Abstract
Background
This study aimed to determine the characteristics of computed tomography perfusion (CTP) patterns and their utility in predicting antiseizure medication (ASM) resistance in patients with nonconvulsive status epilepticus (NCSE).
Methods
We retrospectively reviewed patients diagnosed with NCSE at Inje University Haeundae Paik Hospital Epilepsy Center between March 2015 and March 2022. CTP patterns were analyzed for those patients. A hyperperfusion pattern (HPP) in CTP was defined as a region of both increased cerebral blood flow and cerebral blood volume that did not necessarily follow the vascular territories. The primary endpoint was the responses to ASMs according to CTP patterns.
Results
Fourteen (74%) of the 19 included patients met the criteria for definite NCSE, with the remaining 26% showing nonepileptiform activities with fluctuating quasirhythmic delta activities at >0.5 Hz on electroencephalogram. Three of the four patients who had HPPs with thalamic involvement were refractory to ASMs, whereas all eight patients who had HPPs without thalamic involvement were responsive to ASMs (p = 0.018). Although HPPs themselves were not associated with ASM responses, HPPs with thalamic involvement were associated with resistance to ASMs.
Nonconvulsive status epilepticus (NCSE) is challenging to diagnose due to its diverse presentations and prognoses.1 Performing prompt diagnosis and treatment of NCSE is critical due to its high mortality rate of up to 18%.2
Diagnosing NCSE solely based on electroencephalogram (EEG) can be difficult since its patterns are still debated among experts despite a consensus document having been published.3 Different EEG criteria have been suggested in recent decades in efforts to make diagnoses of NCSE more consistent among neurologists.4,5 The Salzburg consensus criteria for NCSE were proposed at the fourth London-Innsbruck Colloquium on Status Epilepticus held in Salzburg in 2013.6 These criteria were subsequently validated in 2014 with a sensitivity of 98% and a specificity of 90% in diagnosing NCSE.7 However, a group from the Netherlands reported an external retrospective validation in 2019 that showed lower accuracy, with a sensitivity of 67%, specificity of 89%, negative predictive value of 95%, and positive predictive value of 47%.8 There is therefore a need to identify additional modalities that could potentially aid in the diagnosis of NCSE when EEG findings are questionable.
Previous studies have shown that computed tomography perfusion (CTP) could be a reliable and sensitive tool for differentiating seizures from stroke mimics.9,10 Also, homolateral thalamic hyperperfusion along with cortical multilobar hyperperfusion in a patient with an acute-onset neurologic deficit is strongly suggestive of the presence of NCSE and is related to sustained ictal patterns.11
While there have been reports of an association between changes in CTP patterns and EEG ictal-interictal continuum patterns or electrographic seizure (ESz), no consensus has been established regarding the use of CTP in NCSE patients.12,13 Furthermore, to the best of our knowledge, no previous study has investigated the relationship between a hyperperfusion pattern (HPP) in CTP and antiseizure medication (ASM) responses in patients with NCSE. Therefore, this study investigated the characteristics of CTP patterns in NCSE patients and their association with ASM responses with the aim of achieving better prognostication.
We retrospectively reviewed patients diagnosed with NCSE at Inje University Haeundae Paik Hospital epilepsy center between March 2015 and March 2022. The institutional review board has approved this study. Inclusion criteria were 1) ≥20 years old and 2) having undergone CTP with or without diffusion-weighted magnetic resonance imaging (DWI). Exclusion criteria were 1) chronic epilepsy; 2) hypoxic-ischemic encephalopathy (HIE); 3) large structural damage; 4) de novo absence status epilepticus (SE) due to benzodiazepine (BDZ) withdrawal; 5) epilepsia partialis continua (EPC); or 6) incomplete medical records.
We used the definition in the standardized critical-care EEG criteria of the American Clinical Neurophysiology Society for ESz as 1) >25 epileptiform discharges at >2.5 Hz for ≥10 seconds; 2) any patterns with definite evolution lasting ≥10 seconds; or 3) any time-locked EEG patterns with a definite clinical event and clinical improvement with a parenteral ASM.7 NCSE was diagnosed when a patient had an altered mental status and their EEG findings were consistent with electrographic SE 1) ≥10 minutes continuously or 2) a total duration of ≥20% during any 60-minute recording period. However, a diagnosis of NCSE was challenging based solely in EEG when there were quasirhythmic delta activities at >0.5 Hz with fluctuation but no definitive evolution.14
All EEG recordings were performed using the twin EEG system (Grass Technologies, West Warwick, RI, USA) with 19 electrodes placed on the scalp according to the international 10-20 system. Their impedances remained below 5 kΩ before starting the recording and when measured again at the end of the recording session under the following settings: low-pass filter = 0.5 Hz, high-pass filter = 70 Hz, and sampling rate = 250 Hz.
The initial EEG recordings were made for at least 60 minutes. Intravenous sedatives were discontinued at least 30 minutes prior to performing EEG and were not administered during the EEG recordings. EEG recordings were made at least twice within 1 week, and the follow-up recordings were carefully reviewed to evaluate ASM responses. We also performed continuous EEG if the initial routine EEG showed nonepileptiform discharges.
