Abstract
The vestibular cortex is a distributed network of multisensory areas that plays a crucial role in balance, posture, and spatial orientation. The core region of the vestibular cortex is the parietoinsular vestibular cortex (PIVC), which is located at the junction between the posterior insula, parietal operculum, and retroinsular region. The PIVC is connected to other vestibular areas, the primary and secondary somatosensory cortices, and the premotor and posterior parietal cortices. It also sends projections to the vestibular nuclei in the brainstem. The PIVC is a multisensory region that integrates vestibular, visual, and somatosensory information to create a representation of head-in-space motion, which is used to control eye movements, posture, and balance. Other regions of the vestibular cortex include the primary somatosensory, posterior parietal, and frontal cortices. The primary somatosensory cortex is involved in processing information about touch and body position. The posterior parietal cortex is involved in integrating vestibular, visual, and somatosensory information to create a representation of spatial orientation. The frontal cortex is involved in controlling posture, and eye movements. The various methods used to stimulate the vestibular receptors in neuroimaging studies include caloric vestibular stimulation (CVS), galvanic vestibular stimulation (GVS), and auditory vestibular stimulation (AVS). CVS uses warm or cold water or air to stimulate the semicircular canals, GVS uses a weak electrical current to stimulate the vestibular nerve, and AVS uses high-intensity clicks or short tone bursts to stimulate the otolithic receptors.
Developments in functional neuroimaging techniques are improving our understanding of the anatomical and physiological organization of the vestibular cortex in humans. Unlike other sensory modalities, which have dedicated primary visual, auditory, and somatosensory cortices, the vestibular cortex integrates multimodal sensory information.1 The most-central region is the parietoinsular vestibular cortex (PIVC), which is part of the perisylvian area that includes the insular, parietal, and temporal lobes.2 Although the precise anatomical location of the PIVC is still unclear, it can be activated in experiments using various vestibular stimulation techniques.
Various vestibular stimulation techniques such as caloric vestibular stimulation (CVS),3,4 galvanic vestibular stimulation (GVS),5-7 and auditory vestibular stimulation (AVS)8-10 have been studied extensively. The method of magnetic vestibular stimulation has also been reported recently, and is attracting the attention of many researchers.11 The purpose of this review article is to review the functional neuroanatomy of the vestibular cortex in humans and to introduce the various methods used to stimulate the vestibular system.
Researchers have identified at least ten different brain areas in animals that respond to vestibular stimulation. These areas are also present in humans. The vestibular areas identified using CVS, GVS, and AVS are mainly located at the depth of the lateral sulcus and in the perisylvian cortex, which is the area around the Sylvian fissure.2 They are also found in the primary somatosensory cortex, posterior parietal cortex (PPC), frontal cortex, extrastriate cortex, cingulate cortex, and hippocampus.12 Together these areas form the vestibular cortex, which is a widely distributed network of multisensory areas.
The human brain is organized into two hemispheres that have specialized functions determined by factors such as evolution, genetics, and development. The most well-known examples of hemispheric specialization are handedness and language lateralization. However, language does not strictly depend on handedness, since most right-handers and nearly 70% of left-handers have a left-hemispheric dominance for language.13 Sensory input is processed in both hemispheres, but lateralization may also depend on the context. For example, functional magnetic resonance imaging (fMRI) and magnetoencephalography have shown that the right auditory cortex is more active during passive listening to sound coming from different locations.14
The vestibular system is unique because it requires constant communication between the right and left sides of the brain at different levels, especially the hemispheres, to create a unified perception of self-motion, gravity, and spatial orientation.15 This unified perception makes it possible to maintain postural balance in response to body accelerations. However, the vestibular cortex-which requires continuous communication between the two hemispheres-exhibits hemispheric lateralization, similar to language and motor dominance.15 Positron-emission tomography has revealed that the vestibular cortical network is dominated by the right hemisphere in right-handers and by the left hemisphere in left-handers.16 This finding was confirmed by fMRI during GVS and AVS.2 A meta-analysis that applied activation-likelihood estimation to all published neuroimaging results for the vestibular system in humans confirmed the dominance of the right hemisphere in right-handers, and found that the core region of the vestibular cortical circuitry is a parietal opercular area.2 Functional connectivity-based parcellation of the vestibular network in both hemispheres in right-handers and left-handers revealed that the vestibular dominance was located in the area of the posterior insula and parietal operculum, whereas the surrounding multisensory vestibular cortical regions were organized symmetrically and connected to the other sensory systems.17
The PIVC is a brain region that plays a crucial role in vestibular processing. It was first identified in monkeys, where it is located in the parietal operculum, posterior insula, and retroinsular region.18 The PIVC is strongly connected to other vestibular areas, the primary and secondary somatosensory cortices, and the premotor and posterior parietal cortices. It also sends projections to the vestibular nuclei in the brainstem.19 The PIVC is a multisensory region that integrates vestibular, visual, and somatosensory information to create a representation of head-in-space motion.1 This information is used to control eye movements, posture, and balance. It plays an important role in bodily awareness, cognition, and the perception of gravity.20 The exact location of the human PIVC is still unclear, but it is probably located in the posterior and anterior insulae, the parietal operculum, and the temporoparietal junction. These regions can be activated using vestibular stimulation methods such as CVS, GVS, and AVS.
