Abstract
Background
Diffuse alveolar hemorrhage (DAH) is a potentially life-threatening condition that can occur due to a variety of disorders. Hence, rapid diagnosis and prompt initiation of appropriate treatment are imperative.
Case
A 55-year-old woman with a deep neck infection underwent emergent tonsillectomy. General anesthesia and surgery proceeded uneventfully. Upon transfer to the post-anesthesia care unit, ongoing respiratory distress and occasional expectoration of blood-tinged sputum were noted. Lung ultrasonography (LUS) revealed multiple B-profiles and irregular pleural lines with subpleural consolidations. Emergent bronchoscopy with bronchoalveolar lavage was diagnostic of DAH. She underwent a comprehensive evaluation for rheumatologic and infectious etiologies of DAH, all of which yielded negative results. The patient was managed with steroids and conservative treatment.
Diffuse alveolar hemorrhage (DAH) is characterized by the accumulation of red blood cells (RBCs) in the alveolar spaces due to injury or disruption of the alveolar microvasculature [1]. The etiologies of DAH have been associated with various disorders ranging from localized damage by inhalation exposure, infection, and drug to systemic diseases including connective tissue diseases or vasculitis. Since DAH can lead to acute respiratory failure, it is considered a potentially life-threatening medical emergency [2]. Thus, early diagnosis and treatment are imperative to prevent the progression of acute respiratory failure.
Bronchoscopy with bronchoalveolar lavage (BAL) is the gold standard for the diagnosis of DAH [3]; however, it is not suitable for prompt diagnosis during the perioperative period. Thus, advanced diagnostic techniques, such as lung ultrasonography (LUS), can aid in the prompt identification and assessment of DAH. Indeed, the majority of experienced anesthesiologists have effectively diagnosed and managed patients without recognizing the specific features of lung ultrasound in DAH. Nevertheless, integrating clinical information with LUS can enhance patient diagnosis and management optimization. Therefore, we present a case of negative-pressure pulmonary edema (NPPE) presenting with DAH that was diagnosed early via LUS.
A 55-year-old female patient presented to the emergency department with neck pain and respiratory distress. Her height, weight, and body mass index were 155 cm, 50 kg, and 20.8 kg/m2, respectively. This case study was approved by the Institutional Review Board of our institution (IRB 2023-06-004). Written informed consent was obtained from the patient before publication of this case report. She had had chronic sleep apnea syndrome and enlarged tonsils for 10 years, with no other medical conditions. The initial assessment revealed significant swelling in the neck region and difficulty in swallowing. The patient was alert, with non-invasive blood pressure (NIBP) of 139/85 mmHg, heart rate of 91 beats/min, respiratory rate of 20 min, and body temperature of 36.8°C. Chest radiography revealed a normal pattern (Fig. 1). Computed tomography (CT) of the neck showed a substantial abscess in the left palatine tonsil and parapharyngeal space, with no signs of airway obstruction or tracheal deviation. An emergency tonsillectomy was performed to alleviate airway obstruction and facilitate proper drainage of the infected site. In the preoperative laboratory tests, leukocytosis (15.7 × 109 L, reference range: 4–10 × 109 L) and elevated C-reactive protein levels (29.09 mg/dl, reference range: 0–0.5 mg/dl) were detected; the remaining evaluations, including complete blood count and coagulation tests, were unremarkable.
