This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Atypical small acinar proliferation (ASAP) cases typically require rebiopsy, which are invasive and associated with increased risk of complications. Our aim in this study was to determine the importance of laboratory and histological findings and E-26 transformation-specific-related gene (ERG) expression in the diagnosis of malignancy.
Methods
Between March 2016 and March 2022, 84 patients who were diagnosed with ASAP on biopsy or rebiopsy were included in the study. Clinical-laboratory features of age, serum prostate-specific antigen level, and histopathological features were compared and included multifocality, number of suspicious acini, nuclear enlargement, nucleolar prominence, hyperchromasia, cytoplasmic amphophilia, luminal amorphous acellular secretion, crystalloid presence, infiltrative appearance, inflammation, atrophy, α-methyl acyl-CoA racemase, p63, and/or high molecular weight cytokeratin were analyzed. In addition, ERG expression was evaluated immunohistochemically.
Results
Statistically significant correlation was found between nucleolar prominence, nuclear hyperchromasia, crystalloid presence, infiltrative pattern, and prostate cancer (p < .001). In 19 of 84 cases (22.6%) ERG was positive in the nucleus. Prostate cancer was diagnosed at rebiopsy in 15 of the 19 ERG-positive cases (78.9%). A statistically significant correlation was found between ERG positivity and prostate cancer (p= .002).
Conclusions
Our findings suggest that evaluation of these markers during initial transrectal ultrasound biopsies may decrease and prevent unnecessary prostate rebiopsy.
Keywords: Atypical small acinar proliferation, Neoplasms, ERG, Prostate
Atypical small acinar proliferation (ASAP) is comprised of small foci of atypical prostate glandular tissue with some features of adenocarcinoma and without a diagnosis of malignancy. ASAP has been highlighted in several studies as an independent predictive factor of prostate cancer (PCa) [
1] and is found in approximately 4%–5% of prostate biopsy specimens [
2]. The cancer detection rate for ASAP on second biopsy varies between 21% and 51% [
3]. Repeat biopsies are not performed in about one-third of the cases due to multiple factors including age and clinical status [
4]. Therefore, a reduction in the rate of ASAP diagnosis during initial biopsy interpretation may decrease the need for rebiopsy and limit the burden of new diagnostic procedures.
One of the earliest methods of detection of PCa is increased serum prostate-specific antigen (PSA), which has been screened since the 1980s [
5]. At the molecular level, numerous genetic alterations have been characterized in PCa such as PTEN loss and c-myc amplification. The E-26 transformation-specific-related gene (ERG) is the most rearranged member of the ETS family, with the most frequent genetic event resulting from a deletion of the segment of DNA between the
ERG and
TMPRSS2 genes [
6].
ERG gene rearrangements are highly specific for PCa and do not occur in benign prostate glands [
6].
TMPRSS2-ERG gene fusion is the most common genetic rearrangement in PCa, although the prevalence varies by population. About 60% of PCa cases have a
TMPRSS2-ERG gene fusion [
7], leading to formation of the ERG oncoprotein, which results in cellular proliferation. Immunohistochemical methods reliably determine ERG expression, which is highly sensitive and specific for PCa. Immunohistochemistry for ERG correlates strongly with
ERG gene rearrangements by fluorescence in situ hybridization (sensitivity 95.7% to 100%, specificity 96.5% to 100%) [
6]. Because benign prostate tissue and stromal cells do not stain for ERG, the immunohistochemistry of ERG expression increases the detection of PCa and plays an important role in its diagnosis.