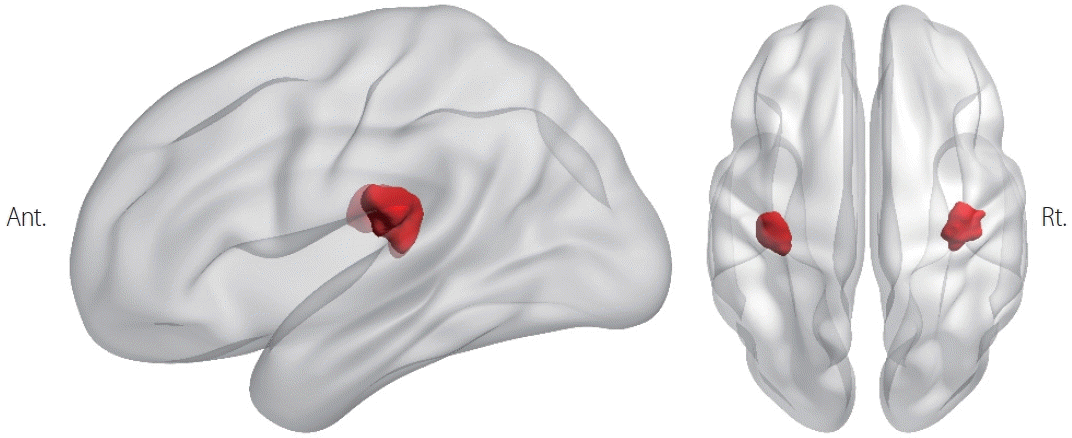
Two recent studies used a technique called coordinate-based activation-likelihood estimation meta-analysis to identify the core vestibular cortex in humans.2,21 This technique combines the results of many different studies to identify areas of the brain that are consistently activated by vestibular stimulation. One study found that a small area in the retroinsular region was activated by all three methods of vestibular stimulation, suggesting that it is the human equivalent of the monkey PIVC.2 This finding is supported by intracranial stimulation studies in humans showing that vestibular responses can be evoked by stimulating the posterior insula.22 Another study found that the so-called parietal operculum region 2 (OP2) may also be part of the human PIVC.12 The posterior insular region and OP2 have shown the most-consistent activation across different studies, and are connected to all of the other vestibular regions. The retroinsular region and OP2 are considered to be the human homologs of the PIVC in monkeys, based on the results of multiple stimulation studies and meta-analyses (Fig. 1). The areas marked in red in that figure correspond to the posterior insula and OP2 according to the Julich probabilistic atlas.23
Functional neuroimaging studies have shown that vestibular stimulation activates the primary somatosensory cortex, a region of the brain that processes information about touch and body position.6,24 This region is likely homologous to area 2v in monkeys, which is a vestibular area located in the primary somatosensory cortex.25 Human neuroimaging studies have also revealed activations in the primary somatosensory cortex in regions that could be equivalent to areas 3aHv and 3aNv in monkeys.26,27 These areas are located in the somatosensory representations of the hand/arm and neck/trunk, respectively.28,29 The vestibular projections to the primary somatosensory cortex may function to precisely code the positions of different body segments in space through multisensory convergence and thereby achieve better postural control.30 Vestibulo-somesthetic convergence in the somatosensory cortex may also account for the influence of vestibular stimulation and vestibular diseases on tactile and proprioceptive perceptions.31 In other words, the vestibular system sends signals to the primary somatosensory cortex that help the brain to integrate information about touch, body position, and body movements. This allows us to maintain balance and posture, and to accurately perceive the world around us.
