Abstract
Objective
This study aims to determine the optimal dose of recombinant-human bone morphogenic protein-2 (rhBMP-2) for successful bone fusion in minimally invasive lateral lumbar interbody fusion (MIS LLIF). Previous studies show that rhBMP is an effective alternative to autologous iliac crest bone graft, but the optimal dose remains uncertain. The study analyzes the fusion rates associated with different rhBMP doses to provide a recommendation for the optimal dose in MIS LLIF.
Methods
Ninety-three patients underwent MIS LLIF using demineralized bone matrix (DBM) or a mixture of rhBMP-2 and DBM as fusion material. The group was divided into the following three groups according to the rhBMP-2 usage : group A, only DBM was used (n=27); group B, 1 mg of rhBMP-2 per 5 mL of DBM paste (n=41); and group C, 2 mg of rhBMP-2 per 5 mL of DBM paste (n=25). Demographic data, clinical outcomes, postoperative complication and fusion were assessed.
Results
At 12 months post-surgery, the overall fusion rate was 92.3% according to Bridwell fusion grading system. Groups B and C, who received rhBMP-2, had significantly higher fusion rates than group A, who received only DBM. However, there was no significant increase in fusion rate when the rhBMP-2 dosage was increased from group B to group C. The groups B and C showed significant improvement in back pain and Oswestry disability index compared to the group A. The incidence of screw loosening was decreased in groups B and C, but there was no significant difference in the occurrence of other complications.
Conclusion
Usage of rhBMP-2 in LLIF surgery leads to early and increased final fusion rates, which can result in faster pain relief and return to daily activities for patients. The benefits of using rhBMP-2 were not significantly different between the groups that received 1 mg/5 mL and 2 mg/5 mL of rhBMP-2. Therefore, it is recommended to use 1 mg of rhBMP-2 with 5 mL of DBM, taking both economic and clinical aspects into consideration.
The efficacy of intervertebral body fusion is pivotal in spnal fusion surgery; failure during this stage can lead to undesirable complications such as pseudoarthrosis, implant migration, or the loosening of pedicle screws [14].
Autologous iliac crest bone grafts have traditionally been the “gold standard” for achieving robust bone fusion in posterior lumbar interbody fusion and transforaminal lumbar interbody fusion (TLIF) procedures [1]. However, the limited availability of autologous bone grafts and the risk of associated complications, including hematoma, infection, and pain, have necessitated the exploration of alternative materials [2]. Therefore, several alternative materials have been introduced to replace iliac crest bone graft. Recombinant-human bone morphogenic protein-2 (rhBMP-2) is an artificial material with the osteoinductive property and is known to be the only one among various osteogenic factors (epidermal growth factor, Insulin-like growth factor, fibroblast growth factor, etc.) that can initiate the entire process of new bone formation [22]. Research has demonstrated that rhBMP-2, when applied to an absorbable collagen sponge, achieves a superior fusion rate of 94.5%, outperforming the 88.7% rate associated with autologous bone grafts [7].
Yet, the effective dosage range for rhBMP-2 remains a contentious point. According to Hofstetter et al. [15], dosages can range from 1.4–12 mg/level, with an average of 4 mg/level and an associated fusion rate of 95% (95% confidence interval [CI], 92.8–96.5%). This result mirror that of the 2020 meta-analysis by Lytle et al. [21], which reported a fusion rate of 94% (95% CI, 91.4–95.8%) associated with an rhBMP-2 dosage range of 1.28 to 12 mg/level. However, early studies also indicated potential complications related to dosage [9,28].
This data underscores the necessity for suggesting a recommendation on the optimal rhBMP-2 dosage. In the research of Lytle et al. [21], the rate of fusion in minimally invasive lateral (MIS) TLIF start to plateau with dosages exceeding 1.01 to 2 mg/level, achieving a fusion rate of 95.2% (95% CI, 94.1–96.3%) with an average dosage of 1.28±0.083 mg/level. To address the current lack of research on the optimal rhBMP-2 dosage for MIS LLIF procedures, this study has been conducted with the aim of suggesting the optimal dosage of rhBMP-2.
We reviewed electronic medical records of a total 93 patients who underwent MIS LLIF and bilateral pedicle screw fixation (PSF) surgery by two neurosurgeons from October 2015 to July 2021. All data were anonymized, and the study was approved by the Institutional Review Board of Chung-Ang University Hospital and performed in compliance with ethical guidelines (approval No. 2306-006-19473).