The aim of the study was to determine the importance of laboratory and histopathological findings of patient age, serum PSA level, multifocality, suspicious acini number, nuclear enlargement, nucleolar prominence, hyperchromasia, cytoplasmic amphophilia, luminal amorphous acellular secretion, presence of crystalloids, infiltrative appearance, inflammation, atrophy, and ERG expression in the diagnosis of malignancy and to understand which cases were diagnosed ASAP with transrectal ultrasound (TRUS)–guided prostate needle biopsy and which of these histopathological findings are more valuable to predict malignancy. For this purpose, we investigated the relationships between the above-mentioned histopathological parameters and ERG expression, which is diagnosed as ASAP in the first prostate biopsy and benign or malignant on rebiopsy.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 1,523 patients who had undergone TRUS-guided prostate needle biopsy between March 2016 and March 2022. Out of these, we diagnosed 100 patients with ASAP, but we excluded 16 of them for various reasons, including consultations, placement outside of serial sections, lack of archival and laboratory data, or no rebiopsy. Finally, we identified 84 ASAP cases with sufficient clinical data.
Histopathological evaluation
The cases were selected retrospectively and re-evaluated in terms of the following parameters: age, serum PSA level, multifocality, number of suspicious acini, nuclear enlargement, nucleolar prominence, hyperchromasia, cytoplasmic amphophilia, luminal amorphous acellular secretion, presence of crystalloids, infiltrative appearance, inflammation, atrophy, and immunohistochemistry (α-methyl acyl-CoA racemase [AMACR], p 63 +/– high molecular weight cytokeratin [HMWCK]).
Immunohistochemical technique and evaluation of immunohistochemistry
Six 4-μm-thick tissue sections were placed on each slide. Samples were deparaffinized, and ERG immunohistochemical staining was performed on the Ventana Benchmark ULTRA automatic staining device (Ventana Medical Systems, Inc., Tucson, AZ, USA). The EPR 3864 clone of the ERG antibody was used in immunohistochemical staining.
Stained sections were evaluated under the Olympus light microscope (Tokyo, Japan). ERG expression is normally observed in lymphocytes and vascular endothelium, which were used as positive internal controls. The presence of ERG nuclear staining was considered positive.
Statistical analysis
The Statistical Package of Social Science (SPSS) 22.0 statistical package program (IBM Corp., Armonk, NY, USA) was used in the analysis of data. Descriptive statistics of evaluation results are presented as numbers and percentages for categorical variables and mean, standard deviation, median, minimum, and maximum for numerical variables. The conformity of the groups to the normal distribution was determined by Kolmogorov-Smirnov test. We performed comparisons of numerical variables between two independent groups using the Student’s t test when the normal distribution conditions met. We evaluated the data using the Mann-Whitney U test when the normal distribution conditions were not fulfilled. We used the ANOVA test when comparing three or more independent groups and meeting the normal distribution condition. When the normal distribution condition was not met, the Kruskal-Wallis test was used. Post hoc comparisons were tested with Bonferroni correction if significant differences were observed in the Kruskal-Wallis test. The chi-square test was used to compare qualitative data. Statistical significance was accepted as p<.05.
RESULTS
Between March 2016 and 2022, a total of 1,523 TRUS-guided prostate needle biopsies were performed in our department, and 100 of them were diagnosed ASAP. The incidence of ASAP was 5.67%. The depletion of the ASAP area in serial sections and/or the need for consultation led to the exclusion of 16 out of the 100 ASAP cases. Ultimately, 84 cases were included in the study. Two of these cases had 24-quadrant biopsies, and the rest had 12 quadrants.
The mean age of patients in the study was 66.36±6.98 years (min–max, 51–87). Overall, 27.3% (n = 18) of the cases with malignancy in the initial biopsy were aged 60 years or over, and 16.7% (n = 3) were younger than 60 years. This difference in age between the benign and malignant groups was not significant (p=.066).
The mean time to the initial biopsy was 5.58 ± 2.62 (min–max, 2–15 months) months. The results of 84 cases were evaluated as benign in 66.7% (n = 56), ASAP in 8.3% (n = 7), and prostatic adenocarcinoma in 25% (n= 21). Initial biopsy materials were obtained by 12-quadrant biopsies (72 of 84 patients) and 24-quadrant biopsies (12 of 84 patients).