The PPC contains a vestibular region that is distinct from the vestibular region of the primary somatosensory cortex. This PPC vestibular region is activated by stimulating either the otolith (using AVS) or semicircular canals (using CVS), suggesting that it integrates information from both types of vestibular receptor.9,24 Two multisensory areas in the PPC are thought to be part of the PPC vestibular region: the ventral intraparietal area (VIP) and the medial intraparietal area.32,33 VIP neurons respond to vestibular, visual, and tactile stimuli, and they create a representation of three dimensional body rotations and translations. The VIP is strongly connected to the motor cortex and the PIVC, suggesting that it plays a role in coordinating body movements and self-consciousness. CVS also activates Brodmann area 7 in the lateral superior parietal cortex and precuneus.34 These areas are also involved in multisensory processing and self-consciousness.
Neuroimaging studies have shown that vestibular stimulation activates several regions of the frontal cortex, including the primary motor cortex, premotor cortex, supplementary motor area, dorsolateral prefrontal cortex, and middle/superior frontal gyri.3,5,6,27 The primary motor cortex and premotor cortex are involved in controlling body movements, posture, and eye movements. The supplementary motor area is involved in planning and executing complex movements. The dorsolateral prefrontal cortex and middle/superior frontal gyri are involved in cognitive functions such as attention, decision-making, and spatial planning. Vestibular projections to the frontal cortex are thought to play a role in coordinating body movements, posture, and eye movements in response to vestibular stimulation.
The visual and vestibular systems are two essential sources of information for the perception of self-motion. The dorsal portion of the medial superior temporal area (MSTd) in the macaque cortex processes visual motion and integrates it with vestibular information to create a unified representation of self-motion.35 Human neuroimaging investigations have revealed that GVS and CVS activate the cortical visual area, known as the human homolog of the medial superior temporal area (hMST) in monkeys.35 Activations were found only in the anterior portion of the medial superior temporal area, indicating that this area contains at least two subregions, only one of which receives vestibular signals. This finding is supported by electrophysiological data in monkeys, showing that the MSTd contains neurons that respond to natural vestibular stimulation and optic flow. Thus, the anterior portion of the hMST that is activated during vestibular stimulation could be the human homolog of the MSTd.
The cingulate cortex is a brain region involved in various functions including motor control, attention, emotion, and vestibular processing. One region of the monkey cingulate cortex specifically involved in vestibular processing connects to two other vestibular brain regions: the PIVC and somatosensory area 3a.29 Human neuroimaging studies have shown that vestibular stimulation activates both the anterior and posterior parts of the cingulate cortex.3,5,9,16,26 The anterior cingulate cortex is involved in motor control and attention, while the posterior cingulate cortex is involved in spatial processing and memory.36 The posterior cingulate cortex might be involved in integrating vestibular information with other sensory information to create a unified representation of the body’s motion and position in space.37 The cingulate cortex may also be involved in controlling body movements and posture in response to vestibular stimulation.2
fMRI studies have shown that vestibular stimulation activates the hippocampus, a brain region that is involved in spatial navigation and memory. This suggests that the hippocampus is involved in processing vestibular information to help us navigate our surroundings and remember our location. Electrophysiological recordings in monkeys have also shown that hippocampal neurons respond to vestibular stimulation.38 Additionally, studies have shown that the activity of place cells and head-direction cells in the hippocampus is strongly dependent on vestibular signals.39,40 Damage to or deactivation of the vestibular system impairs the place and direction selectivity of these neurons. Vestibular symptoms such as dizziness are among the most common symptoms accompanying transient global amnesia and transient topographical disorientation due to hippocampal lesions.41,42
CVS as developed by the otologist Robert Bárány revolutionized clinical vestibular research.43 This has recently become a popular research tool again, especially for studying the vestibular cortex. One reason is neuroimaging techniques such as fMRI requiring the subject to keep their head still, which prevents researchers from using more-natural ways to stimulate the vestibular system such as head rotation or platform motion. CVS has emerged as a valuable tool for neuroimaging studies of the vestibular cortex, enabling researchers to investigate its complex processing of vestibular information in a large network of cortical areas.3,15,24,34 CVS uses warm or cold water or air to stimulate the vestibular system. During CVS, water or air at a specific temperature is injected into the external auditory canal. This temperature change causes the endolymph fluid in the semicircular canals to circulate, which stimulates or inhibits the hair cells in the crista ampullaris. Warm water or air increases the firing rate of hair cell afferents in the horizontal semicircular canals, with cold water or air having the opposite effect. This stimulation or inhibition of the hair cells generates the sensation that the head is spinning.