The inclusion criteria encompassed patients who underwent MIS LLIF and PSF and utilized either demineralized bone matrix (DBM) (Grafton; Medtronics, Memphis, TN, USA) alone or DBM in combination with rhBMP-2 on hydroxyapatite (HA) ceramic (Novosis; CGBIO, Seoul, Korea).
Patients with a history of tumors, infection, or acute fractures were excluded. Additionally, exclusion applied to patients missing any follow-up X-ray images at 3-, 6-, or 12-month post-procedure.
The demographic data collected for this study included sex, age, bone mineral density (BMD), body mass index, diabetes status, smoking status, diagnosis, the number of fusion level and clinical outcomes. Clinical outcomes were evaluated using the Numerical rating system (NRS) for back and leg pain, as well as the Oswestry disability index (ODI) score, assessed preoperatively and at 3, 6, and 12 months after surgery. Electronic medical records and 12-month follow-up images were used to evaluate fusion state and postoperative complications.
MIS LLIF and PSF were performed by two surgeons using the same technique. The patient was placed in a true lateral position and immobilized with plaster. The target level was identified using fluoroscopy imaging. A transverse skin incision of 4–5 cm was made with its center approximately 2 cm from the anterior margin of the target disc. The external oblique, internal oblique, and transverse abdominis muscles were bluntly dissected to access the retroperitoneal space. After retracting the peritoneum with retroperitoneal fat, the psoas muscle could be palpated. The anterior part of the psoas muscle was then bluntly detached from the annulus of the disc. The contralateral annulus was opened with Cobb’s elevators after the intervertebral disc was removed. Endplate preparation was then performed using a shaver and curette [19]. The polyether ether ketone cage (Clydesdale; Medtronic, Minneapolis, MN, USA) was implanted in the disc space, filled with bone fusion material. In group A, only DBM was utilized to fill the center of PEEK cage. For group B, the PEEK cage’s center was filled with a composite of 5 mL DBM and 1 mg of rhBMP-2. While for group C, the center of the cage was filled with a mixture of 5 mL DBM and 2 mg of rhBMP-2.
Following cage placement, posterior fixation was achieved using percutaneous pedicle screw system (Longitude system; Medtronic). Additionally, direct decompression was performed, if necessary, based on the surgeon’s discretion.
Postoperative radiographs, acquired at intervals of 3-, 6-, and 12-month post-surgery, were utilized in the assessment of spinal fusion success. The criteria for a successful fusion were established based on the Bridwell fusion grading system. A successful union was defined as achieving a Bridwell grade of I or II, while a Bridwell grade of III or IV indicated a failure in the fusion process [27]. To enhance the reliability of the fusion assessment, we evaluated the fusion status using dynamic X-ray acquired at 6- and 12-month post-surgery. Per the policy of this institute, in an effort to minimize lumbar motion before the completion of fusion, dynamic X-ray was not performed until 6-month post-surgery. Solid fusion was defined by less than 5° of motion and 1 mm of translation. Conversely, dynamic instability was characterized by a difference of ≥3 mm in the measured translation or by more than 10° of motion [5,10,16].
To determine any significant disparities in fusion rates and clinical outcomes among groups A, B, and C at the 3-, 6-, and 12-month postoperative marks, we employed a Pearson’s chi-square test. A p-value of less than 0.05 was interpreted as statistically significant. In cases where significant differences were detected across the three groups, subsequent post hoc tests were performed on group pairs to pinpoint the specific groups manifesting significant differences. All data were analyzed using IBM SPSS Statistics for Windows (version 26; IBM corp., Armonk, NY, USA).
Of the 113 patients who underwent MIS LLIF and PSF, three who had a history of infection and one with an acute fracture were excluded. Sixteen patients were lost to follow-up within 12 months after surgery. Consequently, a total of 93 patients, representing 169 interbody spaces, were selected for the study.