The mean serum PSA level was 7.07±3.87 ng/mL. The serum PSA level was 6.69 ng/mL in the benign group, 6.76 ng/mL in the ASAP group, and 8.17 ng/mL in the malignant group. This difference was not statistically significant (
Table 1).
Histopathological evaluation
Number of suspicious acini
The maximum number of suspicious acini in one person was four. One of four cases with one suspicious acinus was diagnosed with PCa in rebiopsy, compared with 14 of 32 cases with two suspicious acini. Also, rebiopsy results were PCa in six of 30 cases with 3–4 suspicious acini. There was no statistically significant difference between the number of suspicious acini and PCa (p=.562).
Nuclear enlargement
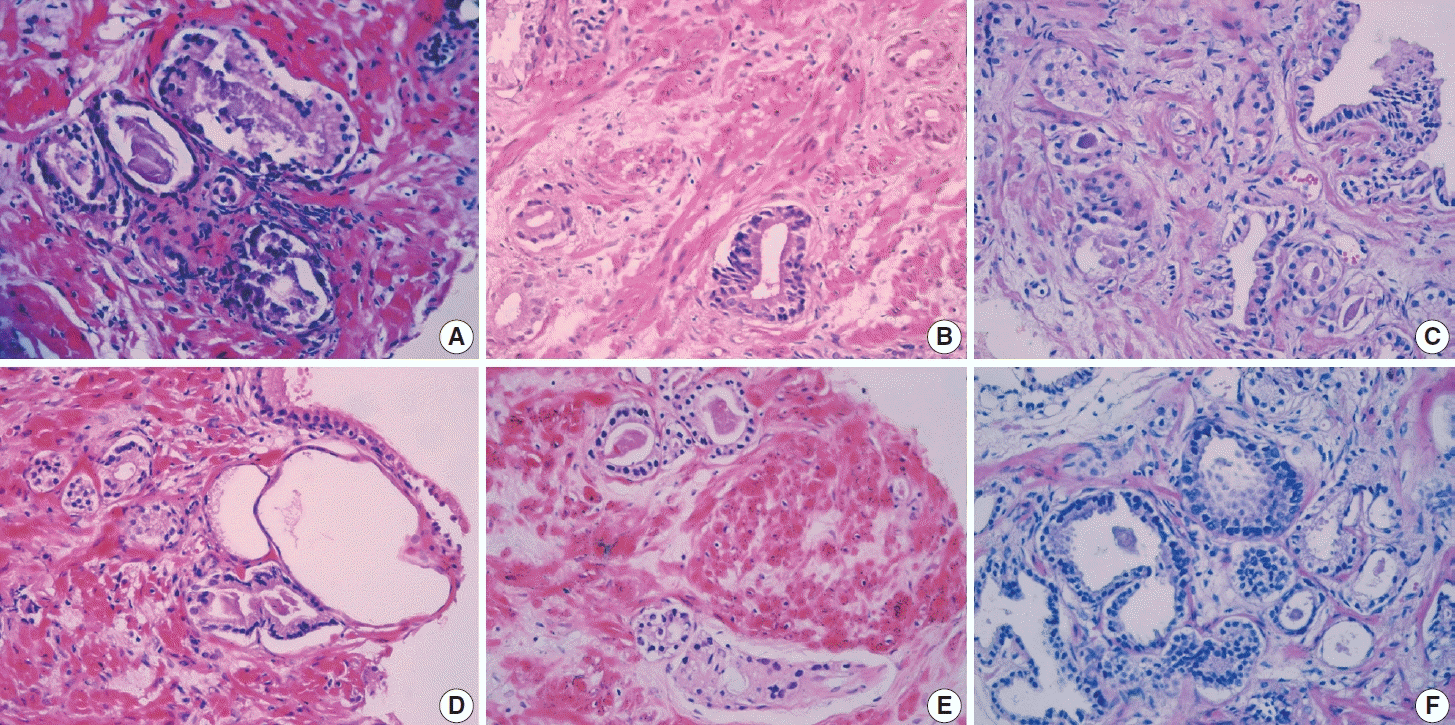
Nuclear enlargement in suspicious foci was assessed through comparison with the surrounding benign epithelium, and ≥ 2 times enlargement was seen in 62.2% of benign rebiopsy cases, 9.5% of ASAP rebiopsy cases, and 28.4% of PCa cases (p=.075) (
Fig. 1E). The p-value was not statistically significant.