CTP was performed within 24 hours of the patient arriving. The perfusion study was based on the following perfusion parameter data sets: cerebral blood flow (CBF), cerebral blood volume (CBV), time to peak (TTP), and mean transit time (MTT). Images were displayed using a false-color scale. CTP patterns were visually analyzed by two neuroradiologists separately and then decided by consensus. An HPP in CTP was defined as a region of increased CBF and CBV along with decreased TTP and MTT, showing a multiterritorial or a single territorial involvement independent of the vascular territories. We did not include hypoperfusion patterns because these have been reported as rare and atypical patterns in NCSE.15,16 If there was increased CBF and CBV at the same time in the thalamic region, we defined this as an HPP with thalamic involvement in CTP. Additionally, DWI signal changes were evaluated qualitatively on images, and defined as being present if there were any bright tissues with restricted diffusion on the images.15,17
A patient recovering consciousness within 3 days after using an ASM and showing improvement in the follow-up EEG was defined as “responsive”. A patient who failed to respond adequately to first- and second-line ASMs was defined as “refractory”, while if seizures persisted after initiating a third-line ASM (anesthetics), this was defined it as “super refractory”.18
The primary endpoint was the ASM responses according to CTP patterns. The secondary endpoints were the Glasgow coma scale (GCS) score at 1 week and the cerebral performance category (CPC) score at discharge. Other variables included age, sex, etiology, DWI patterns, and EEG patterns.
All statistical tests were performed using MedCalc software version 19.1.5 (MedCalc Software Ltd., Ostend, Belgium). In all calculations a p-value of <0.05 was considered statistically significant. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. The Mann-Whitney or Kruskal-Wallis test was applied to numerical variables.
Among 100 patients with NCSE who presented at our center between March 2015 to March 2022, 54 patients were excluded due to 1) history of chronic epilepsy; 2) HIE etiology; or 3) large structural damage. Among the remaining 46 patients, a further 27 patients were excluded due to 1) absence of CTP; 2) EPC; 3) incomplete clinical information; or 4) de novo absence SE secondary to BDZ withdrawal. Finally, 19 patients met both the inclusion and exclusion criteria (Fig. 1).
Table 1 summarizes the clinical demographics of the patients, who ranged in age from 39 to 90 years, and had a female predominance (females:males = 2.8:1). Eleven patients (58%) had acute symptomatic etiologies: three with subarachnoid hemorrhage, two with hypoglycemia, two with hyperglycemia, one with sepsis, one with encephalitis, one with new-onset refractory SE, and one with postoperative state. The remaining patients without an acute symptomatic etiology comprised one (5%) with a remote symptomatic etiology with a previous intracerebral hemorrhage and seven (37%) with cryptogenic etiologies. Their mental status improved significantly after ASM treatment, from an initial median GCS score of 9 to 1 of 12 after 1 week (p = 0.035). Most patients had a favorable outcome at discharge, with 79% showing a CPC score of 1 or 2.
NCSE was diagnosed based on EEG findings alone in 14 (74%) of the 19 patients. The remaining five patients (26%) showed nonepileptiform activities in EEG, which were quasirhythmic delta activities at >0.5 Hz with fluctuation. We additionally performed cEEG in three (60%) of the five patients who showed quasirhythmic delta activities at >0.5 Hz with fluctuation, but no ictal activity was detected during the recordings.
Four of these five patients showed either HPPs in CTP or DWI signal changes, while the fifth patient showed both HPPs in CTP and DWI signal changes (Figs. 2, 3, Table 2). The median time to CTP was 9 hours (interquartile range [IQR] = 4.5-45 hours) after arrival. Nine (64%) of the 14 patients with EEG-confirmed NCSE showed either HPPs in CTP or DWI signal changes (Fig. 2). The diagnostic yield was 74% when only EEG was used to diagnose NCSE. However, we found that the diagnostic yield improved when adding neuroimaging information to the EEG findings: by 21% when adding CTP and by 5% when adding DWI (Table 2).
Twelve (63%) of the 19 patients showed HPPs in CTP, with the other seven (37%) having no HPPs. HPPs themselves were not associated with ASM resistance: 33% with HPPs (4/12) vs. 43% without HPPs (3/7) (p = 1.00). The proportion of patients with acute symptomatic etiologies was higher in the HPP-positive group, but the difference was not statistically significant (75% vs. 29%; p = 0.07). There were no statistically significant differences between patients with and without HPPs in age, sex, CPC score at discharge, or median time to CTP (14 hours [IQR = 6-48 hours] vs. 6 hours [IQR = 3.2-34.5 hours]; p = 0.42).
Seven (37%) of the 19 patients were refractory to ASMs, with the remaining 63% being responsive to ASMs. Levetiracetam was the most frequently used first-line ASM (n = 14) followed by valproate (n = 3) and lorazepam (n = 2). Second-line ASMs were prescribed in 11 patients, with valproate being most common (n = 6), followed by levetiracetam (n = 3), lacosamide (n = 1), and phenytoin (n = 1).