Demographic data are presented in Table 1. The mean ages at the time of surgery for groups A, B, and C were 64.6±9.5, 66.4±10.8, and 67±5.4 years, respectively. The BMD values were -1.4±1.2, -1.5±1.1, and -1.6±1.3, and the proportion of smokers were 25.9%, 21.9%, and 42.8%, respectively. There were no significant differences in demographic data among three groups.
At the 3-month postoperative period, group A exhibited a fusion rate of 12.5%, which was significantly lower than the fusion rates of group B (34.2%) and group C (35.6%). Group B exhibited a significantly higher fusion rate compared to group A, while there was no significant difference between group B and group C. When evaluated 6 months after surgery, Group A demonstrated a fusion rate of 41.7%, while group B and C exhibited markedly higher fusion rates of 73.7% and 82.2%, respectively. Similarly, the fusion rate in group B was statistically higher than that in group A, however, no significant variance was evident between groups B and C. One year following surgery, group A’s fusion rate was observed to be 83.3%, while groups B and C reported fusion rates of 96.1% and 95.6%, respectively. The fusion rate in group B was significantly superior to group A, whereas the difference between group B and group C was statistically insignificant. These results underscore the potential of rhBMP-2 to enhance fusion rates postoperatively (Table 2 and Fig. 1).
Six months after operation, 64.6% of group A achieved solid fusion which was significantly lower than the fusion rates of group B (80.3%) and group C (86.7%). When evaluated 12 months after surgery, group A demonstrated a fusion rate of 87.5%, while groups B and C exhibited markedly higher fusion rates of 98.7% and 100.0%, respectively (Table 3).
The preoperative Numeric rating scale for back pain (NRS-B) scores in groups A, B, and C were determined to be 5.5±1.8, 5.1±2.2, and 4.9±2.1, respectively. At 3 months after surgery, the NRS-B scores for groups A, B, and C were observed to be 3.7±1.6, 3.4±0.8, and 3.5±1.2. At 6 months post-surgery, the NRS-B scores for groups A, B, and C were 2.8±1.3, 2.0±0.8, and 1.7±0.9, respectively. By 12 months post-surgery, the NRS-B scores for these groups were 1.9±0.5, 1.8±0.6, and 1.8±0.4, respectively.
The preoperative NRS for leg pain (NRS-L) scores in groups A, B, and C were initially recorded as 5.5±1.8, 5.1±2.2, and 4.9±2.1, respectively. At 3 months after surgery, the NRS-L scores for groups A, B, and C were 2.6±1.3, 2.4±1.2, and 2.6±1.5. At 6 months after surgery, NRS-L scores for each group were 2.4±1.1, 2.3±1.0, and 2.1±1.2. By 12 months post-surgery, NRS-L scores for group A, group B, and group C were 1.8±0.5, 1.9±0.5, and 1.9±0.6, respectively. Notably, a significant decrease in NRS-B scores at 6 months post-surgery was observed in groups B and C when compared to group A. However, there was no statistically significant difference in NRS-L scores between the three groups at any time point.
Preoperative ODI values for groups A, B, and C were documented as 46.2±18.2, 45.1±14.7, and 47.8±21.8, respectively. At 3 months post-surgery, the ODI values were observed to be 29.2±11.5, 28.3±8.7, and 26.9±13.3. At 6 months post-surgery, the ODI values were recorded as 22.4±9.1, 18.7±7.8, and 16.7±6.6 for groups A, B, and C, respectively. By 12 months post-surgery, ODI values in groups A, B, and C were 18.8±8.8, 17.5±6.7, and 16.4±5.8, respectively. Similarly, to the NRS-B scores, the ODI values showed significant improvement in groups B and C when compared to group A at 6 months after surgery (Table 4).
In group A, complications were observed in eight patients (29.6%), while in groups B and C, complications occurred in seven patients (17.1%) and five patients (20%), respectively. Cage subsidence was noted in 10 levels (20.8%) affecting five patients (18.5%) in group A at 12 months post-surgery. In group B, cage subsidence was identified in 10 levels (13.1%) involving six patients (14.6%). For group C, cage subsidence occurred in eight levels (17.8%) among five patients (20%). No significant differences were found in the incidence of cage subsidence between the groups. Additionally, there were no reported cases of surgical site infection or wound dehiscence.
Screw loosening was observed in eight patients (29.6%) in group A, while in groups B and C, it occurred in two patients (4.9%) and two patients (8.0%), respectively. rhBMP-2 has been shown to aid in reducing the occurrence of screw loosening with statistical significance (Table 5).
