Abstract
Background
This study investigated the relationship between intraoperative requirement for an inhalational anesthetic (sevoflurane) or an opioid (remifentanil) and postoperative analgesic consumption.
Methods
The study included 200 adult patients undergoing elective laparoscopic colectomy. In the sevoflurane group, the effect-site concentration of remifentanil was fixed at 1.0 ng/ml, while the inspiratory sevoflurane concentration was adjusted to maintain an appropriate anesthetic depth. In the remifentanil group, the end-expiratory sevoflurane concentration was fixed at 1.0 vol.%, and the remifentanil concentration was adjusted. Pain scores and cumulative postoperative analgesic consumptions were evaluated at 2, 6, 24, and 48 h after surgery.
Results
Average end-tidal concentration of sevoflurane and effect-site concentration of remifentanil were 2.0 ± 0.4 vol.% and 3.9 ± 1.4 ng/ml in the sevoflurane and remifentanil groups, respectively. Cumulative postoperative analgesic consumption at 48 h postoperatively was 55 ± 26 ml in the sevoflurane group and 57 ± 33 ml in the remifentanil group. In the remifentanil group, the postoperative cumulative analgesic consumptions at 2 and 6 h were positively correlated with intraoperative remifentanil requirements (2 h: r = 0.36, P < 0.001; 6 h: r = 0.38, P < 0.001). However, there was no significant correlation in the sevoflurane group (r = 0.04, P = 0.691).
Conclusions
The amount of intraoperative requirement of short acting opioid, remifentanil, is correlated with postoperative analgesic consumption within postoperative 6 h. It may be contributed by the development of acute opioid tolerance. However, intraoperative sevoflurane requirement had no effect on postoperative analgesic consumption.
Acute postoperative pain often is inadequately managed in clinical practice. In previous reports [1,2], more than 80% of surgical patients experience acute postoperative pain, and more than 75% of these experience moderate, severe, or extreme pain during the immediate postoperative period. Inadequately controlled postoperative pain can lead to complications and increase postoperative morbidity and mortality [1,3]. Appropriate postoperative pain management leads to shortened hospital stays, reduced hospital costs, and increased patient satisfaction as well as a decrease in perioperative morbidity and mortality [4,5]. Therefore, effective management of acute postoperative pain is important after surgery. The cornerstone of pharmacological regimens after major surgery is opioids, including morphine and fentanyl. However, opioids have significant side effects, such as nausea and vomiting, pruritus, urinary retention, constipation, sedation, and respiratory depression which limit their use [6].
Pain is a highly subjective and multidimensional experience that consists of physiological, emotional, and behavioral components [7,8]. Individual variability in any of these factors can lead to different pain experiences and variable responses to therapies [9,10]. The consumption of analgesics in intravenous (IV) patients-controlled analgesia (PCA) should be properly controlled to prevent side-effects caused by over-dosing and severe pain caused by under-dosing. Therefore, identification of predictive risk factors of postoperative pain can lead to more individualized and effective pain management strategies. This approach will prevent over-dosing of analgesics in low-risk patients and thus reduce the risk of adverse effects from postoperative analgesic medications. However, it is very difficult to anticipate a patient’s intensity of pain because pain is a highly personal and subjective experience.
In the current trend of balanced anesthesia, antinociception is the main component of general anesthesia [11]. Anesthetic requirements might be affected by individual nociception during the surgical procedure. Wang et al. [12] reported that patients’ preoperative pain sensitivity was correlated with their stress response during endotracheal intubation and skin incision. Pain tolerance was negatively correlated with changes in blood pressure (BP), heart rate (HR), and plasma norepinephrine level before and after intubation and skin incision. This finding suggests that higher pain sensitivity may be associated with increased anesthetic requirement to control sympathetic stimulation during the surgical procedure.
The authors speculated that intraoperative anesthetic requirement is a predictive factor of postoperative pain intensity. If such a correlation exists, then patients can be adequately managed with a personalized analgesia. Moreover, the intraoperative anesthetic consumption is recorded on the anesthetic record, so anesthesiologists and surgeons can easily find and measure it. The authors hypothesized that patients who require a larger amount of anesthetics to maintain an appropriate depth of anesthesia during the operation will consume more analgesics to achieve adequate postoperative analgesia. The purpose of the current prospective study was to investigate the relationship between intraoperative requirements for the inhalational anesthetic sevoflurane or the opioid remifentanil and postoperative analgesic consumption in patients undergoing laparoscopic colectomy.