Only three (25%) of the 12 patients with HPPs showed thalamic involvement. Three of the four patients who had HPPs with thalamic involvement were refractory to ASMs, whereas all eight patients who had HPPs without thalamic involvement were responsive to ASMs (p = 0.018). HPPs with thalamic involvement might therefore be a significant variable for predicting resistance to ASMs. In addition, ASM responses were associated with better GCS scores at presentation and 1 week, and with a better CPC score at discharge (p = 0.013, 0.027, and 0.009; respectively) (Table 3).
As neurologists, we frequently encounter patients with an altered mental status. It is critical to make prompt clinical judgements and investigations in order to ensure that an NCSE diagnosis is not missed. Our study group may represent the typical NCSE patients that are encountered in hospital settings. In order to maximize the homogeneity of the patient group with NCSE in this study, we excluded specific conditions by applying strict criteria. It is therefore possible that the clinical characteristics of our study group differed from those in other studies.15,16 In our study, acute symptomatic etiology was not related to ASM responses, and the proportion of patients with HPPs was higher than in other studies.12 Nonetheless, the female predominance observed in our study has also been seen in other studies.19,20 We do not have a clear explanation for this sex difference, but it might indicate that the X-chromosome plays a role in the pathogenesis of NCSE.21
Recent studies have revealed that neurovascular coupling is an important mechanism correlated with the high energy demands of the brain by furnishing energy substrates from the blood.22-24 This is responsible for the activity-dependent changes in the CBF. There is a functional and anatomical epileptic network connected to the cortical and subcortical brain structures, with one activity affecting all of the other components.25 The thalamus has widespread connections to the cortex, including to the temporal, parietal, and insular cortices.26 Hypothetically, there are extensive neuronal connections between the cortex and the thalamus, which are also known as functional connectivity.27 The hippocampus is a seizure-vulnerable structure in the brain.28 After seizure events, the neuronal excitability of the hippocampus and the thalamus can affect cortical regions.29 This leads to increased blood supply in those territories, and thalamic involvement could increase the damage in seizure-vulnerable structures of the brain, since the thalamus is thought to have more neuronal connections in the epileptic network. In addition, deep brain stimulation of the thalami has been used as a treatment option for refractory epilepsy,30 which indicates that the thalamus is a pivotal anatomical structure in the epileptic network. The involvement of the thalamus in the pathogenic process of a disease is probably linked to treatment resistance. Only one-fourth (3/12) of our patients who showed HPPs had thalamic involvement, which might have been due to the genetic predisposition, with some groups potentially being more susceptible to insult in the epileptogenic network.31
The reason for only some patients showing HPPs may be the diversity of cerebral collaterals and the complexity of thalamocortical circuits.32,33 Also, our study revealed discrepancies between HPPs and DWI signal changes, which perhaps indicates different underlying mechanisms. HPPs reflect a perfusion increase secondary to neuronal excitation rather than neuronal hyperexcitation itself. During ictal activity, the extracellular space in the cortex shrinks due to water flux into cells.34,35 The extracellular space expands in areas remote from the neuronal activity with a decrease in cortical diffusion, which increases the subcortical white-matter diffusion.36,37 Together these findings indicate that HPPs with thalamic involvement might be related to ASM resistance.
Previous studies have shown that NCSE can be diagnosed based only on EEG findings in almost 90% of cases,38,39 but it is still challenging to diagnose NCSE in patients with nonepileptiform discharges in EEG. Five (26%) of the 19 patients in our study showed quasirhythmic delta activities in both routine EEG and cEEG. We concluded that it is almost impossible to diagnose NCSE solely based on EEG in this patient group, and hence subsequent neuroimaging is needed to increase the diagnostic yield.
Since this was a retrospective study conducted at a single hospital, it is possible that selection bias was present. In addition, it was challenging to collect sufficient patients with NCSE given the rarity of this disease.40 We were motivated to perform this study by many neurologists around the world working hard to find better solutions for those suffering from NCSE. Even though this study involved only 19 patients with NCSE, post-hoc analysis showed that the statistical power exceeded 90%. Therefore, the possibility of type-1 errors could be rejected, making it reasonable to conclude that our statistically significant findings were not incidental.
The present findings support future prospective studies involving larger numbers of patients aimed at validating the utility of CTP in NCSE patients for predicting ASM responsiveness and for increasing the diagnostic yield when EEG alone is not sufficient. Many neurologists are working hard to fully understand NCSE, which is a diagnostically and therapeutically challenging disease. CTP could be a valuable tool for both increasing the diagnostic yield and predicting ASM responsiveness in NCSE patients.
REFERENCES
1. Walker M, Cross H, Smith S, Young C, Aicardi J, Appleton R, et al. Nonconvulsive status epilepticus: epilepsy research foundation workshop reports. Epileptic Disord. 2005; 7:253–296.