During the follow-up period, no patients reported incidents of retrograde ejaculation or post-operative infections. Follow-up computed tomography (CT) and magnetic resonance imaging (MRI) were conducted for patients who continued to experience radiculopathy over an extended period post-surgery; these scans revealed no evidence of seroma or ectopic bone formation.
Frequently, rhBMP-2 is employed by surgeons to mitigate the risk of pseudarthrosis, particularly when risk factors such as tobacco usage, compromised bone quality, and multilevel fusions are present [21,29]. Nonetheless, a standardized dosage of rhBMP for any of spinal fusion surgery approach remains elusive, and research on this topic is notably sparse [21].
As delineated in the guidelines for fusion procedures for degenerative disease of the lumbar spine, authored by Kaiser et al. [17], previous studies have reported a fusion rate of 76.9% with DBM at 12 months after surgery and 75% at 24 months post-surgery. Numerous prior studies have examined the fusion rate in lumbar fusion surgery using rhBMP-2. The literature consistently shows that the use of rhBMP-2 enhances the fusion success rate when compared to employing iliac crest bone graft or DBM alone [4,8,13].
While study designs show heterogeneity in terms of operative technique, type of bone extender used together, range of follow up periods, dosage of rhBMP-2, the fusion rate ranged from 95.8–100% across studies [9,25,27,29]. These results are consistent with the results of our investigation. The rhBMP-2 dosage per level in previous studies varied widely, from 0.16 mg/level to 12 mg/level [21]. However, there have been well-documented dose related complications with the use of rhBMP-2 such as heterotopic bone growth or cage subsidence. Singh et al. [26] conducted a review of 573 patients who underwent MIS TLIF with either a small (4.2 mg) or a large (12 mg) kit of rhBMP-2 per patient and noted 327 patients (57.0%) had transient post-operative radiculitis and 1.7% required revision surgery due to symptomatic radiculitis. Yet, reports of heterotopic bone growth or radiculitis following the utilization of a smaller dose (less than 2 mg) of rhBMP-2 remain scant. Therefore, it is not judicious to unconditionally administer a large dose of rhBMP-2 to augment the fusion rate.
Pertaining to the transforaminal approach, there is a report that there is no substantial alteration in fusion rate when the rhBMP-2 dosage exceeds 1 mg/level [21]. However, studies examining the usage of BMP in a lateral approach are relatively sparse, with no established guidelines on optimal usage so far. Our study aimed to suggest the minimal effective dose of rhBMP-2 with HA carrier in MIS LLIF. As reflected in our findings, the fusion rate at each evaluation point was significantly higher in rhBMP-2 groups than the group that used only DBM. This result shows that the application of rhBMP-2 not only enhances the final fusion rate, but also promotes the early fusion. However, elevating the dosage of rhBMP-2 from 1 mg/5 mL to 2 mg/5 mL did not result in a significant improvement in either overall fusion rate or early fusion.
Previous research has shown that if pseudoarthrosis manifests after lumbar interbody fusion surgery, it may precipitate instability of the lumbar joint, potentially leading to complications such as back pain and screw loosening [11,18,24]. In the current study, at 6 months postoperatively, patients treated solely with DBM exhibited a fusion success rate of less than 50%. In contrast, patients who received rhBMP-2 demonstrated a high fusion rate of over 70%―a rate akin to the 12-month postoperative fusion rate in the DBM group. These radiological outcomes corresponded with the clinical results of our study. Six months post-surgery, the back pain and disability levels reported by patients receiving rhBMP-2 were similar to those reported by the DBM-only patients at the 12-month mark. Considering many patients anticipate rapid pain relief after surgery, these findings suggest that usage of rhBMP-2 on HA ceramic can provide clinical effectiveness.
Pedicle screw loosening represents a delayed postoperative complication that may coincide with pseudoarthrosis, adjacent level degeneration, and progressive kyphosis, potentially resulting in chronic back pain [18,23]. Previous studies indicate that an increased range of movements in a spinal segment can amplify stress on the screw-bone interface, which may contribute to pedicle screw loosening [3,20,23]. Earlier research suggests that screw loosening typically manifests around 5 to 8 months post-surgery, and it is posited that achieving early fusion, approximately the 6-month mark, could alleviate segmental stress and subsequently diminish the incidence of screw loosening [18,23]. This is in line with our study’s findings, which showed a statistically significant decline in the incidence of screw loosening when rhBMP-2 was applied.