The patient was transferred to the operating room without premedication. Standard monitoring, including pulse oximetry, NIBP, end-tidal carbon dioxide concentration, and electrocardiography (ECG), was initiated during the surgery. The initial vital signs were within the normal range. The following intravenous medications were administered for the induction of anesthesia: 2 mg/kg of propofol, 0.6 mg/kg of rocuronium, and 1 mcg/kg of fentanyl. Anesthesia was maintained using sevoflurane at a concentration of 1.5–2 volume percentage in a 50% oxygen mixture with continuous infusion of remifentanil at a rate of 0.05–0.15 µg/kg/min. General anesthesia was administered, with intraoperative fluid infusion at a rate of 120 ml/h, and total input/output was 140 ml/100 ml indicating the absence of volume overload. The ventilator was set in volume control mode, with a tidal volume of 400 ml, a respiratory rate of 12 min, and a peak inspiratory pressure of approximately 10–12 cmH2O. The induction of anesthesia proceeded without any complications such as airway damage or difficult intubation. The duration of the surgery was approximately 40 min. No complications related to surgery or anesthesia, such as bleeding, hypotension, or desaturation, occurred throughout the entire perioperative period. Prior to extubation, we were informed by the surgeon that significant airway edema was not expected. Through the cuff-leak test, which assesses the leakage of air around the upper airway above the cuff, we indirectly confirmed the absence of airway edema in our patient [4]. Even during endotracheal tube suctioning, no evidence of pink frothy secretions was observed, and no significant issues with heart rate or breathing upon awakening were displayed. After complete recovery of consciousness and spontaneous breathing with normal ventilation pressure were confirmed, the patient was gently and gradually extubated without incidents such as endotracheal tube biting or immediate post-extubation respiratory distress. Upon transfer to the post-anesthesia care unit (PACU), ongoing respiratory distress and occasional expectoration of blood-tinged sputum were noted, which was initially suspected to be the result of aspiration during surgery. However, pulse oxygen saturation dropped to approximately 92% despite oxygen supplementation, and bilateral coarse inspiratory crepitation was auscultated. Therefore, we conducted bedside LUS immediately at the PACU. The patient was examined in the supine position, with the probe being placed on the bedside lung ultrasound in emergency (BLUE) point, especially on the anterior chest (Fig. 2). In BLUE protocol, two hands are applied as follows: the upper little finger is positioned just below the clavicle, with fingertips aligning with the middle line, and the lower hand is placed just below the upper hand. Thus, the "upper BLUE point" is positioned at the midpoint of the upper hand (zone 1), while the "lower BLUE point" is situated at the center of the lower palm (zone 2). The “posterolateral alveolar and/or pleural syndrome point”, a helpful indicator for differentiating pneumonia, is formed by the lower BLUE-point and posterior axillary line (zone 3) [5]. LUS revealed multiple B-profiles with the convex probe, and the linear probe confirmed irregular pleural lines with subpleural consolidations, commonly seen in conditions such as acute respiratory distress syndrome or pneumonia (Fig. 3). Confirming that it was not simple pulmonary edema, we immediately performed chest radiography and CT. Chest radiography revealed diffuse and extensive pulmonary consolidations predominantly in the mid-zones. High–resolution CT (HRCT) of the chest revealed extensive ground-glass opacities and consolidation distributed throughout both lungs without evidence of pulmonary embolism. To differentiate between pulmonary hemorrhage and other lung diseases, bronchoscopy and BAL were performed. Bronchofiberscopy confirmed bleeding on both sides of the bronchi, with sequential BAL samples collected from the lingular region showing an increased RBC count (Fig. 4). These findings confirmed a diagnosis of DAH. Hemosiderin-laden macrophages were not detected through cytology, and all cultures yielded negative results. Serological markers for viral hepatitis, vasculitis, HIV, and connective tissue disease were negative, and the ECG and echocardiography results were normal.
The patient was managed conservatively with oxygen supplementation and steroid therapy. The general condition improved rapidly following this treatment approach. In subsequent LUS and HRCT, pulmonary edema resolved completely after 4 days. The patient was discharged without any further episodes of dyspnea or hemoptysis after 7 days, and a follow-up chest radiograph acquired after 2 weeks revealed complete resolution of the bilateral infiltrates.
Our report demonstrates the feasibility of using LUS for the early diagnosis of DAH caused by NPPE following tonsillectomy. Although there have been reports on the use of LUS for DAH diagnosis and monitoring procedures in DAH, its application for the early diagnosis of DAH in postoperative patients is not well established. To the best of our knowledge, this is the first report on the use of LUS in postoperative DAH.