2. Hauf M, Slotboom J, Nirkko A, von Bredow F, Ozdoba C, Wiest R. Cortical regional hyperperfusion in nonconvulsive status epilepticus measured by dynamic brain perfusion CT. AJNR Am J Neuroradiol. 2009; 30:693–698.

3. Sutter R, Kaplan PW. Electroencephalographic criteria for nonconvulsive status epilepticus: synopsis and comprehensive survey. Epilepsia. 2012; 53 Suppl 3:1–51.

4. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996; 47:83–89.

5. Litt B, Wityk RJ, Hertz SH, Mullen PD, Weiss H, Ryan DD, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia. 1998; 39:1194–1202.

6. Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non-convulsive status epilepticus--approach to clinical application. Epilepsy Behav. 2015; 49:158–163.

7. Leitinger M, Trinka E, Gardella E, Rohracher A, Kalss G, Qerama E, et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol. 2016; 15:1054–1062.

8. Goselink RJM, van Dillen JJ, Aerts M, Arends J, van Asch C, van der Linden I, et al. The difficulty of diagnosing NCSE in clinical practice; external validation of the Salzburg criteria. Epilepsia. 2019; 60:e88–e92.

9. Davies E, Elnagi F, Smith T. CT perfusion: stroke, seizure or both? BMJ Case Rep. 2021; 14:e245723.

10. Giovannini G, Malagoli M, Turchi G, Miani A, Orlandi N, Vaudano AE, et al. Cortical and thalamic hyper-perfusion in non-convulsive status epilepticus. Relationship between perfusion CT patterns and Salzburg EEG criteria. Seizure. 2021; 92:10–17.

11. Khoo CS, Kim SE, Lee BI, Shin KJ, Ha SY, Park J, et al. Characteristics of perfusion computed tomography imaging in patients with seizures mimicking acute stroke. Eur Neurol. 2020; 83:56–64.

12. Gugger JJ, Llinas RH, Kaplan PW. The role of CT perfusion in the evaluation of seizures, the post-ictal state, and status epilepticus. Epilepsy Res. 2020; 159:106256.

13. Sheikh IS, Park S, Ali I. Using CT perfusion in the interictal state. J Clin Neurophysiol. 2021; 38:e25–e28.

14. Leitinger M, Trinka E, Zimmermann G, Beniczky S. Salzburg criteria for nonconvulsive status epilepticus: details matter. Epilepsia. 2019; 60:2334–2336.

15. Gelfand JM, Wintermark M, Josephson SA. Cerebral perfusion-CT patterns following seizure. Eur J Neurol. 2010; 17:594–601.

16. Lucas L, Gariel F, Menegon P, Aupy J, Thomas B, Tourdias T, et al. Acute ischemic stroke or epileptic seizure? Yield of CT perfusion in a “code stroke” situation. AJNR Am J Neuroradiol. 2021; 42:49–56.

