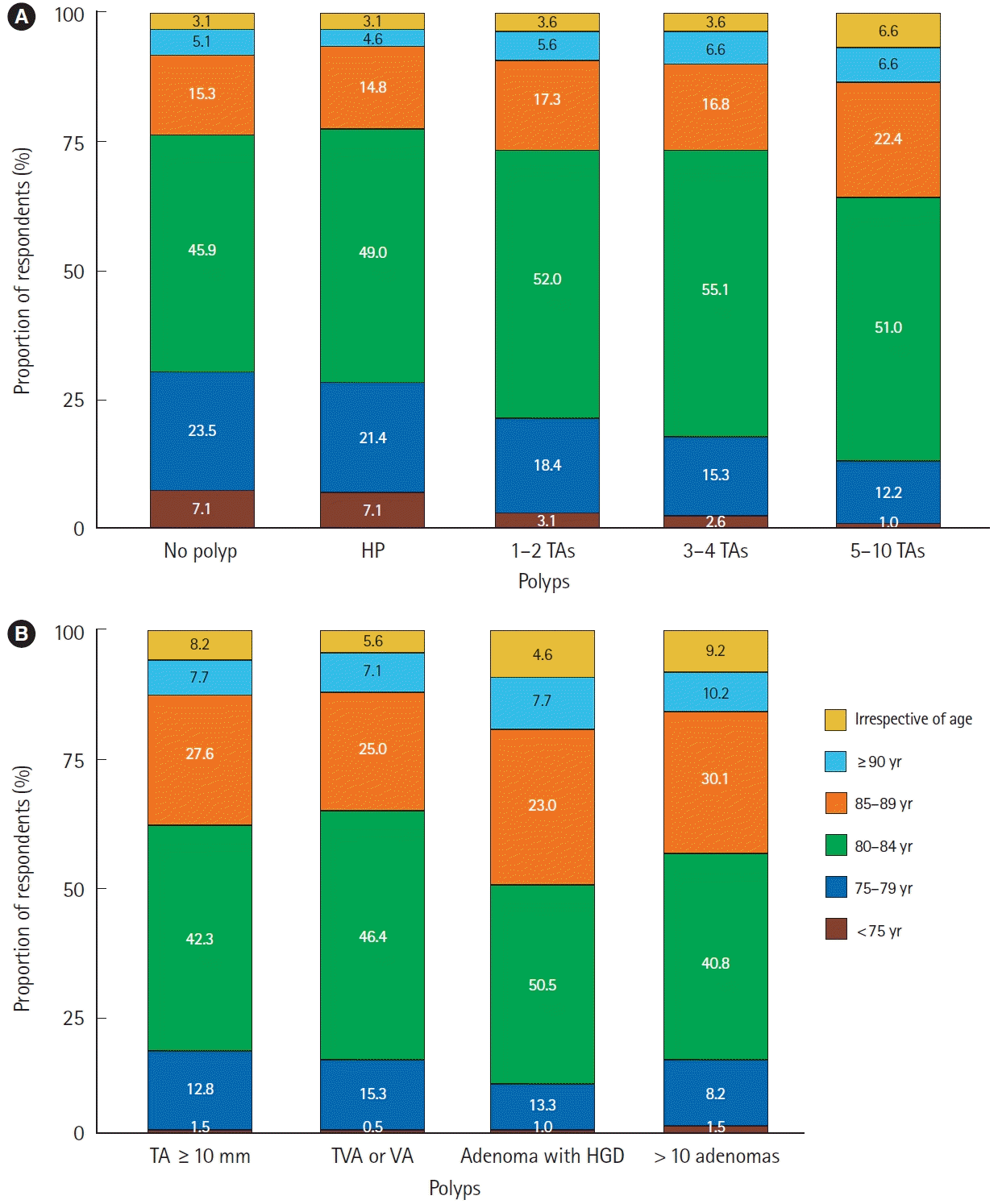
Fig. 5.
Major factors influencing the post-polypectomy surveillance interval compared with the previous study’s results [11]. CRC, colorectal cancer; ADR, adenoma detection rate. aP<0.05, bP<0.01, cP<0.001.