The classical clinical presentation of DAH is characterized by progressive dyspnea, hemoptysis, and chest infiltrates that cannot be attributed to other causes [6]. However, it is important to acknowledge that these features are non-specific and may vary. For example, hemoptysis may be absent even in cases of significant pulmonary hemorrhage, resulting in anemia [7]. The diagnosis of DAH requires imaging studies, such as chest radiography, HRCT, or BAL. However, chest radiography findings may lack specificity, and it may not be feasible to immediately use HRCT or BAL in all cases, such as the present case, which had a rapid postoperative onset. Consequently, the diagnosis of DAH remains challenging.
Over the past decade, LUS has undergone significant advancements and offers notable advantages, such as the absence of radiation exposure and bedside accessibility. The BLUE (Bedside Lung Ultrasound in Emergency) protocol, which is fully based on pathophysiology, is a suitable tool for immediate diagnosis of acute respiratory failure, pneumothorax, or pulmonary edema. LUS has been found to have good sensitivity and specificity for detecting these conditions, but the exact values can vary (the sensitivity and specificity of 80-95% and 85-95% for lung edema; 80-95% and 85-95% for ARDS; 80-90% and 80-95% for pneumonia) [5]. Thus, it has emerged as a valuable tool for the management of postoperative complications and respiratory failure in intensive care settings. However, reports on its specific role in the diagnosis or suspicion of NPPE-induced DAH after surgery are limited. In the present case, we identified an increased number of B-lines and homogenous, hypoechoic subpleural consolidations using LUS [8]. According to the general approach of the BLUE protocol, these findings could raise suspicion of pneumonia. However, in the case of this patient, the absence of antecedent pneumonia symptoms, the precipitous onset of dyspnea subsequent to tonsillectomy, and the confluence of risk factors for pulmonary hemorrhage led to a less common but plausible consideration of DAH. This suspicion was further supported by the CT and bronchoscopy findings. Thus, our study emphasizes the importance of comprehensively interpreting the results of LUS in conjunction with the medical history and symptoms.
Previous studies have linked non-immunological cases of postoperative DAH to various factors, such as exposure to anesthetic gases, anticoagulant use during neurosurgery, and NPPE [9-11]. We hypothesized that the present case possesses multiple factors that contributed to the development of DAH. We first considered NPPE as this patient's underlying etiologic mechanism of DAH. NPPE is generally classified into two main types. Type 1 NPPE occurs in cases of acute airway obstruction, such as post-extubation laryngospasm, and is associated with vigorous inspiratory efforts. Type 1 NPPE typically originates from a significant acute obstruction in the upper airway, such as endotracheal tube biting while intubated, laryngospasm, or excessive secretions. The exertion of respiratory efforts generates robust negative pressures, resulting in the movement of fluid from pulmonary capillaries to interstitial and alveolar spaces [12]. It is more frequently observed in healthy and young men with larger muscle mass, resulting in significant intrapleural negative pressure fluctuations. Type 2 NPPE occurs after the sudden relief of chronic airway obstruction, commonly seen after procedures like adenoidectomy. Surgical intervention, such as tonsillectomy, to address chronic upper airway obstruction can cause a rapid decrease in the intrathoracic pressure, leading to a sudden reduction in the auto-positive end-expiratory pressure and the generation of negative intrathoracic pressure [13]. This results in fluid transudation into the interstitial and alveolar spaces, which can alter the pulmonary epithelial and microvascular membranes and potentially cause hemorrhage. In our case, since the characteristic symptoms or signs of Type 1 did not manifest immediately during the perioperative period, we suspected the DAH was due to Type 2 NPPE. However, since Type 1 NPPE can still occur several minutes to hours after surgery, completely excluding Type 1 was not entirely feasible. This aspect serves as a crucial clue, reminding anesthesiologists that NPPE may still be relevant not only immediately after surgery but also in the postoperative period. Second, sevoflurane may be one of the causes of DAH, in addition to Type 2 NPPE. Studies have shown that sevoflurane can decrease the platelet aggregation rate during the perioperative period, resulting in intra-alveolar bleeding. Moreover, high concentrations of sevoflurane can increase the risk of alveolar wall damage and potentially interfere with phospholipid interactions in the lung surfactant monolayer [14]. Third, the activation of neutrophils triggers an inflammatory response during the process of deep neck infection, leading to an increase in vascular permeability, which exacerbates negative pressure pulmonary edema and worsens DAH.