Most previous studies have utilized products that use collagen sponge as a carrier for rhBMP-2 transportation. However, the binding affinity between the collagen sponge and rhBMP-2 is weak, leading to an excessive and rapid release shortly after the insertion of rhBMP-2. This initial rapid release has been reported to potentially increase the risk of various early complications associated with rhBMP-2 [6]. In our study, we utilized products that use HA ceramic as a carrier, a factor that might be the reason for the lower incidence of complications related to rhBMP-2 compared to previous studies.
We sought to establish the minimal effective dose of rhBMP-2 on HA ceramic product in MIS LLIF. As illustrated in the Fig. 2, the fusion rate at each evaluation point was significantly higher in the rhBMP-2 groups. However, escalating the dosage of rhBMP-2 from 1 mg/5 mL to 2 mg/5 mL did not yield a significant improvement in fusion rate. The findings suggest that the fusion rate begins to plateau with doses exceeding 1 mg/5 mL.
The study has several limitations. Firstly, the research design was a retrospective analysis of prospectively collected data, leading to a lack of randomization. This could potentially introduce selection bias and confounding factors. Secondly, we only conducted CT scans or MRI scans for patients who persistently reported symptoms during outpatient visits. This implies the potential discrepancy between the fusion rate we assessed and the actual fusion rate, as well as the limitation that we could not identify asymptomatic rhBMP-2 related complications. However, we were unable to implement routine advanced image scans in our institution due to the potential health risks of increased radiation exposure and the associated financial costs. Thirdly, the observation period of our study was relatively short to assess the result of spinal fusion surgery. To obtain more conclusive result, it is needed to conduct a follow-up study spanning a longer duration, exceeding 2 years, to evaluate the subjects of this study. Forthly, despite our study demonstrating a statistically significant correlation between early fusion success rate and pain improvement, no causal relationship can be inferred from the present results. To our knowledge, no previous studies have substantiated a correlation between successful bone fusion and back pain improvement. Fifth, the MIS LLIF procedures in this study were performed at a single institution by two surgeons. Although this design is still subject to variation between surgeons, it does control for discrepancy across multiple hospitals, which has been proven to be highly variable [12].
Our study illustrates that the application of rhBMP-2 with HA ceramic in LLIF surgery yields both early fusion success and an enhanced final fusion rate. This implies that rhBMP-2 may expedite pain relief and facilitate patients’ rapid return to daily activities. Furthermore, early fusion at the 6-month postoperative stage may potentially decrease the incidence of complications such as screw loosening, minimize the need for revision surgery, and augment pain relief.
Intriguingly, the advantages derived from application of rhBMP-2 with HA ceramic did not significantly differ between the groups administered 1 mg/5 mL and 2 mg/5 mL. As such, a dose of 1 mg/5 mL might be optimal considering both economic and clinical perspectives.
These insights hold considerable implications for clinical practice. The utilization of rhBMP-2 with HA ceramic can heighten the success rate of LLIF surgery, diminish the probability of complications, and thereby, enhance patient outcomes and quality of life. Thus, this study endorses the use of rhBMP-2 with HA ceramic as a viable option in spinal fusion surgery.
Notes
Conflicts of interest
Seung Won Park has been editorial board of JKNS since November 2014. He was not involved in the review process of this original article. No potential conflicts of interest relevant to this study exist.
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
1. Adams CL, Ogden K, Robertson IK, Broadhurst S, Edis D. Effectiveness and safety of recombinant human bone morphogenetic protein-2 versus local bone graft in primary lumbar interbody fusions. Spine (Phila Pa 1976). 39:164–171. 2014.

2. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. (329):300–309. 1996.

3. Bisschop A, van Engelen SJ, Kingma I, Holewijn RM, Stadhouder A, van der Veen AJ, et al. Single level lumbar laminectomy alters segmental biomechanical behavior without affecting adjacent segments. Clin Biomech (Bristol, Avon). 29:912–917. 2014.

4. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976). 27:2662–2673. 2002.

5. Boden SD, Wiesel SW. Lumbosacral segmental motion in normal individuals. Have we been measuring instability properly? Spine (Phila Pa 1976). 15:571–576. 1990.
6. Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 32:5241–5251. 2011.

7. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 15:337–349. 2002.

8. Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 16:113–122. 2003.

9. Carreon LY, Glassman SD, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: a cost-utility study. Spine (Phila Pa 1976). 34:238–243. 2009.

10. Choudhri TF, Mummaneni PV, Dhall SS, Eck JC, Groff MW, Ghogawala Z, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 21:23–30. 2014.

11. Chun DS, Baker KC, Hsu WK. Lumbar pseudarthrosis: a review of current diagnosis and treatment. Neurosurg Focus. 39:E10. 2015.

12. Desai A, Bekelis K, Ball PA, Lurie J, Mirza SK, Tosteson TD, et al. Variation in outcomes across centers after surgery for lumbar stenosis and degenerative spondylolisthesis in the spine patient outcomes research trial. Spine (Phila Pa 1976). 38:678–691. 2013.

13. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 4:527–538. discussion 538-539. 2004.

14. Han S, Park B, Lim JW, Youm JY, Choi SW, Kim DH, et al. Comparison of fusion rate between demineralized bone matrix versus autograft in lumbar fusion : meta-analysis. J Korean Neurosurg Soc. 63:673–680. 2020.

15. Hofstetter CP, Hofer AS, Levi AD. Exploratory meta-analysis on dose-related efficacy and morbidity of bone morphogenetic protein in spinal arthrodesis surgery. J Neurosurg Spine. 24:457–475. 2016.

16. Iii WS, Orías AAE, Shifflett GD, Lee JYB, Siemionow K, Gandhi S, et al. Image-based markers predict dynamic instability in lumbar degenerative spondylolisthesis. Neurospine. 17:221–227. 2020.

17. Kaiser MG, Groff MW, Watters WC 3rd, Ghogawala Z, Mummaneni PV, Dailey AT, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion. J Neurosurg Spine. 21:106–132. 2014.

18. Kim JW, Park SW, Kim YB, Ko MJ. The effect of postoperative use of teriparatide reducing screw loosening in osteoporotic patients. J Korean Neurosurg Soc. 61:494–502. 2018.

19. Ko MJ, Park SW, Kim YB. Effect of cage in radiological differences between direct and oblique lateral interbody fusion techniques. J Korean Neurosurg Soc. 62:432–441. 2019.

20. Lee KK, Teo EC. Effects of laminectomy and facetectomy on the stability of the lumbar motion segment. Med Eng Phys. 26:183–192. 2004.

21. Lytle EJ, Slavnic D, Tong D, Bahoura M, Govila L, Gonda R, et al. Minimally effective dose of bone morphogenetic protein in minimally invasive lumbar interbody fusions: six hundred ninety patients in a dose-finding longitudinal cohort study. Spine (Phila Pa 1976). 44:989–995. 2019.

22. Ozkaynak E, Schnegelsberg PN, Jin DF, Clifford GM, Warren FD, Drier EA, et al. Osteogenic protein-2. A new member of the transforming growth factor-beta superfamily expressed early in embryogenesis. J Biol Chem. 267:25220–25227. 1992.

23. Pearson HB, Dobbs CJ, Grantham E, Niebur GL, Chappuis JL, Boerckel JD. Intraoperative biomechanics of lumbar pedicle screw loosening following successful arthrodesis. J Orthop Res. 35:2673–2681. 2017.

24. Raizman NM, O’Brien JR, Poehling-Monaghan KL, Yu WD. Pseudarthrosis of the spine. J Am Acad Orthop Surg. 17:494–503. 2009.

25. Rihn JA, Makda J, Hong J, Patel R, Hilibrand AS, Anderson DG, et al. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: a clinical and radiographic analysis. Eur Spine J. 18:1629–1636. 2009.

26. Singh K, Nandyala SV, Marquez-Lara A, Cha TD, Khan SN, Fineberg SJ, et al. Clinical sequelae after rhBMP-2 use in a minimally invasive transforaminal lumbar interbody fusion. Spine J. 13:1118–1125. 2013.

27. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 7:301–307. 2007.