This prospective study was approved by the Institutional Review Board (CUH201610025002) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at Clinical Research Information Service - Republic of Korea in the World Health Organization International Clinical Trials Registry Platform (KCT0004743). The study included 200 patients from 20–80 years of age with an American Society of Anesthesiologists physical status of I or II who were undergoing elective laparoscopic colectomy due to colon cancer. Exclusion criteria were administration of analgesics, steroids, sedatives, or antidepressants within 48 hours of surgery and cardiovascular, respiratory, hepatic, renal, or central nervous system disease. We also excluded patients with documented history of postoperative nausea and vomiting (PONV) and hypersensitivity to fentanyl or ketorolac, and patients who inability to use an IV PCA device. The participants were assigned randomly to either the sevoflurane or remifentanil group using a computer-generated block randomization with random permuted block size of 4 to ensure a similar number of patients in each group over time. The NPRS, cumulative analgesic consumption, and any side effects were assessed by one anesthesiologist to prevent interrater bias. This anesthesiologist was not aware of anesthetic technique or study assignment.
The evening before surgery, all patients were taught how to operate the PCA pump and were educated about the postoperative pain assessment method using an 11-point numerical pain rating scale (NPRS), where 0 = no pain and 10 = the worst possible pain. In addition, the authors explained that the target postoperative NPRS score is 2–3 at rest. The anesthetic regimen was standardized for all patients. The patients received 1.0–2.0 mg/kg propofol, 3–5 ng/ml of effect-site concentration of remifentanil, and 1.0 mg/kg rocuronium to induce anesthesia. Remifentanil was administered using a Minto model effect-site target-controlled infusion pump (Orchestra® Base Primea, Fresenius Vial). After tracheal intubation, anesthesia was maintained with sevoflurane and remifentanil. In the sevoflurane group, the effect-site concentration of remifentanil was fixed at 1.0 ng/ml, while the inspiratory sevoflurane concentration was adjusted to maintain the desired anesthetic depth with appropriate BP (within 20% of preoperative value) and bispectral index (BIS) (< 60). In contrast, the end-tidal sevoflurane concentration was fixed at 1.0 vol.% and the remifentanil concentration was adjusted in the remifentanil group. The patient’s vital signs, BIS, end-expiratory sevoflurane concentration, and effect-site concentration of remifentanil were measured every 5 min and recorded at the skin incision (SI), at 10, 30, 60, 90, and 120 min after the SI. If the BIS is higher than 60 in the sevoflurane group, the concentration of sevoflurane is increased and the study is discontinued to prevent the risk of intraoperative awareness. The average end-tidal concentration of sevoflurane and effect-site concentration of remifentanil were calculated as the sum of the concentrations during the surgical periods divided by surgical time. For all patients, a 0.075 mg bolus of palonosetron (Aloxi™, CJ Health Care) was administered after induction of anesthesia to prevent PONV, and each patient was equipped with an IV PCA device (Accumate 1100™, Wooyoung Meditech). The analgesic solution contained 3,600 µg fentanyl and 240 mg ketorolac in 120 ml of saline. All patients received a 0.1 ml/kg bolus of analgesic solution at the time of skin closure. The demand dosage was 2.0 ml with no background infusion and a 5 min lockout interval. After skin closure, administration of sevoflurane and remifentanil was discontinued. The amount of analgesic in PCA device was determined after pilot study. In order to eliminate the need for rescue analgesics other than PCA, demand dose was increased and background infusion was eliminated to prevent over dose of analgesics.
In the PACU, the patients were re-educated about achieving target NPRS using a IV PCA device. Pain intensities at rest and deep breathing were evaluated at 2, 6, 24, and 48 h after surgery. The cumulative postoperative analgesic consumption amounts were recorded at each time point. Side effects of analgesics, such as hypotension, bradycardia, nausea, vomiting, urinary retention, pruritus, somnolence, and respiratory depression, were recorded. Although all patients received palonosetron as an antiemetic prophylaxis, 10 mg of metoclopramide was administered when the patients reported nausea or vomiting in the PACU or ward.
The primary end-point of the study was the relationship between intraoperative sevoflurane or remifentanil requirement and postoperative analgesic consumption. The secondary endpoints were postoperative NPRS, cumulative analgesic consumption, and any side effects of analgesics.