Nucleolar prominence
Forty patients (47.6%) had nucleolar prominence; half of these cases were PCa on rebiopsy (
Fig. 1F). A statistically significant correlation was found between nucleolar prominence and PCa (p<.001).
Hyperchromasia
Nuclear hyperchromasia was seen in 16.6% of the cases, in which PCa was detected on rebiopsy in 63.2% (
Fig. 1B). A significant correlation existed between nuclear hyperchromasia and PCa (p<.001).
Cytoplasmic amphophilia
Amphophilic cytoplasm was seen in 83.3% of case, of which 61.4% were benign, 10% were ASAP, and 28.6% were PCa at rebiopsy (
Fig. 1E). There was no significant correlation between amphophilic cytoplasm and PCa (p=.092).
Luminal amorphous acellular secretion
Luminal acellular amorphous eosinophilic secretions were seen in 55.9% of cases (
Fig. 1D). PCa was detected on rebiopsy in 34% of these cases. There was no significant correlation between luminal amorphous acellular secretion and PCa (p=.089).
Presence of crystalloids
Intraluminal crystalloids were present in 15.4% of the cases, and PCa was detected on rebiopsy in 61.5% of these cases (
Fig. 1A). There was a significant correlation between intraluminal crystalloids and PCa (p=.003).
Infiltrative appearance
PCa was detected in five of six cases (83.3%) with an infiltrative pattern at rebiopsy (
Fig. 1C). A statistically significant correlation was found between infiltrative pattern and PCa (p=.003).
Inflammation
Inflammation of the surrounding tissues was observed in 73.2% of benign cases and 24.4% of PCa cases. There was no statistically significant correlation between inflammation and PCa (p=.171).
Atrophy
Atrophy of the surrounding tissues was present in 23.8% of the cases. In 20% of these cases with atrophy, PCa was detected on rebiopsy. There was no significant correlation between atrophy and PCa (p=.821).
In cases with four histopathological parameters, PCa was detected at a rate of 70.6% in rebiopsy; this rate was 75% in cases with five histopathological parameters. In the presence of six or more parameters, all cases were diagnosed with PCa on rebiopsy. A significant correlation was found between the presence of four or more histopathological parameters and PCa (p<.001).