GVS involves applying weak electrical currents to stimulate the vestibular system. Electrodes are placed on the skin over the mastoid processes to induce current flows through the skin and bone that stimulate the vestibular nerve. GVS stimulates both the otoliths and semicircular canals.5-7,35 This means that GVS can produce more-complex sensations of motion than can CVS, which only stimulates the semicircular canals. The firing rates of the ipsilateral vestibular afferents increase to the cathodal electrode.2 GVS is a safe and effective way to stimulate the vestibular system, but it can cause some minor side effects such as burning, tingling, itching, and pain at the electrode sites. These side effects are usually mild and can be minimized by using large surface electrodes, covering the electrodes with gel or topical anesthesia, and restricting the current amplitude.44
Researchers have stimulated vestibular receptors using high-intensity clicks at 120 dB and short tone bursts at 500 Hz applied at 102 dB for 10 ms to activate the neural pathways that originate from the otolithic receptors.9,10 AVS has been shown to induce activation in several regions of the vestibular cortex, including the PIVC, in a manner similar to CVS and GVS.
The vestibular cortex plays critical roles in balance, posture, and spatial orientation. The development of various vestibular stimulation methods and neuroimaging techniques in recent years has led to a better understanding of the functional neuroanatomy of the vestibular cortex. However, current vestibular stimulation methods are often artificial and hence may not accurately reflect natural vestibular stimuli, and so further studies are needed to investigate how the vestibular cortex responds to natural translation and rotation stimuli.
REFERENCES
1. Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012; 35:185–196.

2. Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. 2012; 212:159–179.

3. Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002; 17:1384–1393.

4. Frank SM, Greenlee MW. An MRI-compatible caloric stimulation device for the investigation of human vestibular cortex. J Neurosci Methods. 2014; 235:208–218.

5. Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol. 2001; 85:886–899.
6. Lobel E, Kleine JF, Bihan DL, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998; 80:2699–2709.
7. Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, et al. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005; 26:721–732.
8. Janzen J, Schlindwein P, Bense S, Bauermann T, Vucurevic G, Stoeter P, et al. Neural correlates of hemispheric dominance and ipsilaterality within the vestibular system. Neuroimage. 2008; 42:1508–1518.
9. Miyamoto T, Fukushima K, Takada T, de Waele C, Vidal PP. Saccular stimulation of the human cortex: a functional magnetic resonance imaging study. Neurosci Lett. 2007; 423:68–72.

10. Schlindwein P, Mueller M, Bauermann T, Brandt T, Stoeter P, Dieterich M. Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage. 2008; 39:19–31.
11. Ward BK, Roberts DC, Otero-Millan J, Zee DS. A decade of magnetic vestibular stimulation: from serendipity to physics to the clinic. J Neurophysiol. 2019; 121:2013–2019.
12. Ibitoye RT, Mallas EJ, Bourke NJ, Kaski D, Bronstein AM, Sharp DJ. The human vestibular cortex: functional anatomy of OP2, its connectivity and the effect of vestibular disease. Cereb Cortex. 2023; 33:567–582.
13. Geschwind N. Specializations of the human brain. Sci Am. 1979; 241:180–199.
14. Woldorff MG, Tempelmann C, Fell J, Tegeler C, Gaschler-Markefski B, Hinrichs H, et al. Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum Brain Mapp. 1999; 7:49–66.
15. Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann N Y Acad Sci. 1999; 871:293–312.
16. Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003; 13:994–1007.
17. Cauzzo S, Singh K, Stauder M, García-Gomar MG, Vanello N, Passino C, et al. Functional connectome of brainstem nuclei involved in autonomic, limbic, pain and sensory processing in living humans from 7 Tesla resting state fMRI. Neuroimage. 2022; 250:118925.
18. Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurones in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis). J Physiol. 1990; 430:537–557.
19. Guldin WO, Mirring S, Grüsser OJ. Connections from the neocortex to the vestibular brain stem nuclei in the common marmoset. Neuroreport. 1993; 5:113–116.
20. Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol. 1994; 35:403–412.
21. zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012; 60:162–169.
22. Mazzola L, Lopez C, Faillenot I, Chouchou F, Mauguière F, Isnard J. Vestibular responses to direct stimulation of the human insular cortex. Ann Neurol. 2014; 76:609–619.
23. Amunts K, Mohlberg H, Bludau S, Zilles K. Julich-Brain: a 3D probabilistic atlas of the human brain’s cytoarchitecture. Science. 2020; 369:988–992.
24. Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, et al. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001; 12:441–449.
25. Schwarz DW, Fredrickson JM. Rhesus monkey vestibular cortex: a bimodal primary projection field. Science. 1971; 172:280–281.
26. Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp Brain Res. 1994; 99:164–169.
27. Emri M, Kisely M, Lengyel Z, Balkay L, Márián T, Mikó L, et al. Cortical projection of peripheral vestibular signaling. J Neurophysiol. 2003; 89:2639–2646.
28. Odkvist LM, Schwarz DW, Fredrickson JM, Hassler R. Projection of the vestibular nerve to the area 3a arm field in the squirrel monkey (saimiri sciureus). Exp Brain Res. 1974; 21:97–105.
29. Guldin WO, Grüsser OJ. Is there a vestibular cortex? Trends Neurosci. 1998; 21:254–259.
30. Ferrè ER, Bottini G, Iannetti GD, Haggard P. The balance of feelings: vestibular modulation of bodily sensations. Cortex. 2013; 49:748–758.
31. Lopez C. A neuroscientific account of how vestibular disorders impair bodily self-consciousness. Front Integr Neurosci. 2013; 7:91.
32. Klam F, Graf W. Discrimination between active and passive head movements by macaque ventral and medial intraparietal cortex neurons. J Physiol. 2006; 574:367–386.
33. Bremmer F, Klam F, Duhamel JR, Ben Hamed S, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci. 2002; 16:1569–1586.
34. Vitte E, Derosier C, Caritu Y, Berthoz A, Hasboun D, Soulié D. Activation of the hippocampal formation by vestibular stimulation: a functional magnetic resonance imaging study. Exp Brain Res. 1996; 112:523–526.
35. Smith AT, Wall MB, Thilo KV. Vestibular inputs to human motion-sensitive visual cortex. Cereb Cortex. 2012; 22:1068–1077.
36. Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001; 2:417–424.
37. Guterstam A, Björnsdotter M, Gentile G, Ehrsson HH. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr Biol. 2015; 25:1416–1425.
38. O’Mara SM, Rolls ET, Berthoz A, Kesner RP. Neurons responding to whole-body motion in the primate hippocampus. J Neurosci. 1994; 14:6511–6523.
39. Smith PF. Vestibular-hippocampal interactions. Hippocampus. 1997; 7:465–471.
40. Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002; 12:291–303.
41. Irving S, Pradhan C, Dieterich M, Brandt T, Zwergal A, Schöberl F. Transient topographical disorientation due to right-sided hippocampal hemorrhage. Brain Behav. 2018; 8:e01078.
42. Spiegel DR, Smith J, Wade RR, Cherukuru N, Ursani A, Dobruskina Y, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017; 13:2691–2703.
43. Lopez C, Blanke O. Nobel Prize centenary: Robert Bárány and the vestibular system. Curr Biol. 2014; 24:R1026–R1028.
44. Nguyen TT, Kang JJ, Oh SY. Thresholds for vestibular and cutaneous perception and oculomotor response induced by galvanic vestibular stimulation. Front Neurol. 2022; 13:955088.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download