Postoperative DAH typically exhibits a quick onset and is characterized by a relatively prompt resolution. If recognized early and managed appropriately, postoperative DAH typically transitions into a reversible state, with clinical and radiological improvement often becoming evident within a week. The utilization of LUS in patients presenting with postoperative respiratory distress despite mask oxygen support and hemoptysis provides an opportunity for the early detection of perioperative complications, such as DAH.
In conclusion, patients anesthetized with sevoflurane and undergoing surgical interventions to alleviate acute airway obstruction are susceptible to DAH, even in the presence of mild NPPE perioperatively. Therefore, anesthesiologists and clinicians should pay special attention to anesthetic management by closely monitoring the patient's condition through an examination using oxygen saturation and LUS after surgery. This may help early recognition of DAH, facilitating an appropriate treatment strategy.
Notes
REFERENCES
1. Tamai K, Tomii K, Nakagawa A, Otsuka K, Nagata K. Diffuse alveolar hemorrhage with predominantly right-sided infiltration resulting from cardiac comorbidities. Intern Med. 2015; 54:319–24.
2. Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010; 137:1164–71.
3. Robbins RA, Linder J, Stahl MG, Thompson AB, Haire W, Kessinger A, et al. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med. 1989; 87:511–8.
4. Zhou T, Zhang HP, Chen WW, Xiong ZY, Fan T, Fu JJ, et al. Cuff-leak test for predicting postextubation airway complications: a systematic review. J Evid Based Med. 2011; 4:242–54.
5. Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015; 147:1659–70.
6. De Prost N, Parrot A, Cuquemelle E, Picard C, Antoine M, Fleury-Feith J, et al. Diffuse alveolar hemorrhage in immunocompetent patients: etiologies and prognosis revisited. Respir Med. 2012; 106:1021–32.

7. Alexandre AT, Vale A, Gomes T. Diffuse alveolar hemorrhage: how relevant is etiology? Sarcoidosis Vasc Diffuse Lung Dis. 2019; 36:47–52.
8. Buda N, Masiak A, Smoleńska Ż, Gałecka K, Porzezińska M, Zdrojewski Z. Serial lung ultrasonography to monitor patient with diffuse alveolar hemorrhage. Ultrasound Q. 2017; 33:86–9.
9. Austin A, Modi A, Judson MA, Chopra A. Sevoflurane induced diffuse alveolar hemorrhage in a young patient. Respir Med Case Rep. 2016; 20:14–5.

10. Choe YS, Kim NY, Lee AR. Diffuse alveolar hemorrhage in patients undergoing neurointervention: a case report. Anesth Pain Med. 2016; 6:e33979.

11. Kim JP, Park JJ, Kim NJ, Woo SH. A case of diffuse alveolar hemorrhage after tonsillectomy -a case report-. Korean J Anesthesiol. 2012; 63:165–8.

12. Bhaskar B, Fraser JF. Negative pressure pulmonary edema revisited: pathophysiology and review of management. Saudi J Anaesth. 2011; 5:308–13.
Fig. 2.
The procedure of lung ultrasonography. The patient was examined in the supine position, with the probe being placed on the bedside lung ultrasound in emergency point, especially on the anterior chest, to confirm the ultrasound findings.
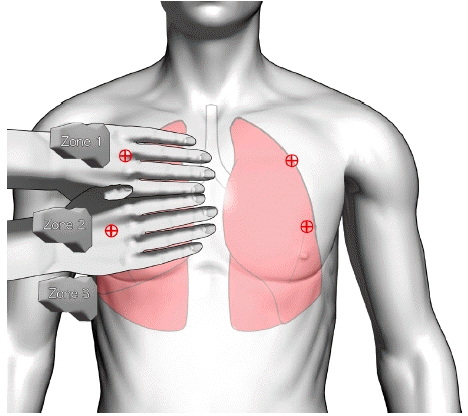
Fig. 3.