Immunohistochemical evaluation
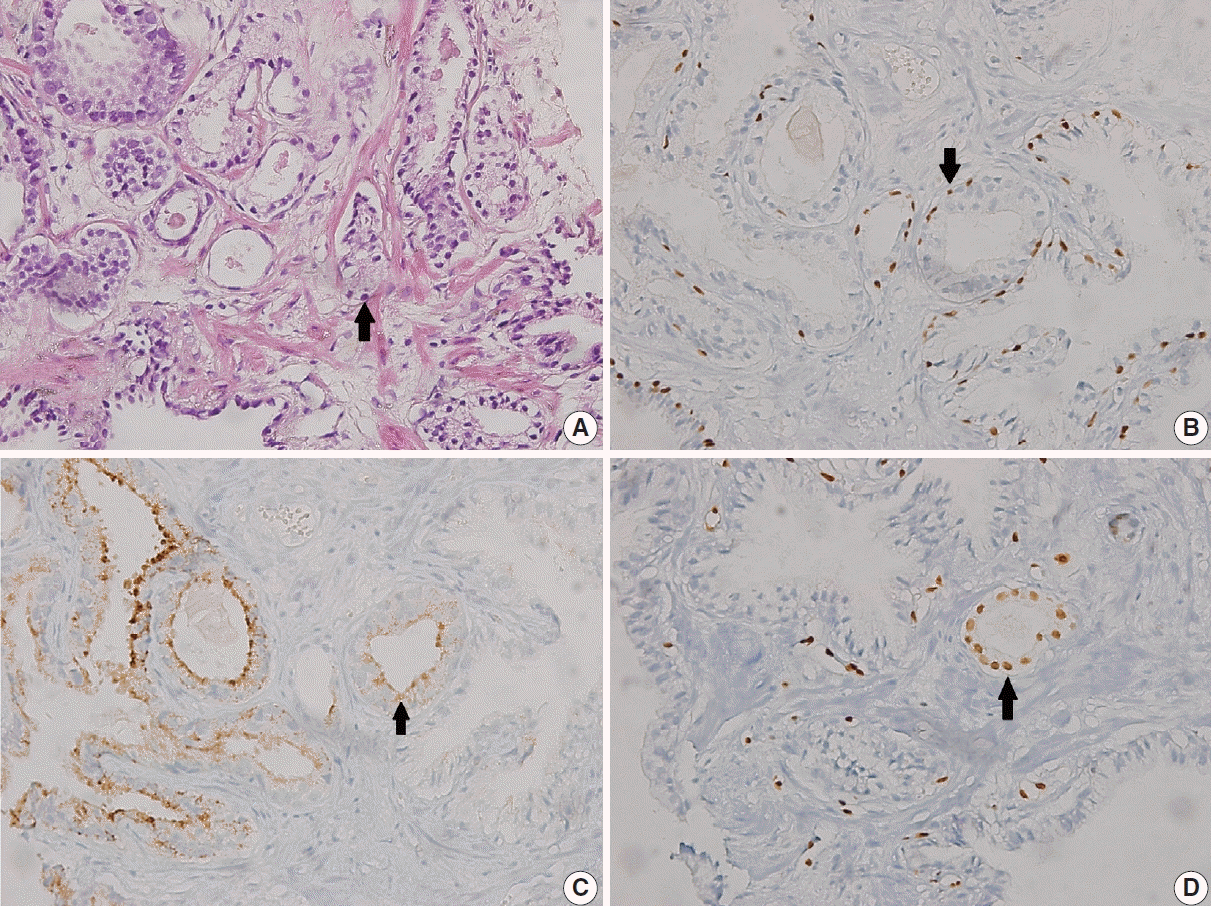
Basal cell markers (p63 and HMWCK) were not observed in any of the cases.
Of the 84 ASAP cases, 21 were diagnosed with PCa at rebiopsy.
In 27 of 84 cases (32.1%), AMACR showed nuclear positivity. PCa was diagnosed at rebiopsy in 15 (55.5%) of these positive cases. A significant correlation was found between AMACR positivity and PCa (p<.001).
In 19 of 84 cases (22.6%), ERG showed nuclear positivity. ERG, p63, and AMACR stains are shown in
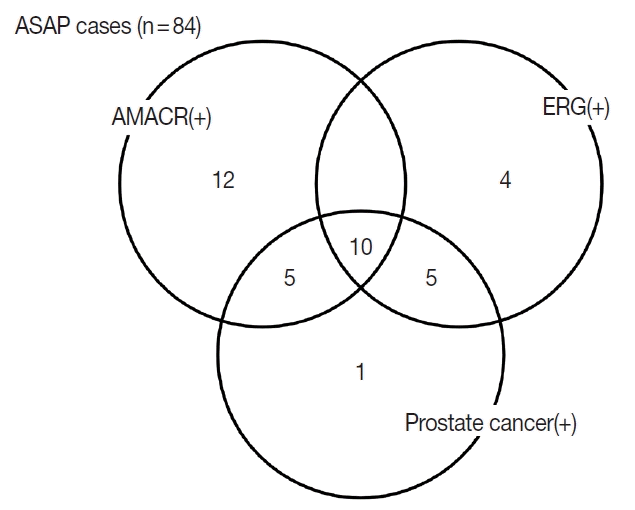
Fig. 2. PCa was diagnosed at rebiopsy in 15 of 19 cases (78.9%) that were positive for ERG. A significant correlation was found between ERG positivity and PCa (p < .001). A Venn diagram of ASAP cases that were positive for PCa and AMACR and ERG is shown in
Fig. 3. There was one patient who was both AMACR and ERG negative and had PCa in rebiopsy; that patient showed four histopathological parameters (nuclear enlargement, nucleolar prominence, cytoplasmic amphophilia, and luminal amorphous acellular secretion).