In general, the postoperative analgesic consumption is very variable according to the degree of pain experienced by each patient, so it is difficult to have a strong correlation between the intraoperative requirement of anesthetics and the postoperative consumption of analgesics. Therefore, we assumed the correlation coefficient between the variables to be 0.35. It was then determined that 62 patients were required to differentiate between a correlation coefficient of 0.35 with a significance level of 0.05 (α = 0.05) and a power of 80% (β = 0.2). Due to the number of patients dropping out of screening or refusing to participate in the study, the minimum number of participants per each group was increased to 100.
The normality and equal variance test of intraoperative sevoflurane and remifentanil requirements and the cumulative analgesic consumption was tested using the Shapiro-Wilk test. Intraoperative sevoflurane and remifentanil requirements passed this test, but cumulative analgesic consumption at 48 h postoperatively did not pass in either study. The correlations between intraoperative sevoflurane or remifentanil requirement and cumulative analgesic consumption were tested using a Spearman rank correlation test. Continuous demographic data and analgesic consumption were tested Student t-test, and NPRS score were analyzed using Mann-Whitney rank sum test after Yates correction. Vital signs and BIS value obtained within the study were analyzed using a one-way repeat measured ANOVA. Continuous values were expressed as mean ± standard deviation, and NPRS score was presented as median (1Q, 3Q). Statistical significance was set at P < 0.05. SigmaPlot 14.0 (Systat Software Inc.) was used for statistical analysis software.
One hundred fifty-two patients were allocated to the two groups, and the subject flow diagram is shown in Fig. 1. Sixty-six patients in the sevoflurane group and 68 patients in the remifentanil group completed the 48-h follow-up and contributed data demonstrating adequate pain relief. The demographic and clinical characteristics of the patients are shown in Table 1.
In the sevoflurane group, the average end-tidal concentration of sevoflurane was 2.0 ± 0.4 vol.% during the operation. In the remifentanil group, the average effect-site concentration of remifentanil was 3.9 ± 1.4 ng/ml.
The postoperative NPRS were kept below 3 for postoperative 48 h in both groups (Table 2). Cumulative postoperative analgesic consumptions at 48 h were 55 ± 26 and 57 ± 33 ml (P = 0.730) in the sevoflurane and remifentanil groups, respectively, and there was no significant difference between the two groups. In the sevoflurane group, cumulative analgesic consumption at 48 h postoperatively had no significant correlation with average end-tidal concentration of sevoflurane (r = 0.04, P = 0.691, Fig. 2A). In the remifentanil group, intraoperative median effect-site concentration of remifentanil was 3.9 ng/ml. There were no significant correlations between intraoperative average effect-site concentration of remifentanil and 48 h cumulative postoperative analgesic consumption (r = 0.02, P = 0.823, Fig. 2B). However, the postoperative cumulative analgesic consumptions at 2 and 6 h were correlated with intraoperative remifentanil requirement (2 h: r = 0.36, P < 0.001, Fig. 3A; 6 h: r = 0.38, P < 0.001, Fig. 3B) in the remifentanil group. In the remifentanil group, the analgesic consumption within postoperative 2 h was significantly higher than in the sevoflurane group, but there was no difference after that time (P < 0.001, Fig. 4). There were no significant correlations between variables by sex (6 h; female 26.6 ± 11.6 ml vs. male 28.0 ± 11.7 ml, P = 0.634, 48 h; female 55.5 ± 31.5 ml vs. male 64.8 ± 33.8 ml, P = 0.220) and age (6 h; r = 0.03, P = 0.830, 48 h; r = -0.14, P = 0.241) in remifentanil group.
During surgery, mean arterial pressures (MAPs) were maintained within 20% of the pre-anesthetic values in both groups. There were no differences in MAPs during surgery in both groups, but HR was lower in the remifentanil group compared to sevoflurane group (Fig. 5). Although BIS values were between 40–60 during the operation, BIS value was higher in the remifentanil group than in the sevoflurane group (Fig. 6). No patient in the sevoflurane group required ephedrine to manage intraoperative hypotension, but four patients in the remifentanil group received ephedrine. In addition, five and three patients discontinued the study due to postoperative analgesic-related complications in the sevoflurane and remifentanil groups, respectively.