An integrated assessment of Type 2 negative-pressure pulmonary edema, presenting as diffuse alveolar hemorrhage, using lung ultrasound (A, B), chest radiograph (C), and high–resolution computed tomography (HRCT) (D) with schematic representations (E–H). The infiltrative opacification pattern observed in the mid-zone on the chest radiograph (C) and the extensive ground-glass opacities and consolidations seen on the HRCT (D) corresponds to multiple B-lines (A, E) and irregular pleural line with subpleural consolidation (B, F) detected on lung ultrasound. The detailed examination of the lung ultrasound scan is represented by the blue box (G) and blue circle (H), with the convex probe (A, E) and linear probe (B, F) placed longitudinally.
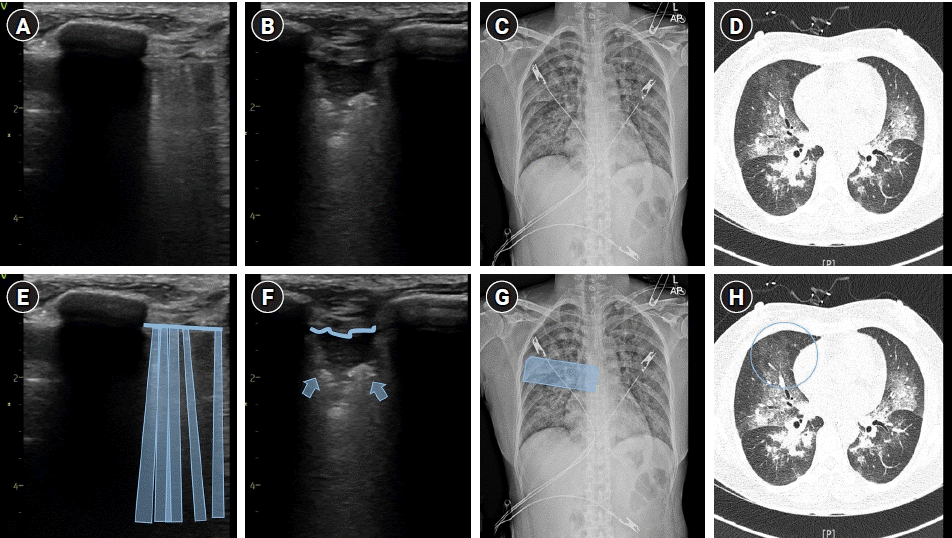
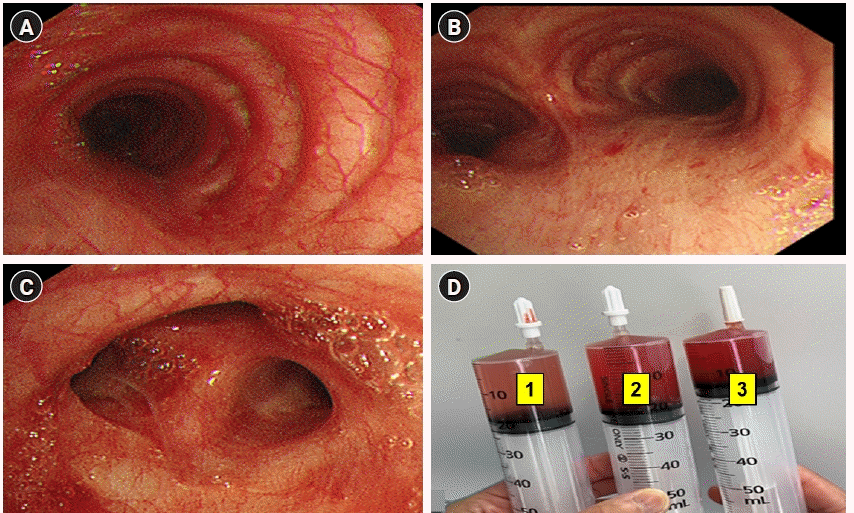
Fig. 4.
(A) Diffuse alveolar hemorrhage (DAH) in bronchoalveolar lavage (BAL), at trachea (B) DAH in BAL, at carina (C) DAH in BAL at the lingual region of the left upper lobe (D) The progressive hemorrhagic BAL in the serial samples (number 1 is the first BAL, number 2 is the second BAL, and number 3 is the third BAL).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download