Comparison of histopathological parameters and immunohistochemical evaluation
A significant correlation was found between AMACR positivity and histopathological parameters of number of suspicious acini (p = .004), nucleolar prominence (p = .030), and hyperchromasia (p=.014).
A significant correlation was found between ERG positivity and histopathological parameters of nucleolar prominence (p< .001), hyperchromasia (p < .001), and infiltrative appearance (p=.002) (
Table 2).
DISCUSSION
Approximately 5% of prostate biopsies are diagnosed as ASAP due to the absence of adequate histomorphological parameters allowing differentiation of the atypical focus from PCa [
8]. In this study, the ASAP rate was consistent with that in the literature (5.67%) [
2,
8].
Several studies have shown a significant relationship between serum PSA level and advanced age for malignancy in ASAP patients [
9]. In our study, 18 of 21 patients who had malignancy on rebiopsy were 60 years or older, demonstrating that advanced age is an important risk factor for PCa. However, we did not find a significant relationship between serum PSA level and malignancy; PSA level was similar in the benign and malignant groups. The presence of intense active chronic inflammation in most benign cases also increases the PSA level [
10].
Some previous studies have reported mild nuclear enlargement in patients diagnosed with ASAP and mild to moderate enlargement with carcinoma [
4]. However, this evaluation criterion can be affected by many factors such as fixation, section thickness, and routine hematoxylin and eosin staining procedures. Therefore, it is a more accurate and reliable method to compare it with a benign epithelium when trying to determine nuclear enlargement. Although nuclear enlargement is often associated with adenocarcinoma, its low sensitivity and limited diagnostic value must be kept in mind [
11].
In our study, nucleolar prominence was the primary significant criteria in histological evaluation of cases diagnosed with ASAP. Some other studies, for example, Varma et al. [
11] observed a prominent nucleolus in 94% of malignant cases and 25% of benign cases and reported no prominent nucleolus in 24% of prostate malignancies obtained by prostate needle biopsies.
One study determined the frequency of infiltrative growth pattern in ASAP cases to be 68%–75% [
12]. The frequency of infiltrative growth pattern in our study was 83.3%, which is consistent with though slightly higher than that study. In addition, a significant correlation with malignancy was found in ASAP cases with an infiltrative growth pattern.
While some studies have suggested that intraluminal crystalloids do not increase the risk of cancer [
4], others have reported that they are frequently observed in cases with malignancies (40.6% in malignant cases vs. 1% in benign cases) [
11]. In our study, the prevalence of intraluminal crystalloids was significantly higher in the malignant group, suggesting a possible role in malignancy in ASAP cases. We also found that intraluminal amorphous eosinophilic materials and cytoplasmic amphophilia were evenly distributed between the groups.