Fig. 1.
Fusion assessment by bridwell grading system. *Post hoc analysis showed a statistically significant difference between group A and group B (p<0.05). **Post hoc analysis showed a statistically significant difference between group A and group C (p<0.05), group B and C showed no statistical difference.
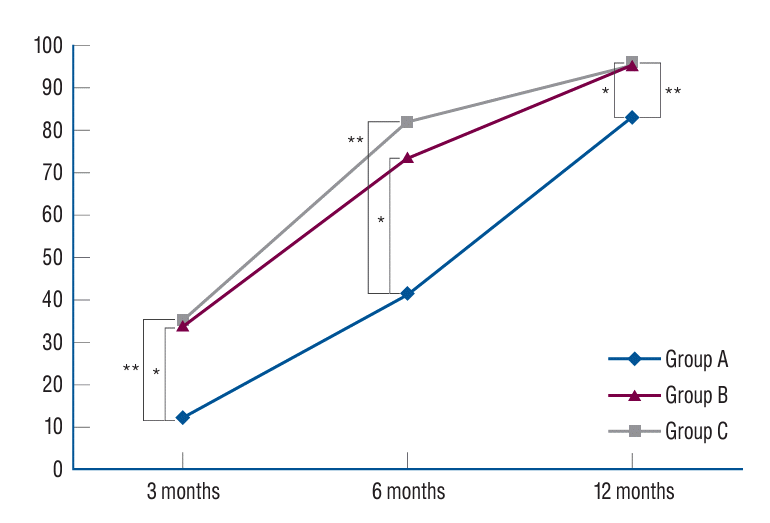
Fig. 2.
Developed figures to show progressive change at each time point that was assessed. Goal of figures are to show continuity in assessment and comparability in the technique of the assessment. A : A 62-year-old female of group A at 3, 6, and 12 months after surgery. B : A 68-year-old male of group B at 3, 6, and 12 months after operation. C : A 59-year-old male of group C at 3, 6, and 12 months.
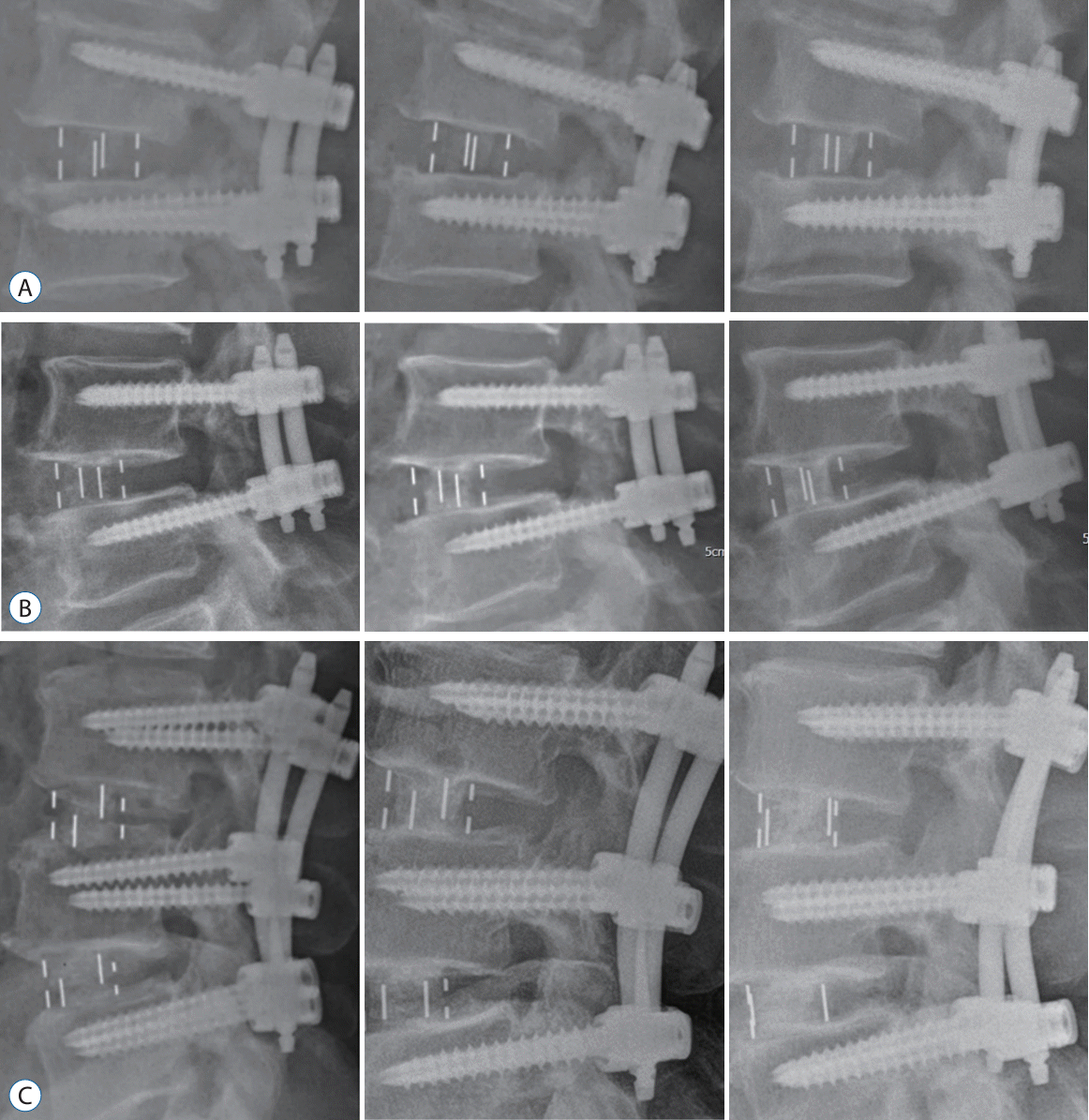
Table 1.
Demographic data
Table 2.
Fusion assessment by bridwell grading system
| Group A (level=48) | Group B (level=76) | Group C (level=45) | p-value | |
|---|---|---|---|---|
| 3 months | 6 (12.5) | 26 (34.2) | 16 (35.6) | 0.016* |
| 6 months | 20 (41.7) | 56 (73.7) | 37 (82.2) | <0.001* |
| 12 months | 40 (83.3) | 73 (96.1) | 43 (95.6) | 0.043* |
Table 3.
Fusion assessment via dynamic X-ray : proportion achieving solid fusion