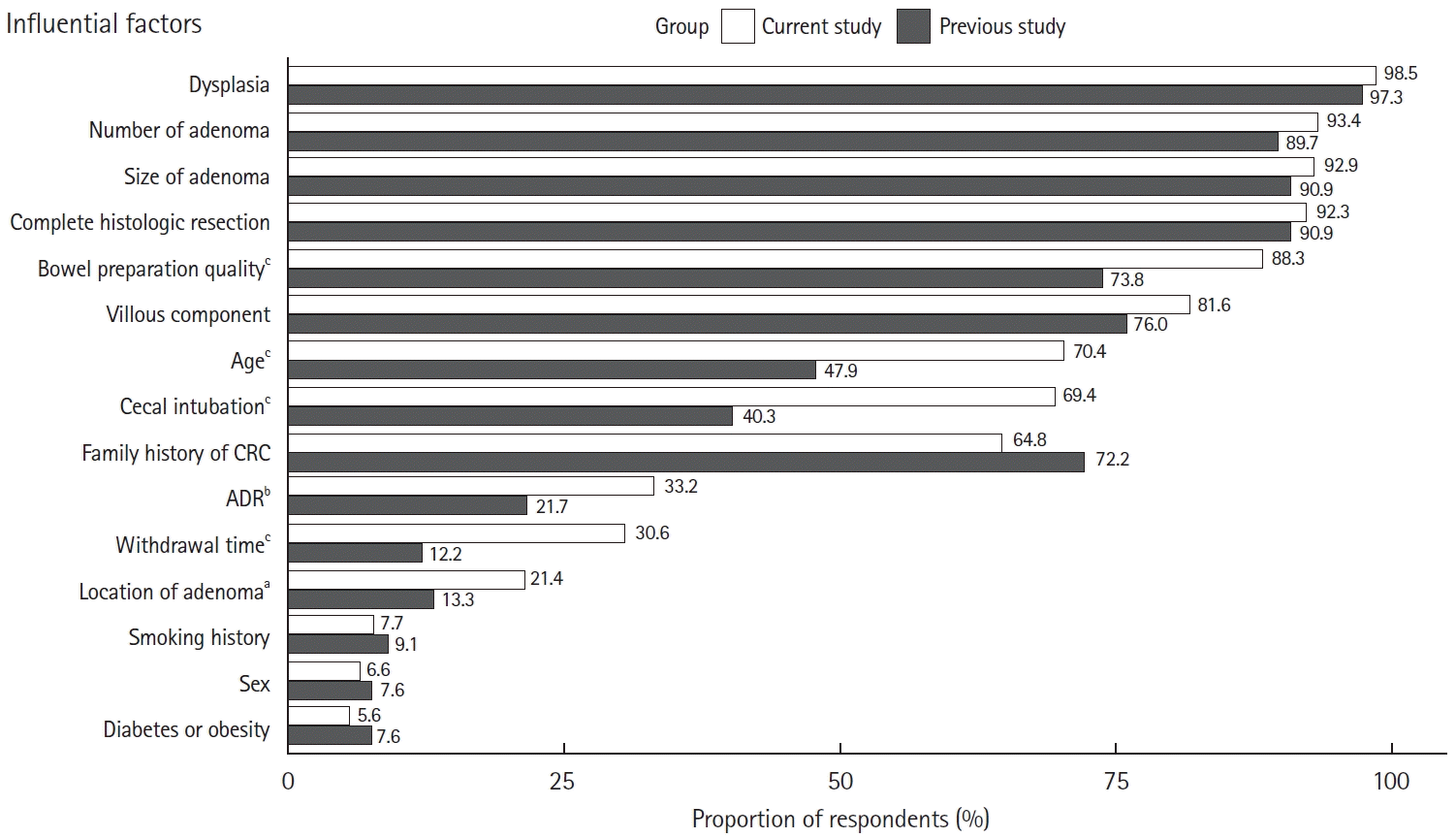
Table 1.
Baseline Characteristics of the Respondents (n=196)
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 149 (76.0) |
| Female | 47 (24.0) |
| Age | |
| < 40 yr | 81 (41.3) |
| 40–49 yr | 83 (42.3) |
| 50–69 yr | 32 (16.3) |
| Specialty board certification | |
| Gastroenterology | 190 (96.9) |
| Othersa | 6 (3.1) |
| Practice hospital | |
| Primary facility | 41 (20.9) |
| Secondary facility | 42 (21.4) |
| Tertiary facility | 113 (57.7) |
| Years in colonoscopy practice | |
| < 3 yr | 49 (25.0) |
| 4–9 yr | 68 (34.7) |
| ≥ 10 yr | 79 (40.3) |
| No. of performed colonoscopies (/mo) | |
| < 49 | 58 (29.6) |
| 50–99 | 75 (38.3) |
| ≥ 100 | 63 (32.1) |
| No. of performed polypectomies (/mo) | |
| < 49 | 81 (41.3) |
| 50–99 | 61 (31.1) |
| ≥ 100 | 53 (27.0) |
| Preferred surveillance test after polypectomy | |
| Colonoscopy | 193 (98.5) |
| Sigmoidoscopy | 10 (5.1) |
| Fecal occult blood test | 6 (3.1) |
| Computed tomography colonography | 1 (0.5) |
| Fecal DNA test | 0 |
| Most influential guidelines determining surveillance | |
| Korean guidelines | 160 (81.6) |
| USMSTF guidelines | 87 (44.4) |
| ESGE guidelines | 41 (20.9) |
| BSG guidelines | 5 (2.6) |
| No influential guidelines | 5 (2.6) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download