In the current study, the authors hypothesized that patients who required more anesthetics to maintain an appropriate depth of anesthesia during the surgical procedure would consume more analgesics for adequate postoperative analgesia. This is the first study to examine the correlations of intraoperative anesthetics requirement and postoperative analgesic consumption. In our results, early postoperative analgesic consumptions were significantly correlated with intraoperative remifentanil requirement in remifentanil group. However, intraoperative sevoflurane requirement was not related to postoperative analgesic consumption. These results suggest that patients receiving high doses of remifentanil during operation may experience more severe pain at early postoperative period. Therefore, it is considered that postoperative pain management should be performed by considering this point.
The current results are similar to the previous study showed that high dose intraoperative remifentanil infusion increased early postoperative analgesic consumption [13]. Although the study showed a significant difference when remifentanil was administered at a high dose ≥ 8 ng/ml, the current study showed a correlation between intraoperative remifentanil dose and postoperative analgesic consumption. Previous studies have demonstrated that opioids can enhance pain sensitivity on their withdrawal, and rapid development of acute tolerance to their analgesic effects during continuous infusions of opioids [14-16]. These effects of opioids seem to develop frequently with short acting opioids such as remifentanil. Also, remifentanil-induced acute opioid tolerance may be related to the relatively large-dose [13,14]. In the current study, remifentanil infusion may contribute to the development of acute opioid tolerance in the remifentanil group.
Previous studies have focused on identifying predictors of postoperative pain to improve pain management [17-19]. Preoperative pain, patient age, type of surgery, and psychological distress have been identified as predictors of postoperative pain [17,18]. Although there was a significant correlation between the requirement of intraoperative remifentanil and the consumption of postoperative within 6 hours after surgery in the remifentanil group, the correlation was weak (correlation coefficient 0.36–0.38). Also, the remifentanil group consumed more analgesics than the sevoflurane group within postoperative 2 h, but it is difficult to say that it is clinically significant. However, it suggests that patients with large amount of intraoperative remifentanil use may experience more severe pain early postoperative period. Although the authors speculated that intraoperative anesthetic requirement is a predictive factor of postoperative pain intensity, intraoperative anesthetic requirement is insufficient as the predictor.
Postoperative pain score can be used as an indicator of postoperative pain intensity when the patient is receiving analgesics constantly. However, some patients in this protocol experienced severe pain and side effects by under-dosing and over-dosing of analgesics, respectively. Therefore, we measured postoperative analgesic consumption as an indicator of postoperative pain intensity after the patients were educated on how to achieve the target pain scores using a IV PCA device. As a result, in this study, most patients scored an appropriate target pain score of 2–3 points, and the amount of appropriate analgesics was used to achieve the target pain score. Although we did not use pain scores as indicator of pain intensity, the amount of postoperative analgesic consumption might be related directly to postoperative pain intensity when the patient used analgesics to achieve a constant pain score.
This study had a limitation. Postoperative pain is complex and multi-dimensional; it consists of physiological, emotional, and behavioral components and is influenced by genetic factors. Several previous studies [17,20-22] have consistently reported that emotional and psychological factors such as preoperative anxiety and pain catastrophizing predict postoperative pain and analgesic consumption, especially in patients who had undergone cancer surgery. Therefore, emotional factors can affect the results of correlational studies on postoperative pain. However, emotional and psychological factors were not considered in the current study.
In conclusion, the amount of intraoperative requirement of short acting opioid, remifentanil, is correlated with postoperative analgesic consumption within postoperative 6 h. It may be contributed by the development of acute opioid tolerance. However, there was no correlation between intraoperative sevoflurane requirement and postoperative analgesic consumption. Therefore, it is necessary to consider that patients who received high dose of remifentanil during operation may experience severe pain at early postoperative period.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Jun Ho Lee. Writing - review & editing: Jun Ho Lee, A Ram Doo, Hyunji Oh, Hyungun Lee, Seonghoon Ko. Conceptualization: Seonghoon Ko. Data curation: Jun Ho Lee, A Ram Doo, Hyunji Oh, Hyungun Lee, Seonghoon Ko. Formal analysis: Jun Ho Lee, Seonghoon Ko. Methodology: A Ram Doo, Hyungun Lee. Investigation: Jun Ho Lee, A Ram Doo, Hyunji Oh, Seonghoon Ko.
REFERENCES
1. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017; 10:2287–98.

2. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003; 97:534–40.

3. Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001; 87:62–72.

4. Tsui SL, Law S, Fok M, Lo JR, Ho E, Yang J, et al. Postoperative analgesia reduces mortality and morbidity after esophagectomy. Am J Surg. 1997; 173:472–8.

5. Li Y, Dong H, Tan S, Qian Y, Jin W. Effects of thoracic epidural anesthesia/analgesia on the stress response, pain relief, hospital stay, and treatment costs of patients with esophageal carcinoma undergoing thoracic surgery: A single-center, randomized controlled trial. Medicine (Baltimore). 2019; 98:e14362.
6. Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand. 2001; 45:795–804.

7. Bachiocco V, Morselli AM, Carli G. Self-control expectancy and postsurgical pain: relationships to previous pain, behavior in past pain, familial pain tolerance models, and personality. J Pain Symptom Manage. 1993; 8:205–14.

8. Maranets I, Kain ZN. Preoperative anxiety and intraoperative anesthetic requirements. Anesth Analg. 1999; 89:1346–51.

9. Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000; 84:65–75.

10. Vahabi S, Abaszadeh A, Yari F, Yousefi N. Postoperative pain, nausea and vomiting among pre- and postmenopausal women undergoing cystocele and rectocele repair surgery. Korean J Anesthesiol. 2015; 68:581–5.

11. Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018; 127:1246–58.

12. Wang H, Cai Y, Liu J, Dong Y, Lai J. Pain sensitivity: a feasible way to predict the intensity of stress reaction caused by endotracheal intubation and skin incision? J Anesth. 2015; 29:904–11.

13. Kim D, Lim HS, Kim MJ, Jeong W, Ko S. High-dose intraoperative remifentanil infusion increases early postoperative analgesic consumption: a prospective, randomized, double-blind controlled study. J Anesth. 2018; 32:886–92.

14. Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000; 93:409–17.
15. Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008; 24:479–96.
16. Colvin LA, Fallon MT. Opioid-induced hyperalgesia: a clinical challenge. Br J Anaesth. 2010; 104:125–7.

17. Sommer M, De Rijke JM, Van Kleef M, Kessels AG, Peters ML, Geurts JW, et al. Predictors of acute postoperative pain after elective surgery. Clin J Pain. 2010; 26:87–94.

18. Ip HYV, Abrishami A, Peng PWH, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009; 111:657–77.
19. Lanitis S, Mimigianni C, Raptis D, Sourtse G, Sgourakis G, Karaliotas C. The impact of educational status on the postoperative perception of pain. Korean J Pain. 2015; 28:265–74.

20. Hayashida M, Nagashima M, Satoh Y, Katoh R, Tagami M, Ide S, et al. Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype. Pharmacogenomics. 2008; 9:1605–16.