| Group A (level=48) | Group B (level=76) | Group C (level=45) | p-value* | |
|---|---|---|---|---|
| 6 months | 31 (64.6) | 61 (80.3) | 39 (86.7) | 0.041 |
| 12 months | 42 (87.5) | 75 (98.7) | 45 (100.0) | 0.028 |
Table 4.
Numeric rating scale (NRS) and Oswestry disability index (ODI)
| Group A | Group B | Group C | p-value | |
|---|---|---|---|---|
| NRS-B | ||||
| Pre | 5.5±1.8 | 5.1±2.2 | 4.9±2.1 | >0.05 |
| 3 months | 3.7±1.6 | 3.4±0.8 | 3.5±1.2 | >0.05 |
| 6 months | 2.8±1.3 | 2.0±0.8 | 1.7±0.9 | 0.04* |
| 12 months | 1.9±0.5 | 1.8±0.6 | 1.8±0.4 | >0.05 |
| NRS-L | ||||
| Pre | 5.5±1.8 | 5.1±2.2 | 4.9±2.1 | >0.05 |
| 3 months | 2.6±1.3 | 2.4±1.2 | 2.6±1.5 | >0.05 |
| 6 months | 2.4±1.1 | 2.3±1.0 | 2.1±1.2 | >0.05 |
| 12 months | 1.8±0.5 | 1.9±0.5 | 1.9±0.6 | >0.05 |
| ODI | ||||
| Pre | 46.2±18.2 | 45.1±14.7 | 47.8±21.8 | >0.05 |
| 3 months | 29.2±11.5 | 28.3±8.7 | 26.9±13.3 | >0.05 |
| 6 months | 22.4±9.1 | 18.7±7.8 | 16.7±6.6 | 0.038* |
| 12 months | 18.8±8.8 | 17.5±6.7 | 16.4±5.8 | >0.05 |
Table 5.
Post operative complications
| Group A | Group B | Group C | p-value | |
|---|---|---|---|---|
| Cage subsidence | 10 (20.8) | 10 (13.1) | 8 (17.8) | 0.64 |
| Screw loosening | 8 (29.6) | 2 (4.9) | 2 (8.0) | 0.03* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download