Immunohistochemistry has limited potential for differentiating ASAP cases from carcinomas in TRUS biopsies, although its sensitivity may be increased if basal cell markers such as p63 and HMWCK are used together [
13]. Immunohistochemical AMACR staining is typically strong and appears diffusely positive in 97%–100% of PCas and 8%–12% of benign cases, while
ERG gene rearrangements were seen in 60% of prostate carcinomas [
4]. This fusion leads to the formation of the ERG oncoprotein, which results in proliferation of prostate carcinoma cells. ERG expression can be reliably determined by immunohistochemical methods and has high sensitivity and specificity for diagnosis of prostate carcinoma. Therefore, maximum diagnostic potential may be achieved through the combined use of hematoxylin and eosin (considering the histopathological parameters) and immunohistochemical analyses (AMACR and ERG and p63 and/or HMWCK) in foci suspicious for malignancy. The incidence of PCa in repeat biopsies following atypical diagnosis is high. In one study, this rate was 45% [
14]. Because of these high rates, patients with atypical biopsies should undergo repeat biopsy. Hence, it is crucial to accurately diagnose these very suspicious cancerous lesions from the outset to prevent the necessity of a subsequent biopsy in these individuals. Utilizing AMACR, ERG, and basal cell markers (HMWCK and/or p63) is very advantageous in diagnosing PCa, especially in unusual instances. ERG immunohistochemistry has potential diagnostic value that may be limited to specific settings. For example, in cases with negative AMACR and negative HMWCK expression, positive ERG immunohistochemistry would strongly support the diagnosis of PCa.
Morphologic features have been found to be associated with an increased risk of cancer. However, the predictive value of morphology alone in differentiating benign from malignant lesions in ASAP is limited as there is significant morphologic overlap between benign mimickers and cancer.
ERG expression has been shown to be a valuable marker in the diagnosis of malignancy in ASAP. ERG-positive cases are more likely to represent underlying carcinoma, particularly those associated with ERG gene rearrangements. ERG-negative cases are more likely to represent benign lesions or non-ERG fusion-driven malignancies. The use of ERG expression in conjunction with morphologic evaluation provides valuable information and improves the diagnostic accuracy in cases with ASAP. While morphology alone can raise suspicion for malignancy, it has limitations due to the overlap between benign and malignant features. ERG expression helps refine the diagnosis and aids in distinguishing between benign and malignant lesions [
15]. ERG immunohistochemical studies, despite their undisputed value in reinforcing PCa diagnosis, will involve additional costs. Therefore, ERG immunohistochemistry in cases of persistent suspicion of PCa could facilitate early diagnosis and may help mitigate the necessity for more expensive and invasive treatments, ultimately reducing patient burden.
ERG testing may not be universally available in all clinical settings. In such cases, histomorphological evaluation remains the primary tool for PCa diagnosis, and the presence of significant atypia and high-grade features should raise concern for malignancy. Pathologists should consider incorporating available markers, including racemase, p63, and HMWCK, with careful morphologic evaluation to maximize diagnostic accuracy in the absence of ERG testing.
Shah et al. found that 45% of cases initially diagnosed as limited PCa but classified as atypical showed positive ERG protein expression [
16]. Additionally, 28% of cases initially classified as atypical based on morphology, AMACR, and basal cell markers were reclassified as cancer due to ERG positivity. ERG expression was moderate to strong and uniform in most cases (80%). Interestingly, ERG expression was noted in benign/prostatic intraepithelial neoplasia glands in 10% of cases, but only in cases with PCa. Notably, two cases were diagnosed as ERG-negative PCa with adjacent benign glands positive for ERG, possibly due to ERG interfocal tumor heterogeneity [
17].
While immunohistochemical markers such as AMACR, p63, and HMWCK can provide additional information in the evaluation of prostate biopsies, their predictive value for cancer in cases with ASAP is less robust than ERG expression. ERG expression analysis in combination with histomorphological evaluation improves the accuracy of diagnosing malignancy in ASAP. However, in the absence of ERG testing, careful evaluation of morphologic features remains crucial in the assessment of atypical glands and the differentiation of benign and malignant lesions. In prostate biopsies, immunohistochemical ERG and AMACR in addition to these histological parameters may reduce inaccurate ASAP diagnoses and prevent unnecessary rebiopsy and related complications.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download