Fig. 1.
CONSORT flow diagram. CONSORT: consolidated standards of reporting trials, PCA: patients-controlled analgesia.
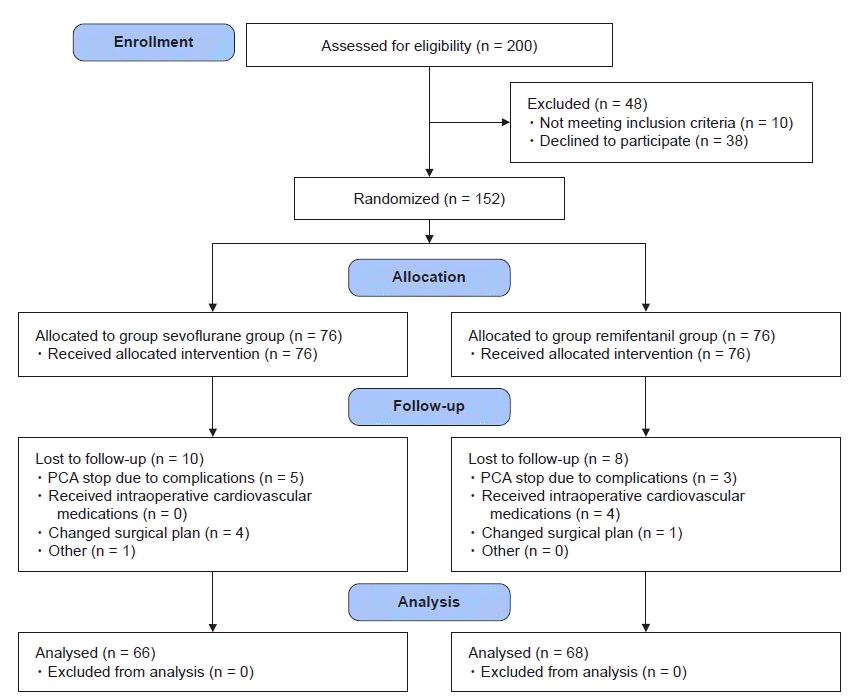
Fig. 2.
Cumulative analgesic consumption at 48 h postoperatively had no significant correlation with intraoperative inhalational anesthetic, sevoflurane, requirement in the sevoflurane group (r = 0.04, P = 0.691, A). In addition, there were no significant correlations between postoperative 48 h cumulative analgesic consumption and intraoperative remifentanil requirement (r = 0.02, P = 0.823, B).
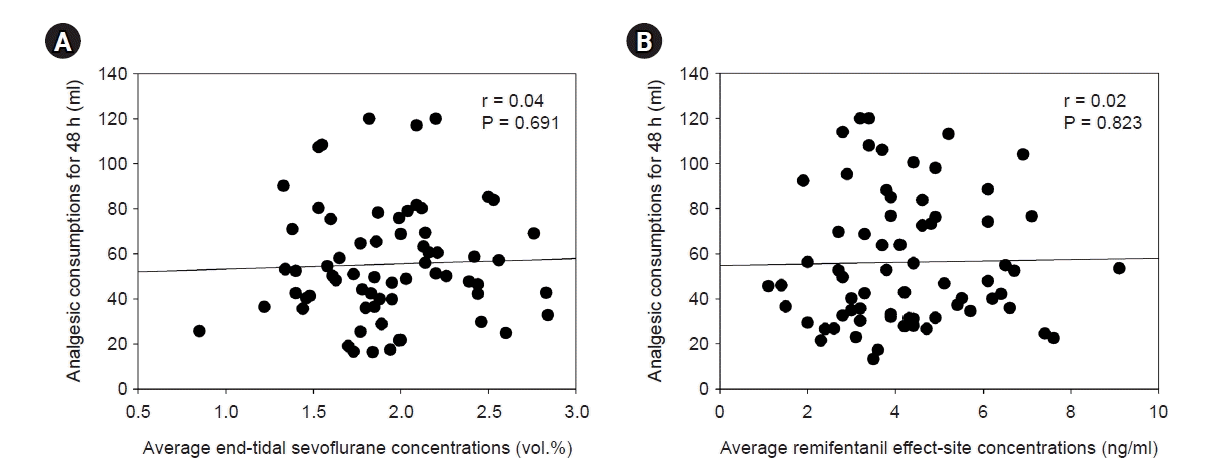
Fig. 3.
The cumulative analgesic consumptions at 2 (r = 0.36, P < 0.001, A) and 6 h (r = 0.38, P < 0.001, B) postoperatively were significantly correlated with intraoperative remifentanil requirement in the remifentanil group.
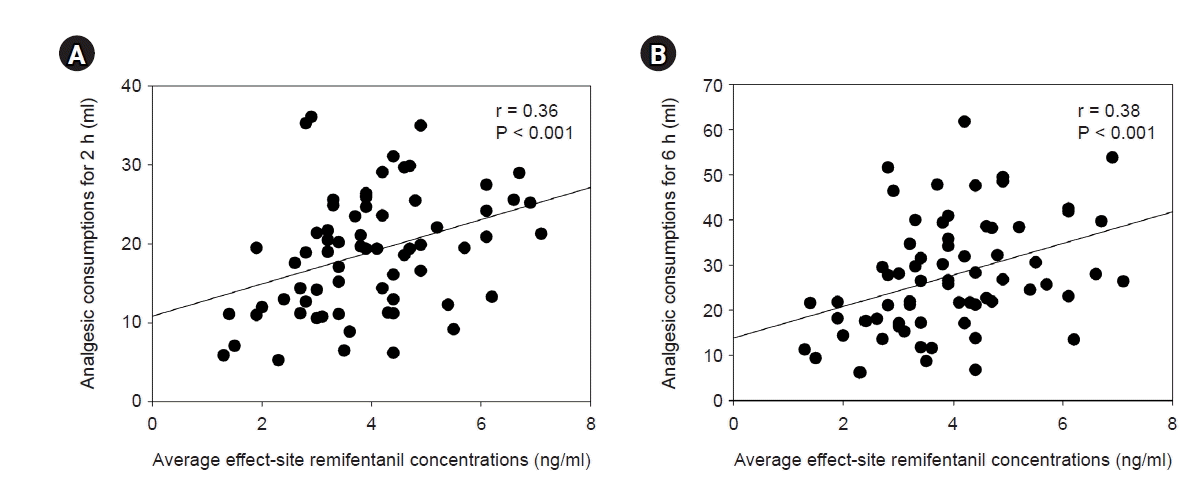
Fig. 4.
In the remifentanil group, the analgesic consumption within postoperative 2 h was significantly higher than in the sevoflurane group, but there was no difference after that time (*P < 0.001).
Fig. 5.
There were no differences in MAP during surgery in both groups, but average HR was lower in the remifentanil group compared to sevoflurane group (*P < 0.001). MAP: mean arterial pressures, HR: heart rate, SI: skin incision, SC: skin closure.
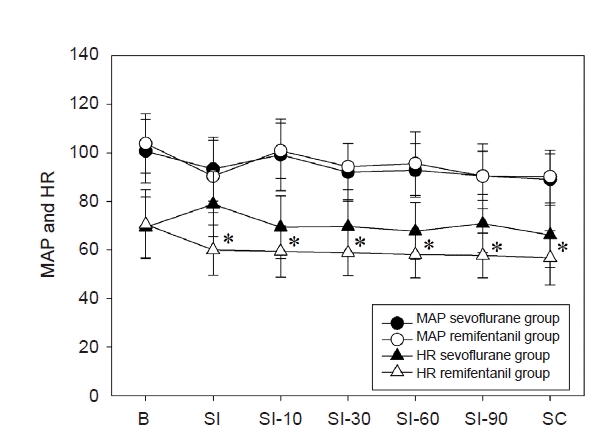
Fig. 6.
BIS value was higher in the remifentanil group than in the sevoflurane group (*P < 0.001). BIS: bispectral index, SI: skin incision, SC: skin closure.
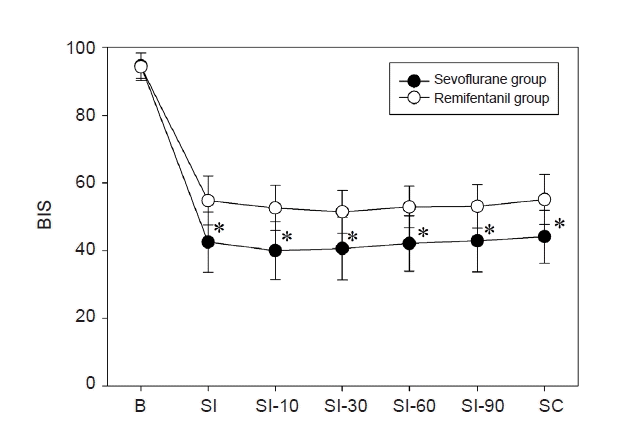
Table 1.
Demographic Data and Clinical Characteristics
Table 2.
Postoperative Numerical Pain Rating Scale




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download