17. Williams J, Mullins G, Delanty N, Bede P, Doherty CP. The spectrum of peri-ictal MRI changes; four illustrative cases. Seizure. 2017; 50:189–193.

18. Samanta D, Garrity L, Arya R. Refractory and super-refractory status epilepticus. Indian Pediatr. 2020; 57:239–253.

19. Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003; 74:189–191.

20. Lee JJ, Park KI, Park JM, Kang K, Kwon O, Lee WW, et al. Clinical characteristics and treatment outcomes of de novo nonconvulsive status epilepticus: a retrospective study. J Clin Neurol. 2021; 17:26–32.

21. Gschwind M, Foletti G, Baumer A, Bottani A, Novy J. Recurrent nonconvulsive status epilepticus in a patient with coffin-lowry syndrome. Mol Syndromol. 2015; 6:91–95.

22. Stackhouse TL, Mishra A. Neurovascular coupling in development and disease: focus on astrocytes. Front Cell Dev Biol. 2021; 9:702832.

23. Phillips AA, Chan FH, Zheng MM, Krassioukov AV, Ainslie PN. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016; 36:647–664.

24. Kaplan L, Chow BW, Gu C. Neuronal regulation of the bloodbrain barrier and neurovascular coupling. Nat Rev Neurosci. 2020; 21:416–432.

25. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002; 43:219–227.

26. Rosenberg DS, Mauguière F, Catenoix H, Faillenot I, Magnin M. Reciprocal thalamocortical connectivity of the medial pulvinar: a depth stimulation and evoked potential study in human brain. Cereb Cortex. 2009; 19:1462–1473.

27. Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol. 2000; 21:1397–1401.
28. Lee DA, Lee J, Kim HC, Park KM, Kim SE. Hippocampal injury in patients with status epilepticus: quantitative analysis of hippocampal volume and structural co-variance network. Seizure. 2022; 95:84–89.

29. Navidhamidi M, Ghasemi M, Mehranfard N. Epilepsy-associated alterations in hippocampal excitability. Rev Neurosci. 2017; 28:307–334.

30. Fisher RS. Deep brain stimulation of thalamus for epilepsy. Neurobiol Dis. 2023; 179:106045.
32. Kremkow J, Alonso JM. Thalamocortical circuits and functional architecture. Annu Rev Vis Sci. 2018; 4:263–285.

33. Rodriguez-Moreno J, Porrero C, Rollenhagen A, Rubio-Teves M, Casas-Torremocha D, Alonso-Nanclares L, et al. Area-specific synapse structure in branched posterior nucleus axons reveals a new level of complexity in thalamocortical networks. J Neurosci. 2020; 40:2663–2679.

34. Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986; 44:619–639.
35. Jabeen SA, Cherukuri P, Mridula R, Harshavardhana KR, Gaddamanugu P, Sarva S, et al. A prospective study of diffusion weighted magnetic resonance imaging abnormalities in patients with cluster of seizures and status epilepticus. Clin Neurol Neurosurg. 2017; 155:70–74.
36. Hochman DW. The extracellular space and epileptic activity in the adult brain: explaining the antiepileptic effects of furosemide and bumetanide. Epilepsia. 2012; 53 Suppl 1:18–25.

37. Mendes A, Sampaio L. Brain magnetic resonance in status epilepticus: a focused review. Seizure. 2016; 38:63–67.

38. Lee DA, Park KM, Kim HC, Khoo CS, Lee BI, Kim SE. Spectrum of ictal-interictal continuum: the significance of 2HELPS2B score and background suppression. J Clin Neurophysiol. 2023; 40:364–370.

39. Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010; 12:382–389.

40. Towne AR, Waterhouse EJ, Boggs JG, Garnett LK, Brown AJ, Smith JR Jr, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000; 54:340–345.
Fig. 1.
Flow chart of patient selection. NCSE, nonconvulsive status epilepticus; HIE, hypoxic-ischemic encephalopathy; CTP, computed tomography perfusion; EPC, epilepsia partialis continua; SE, status epilepticus; BDZ, benzodiazepine.
Fig. 2.
Seizure-related CTP patterns and diffusion-weighted imaging (DWI) signal changes. (A) Hyperperfusion pattern (HPP) with thalamic involvement and seizure-related DWI signal changes. (B) HPP only. CTP, computed tomography perfusion.
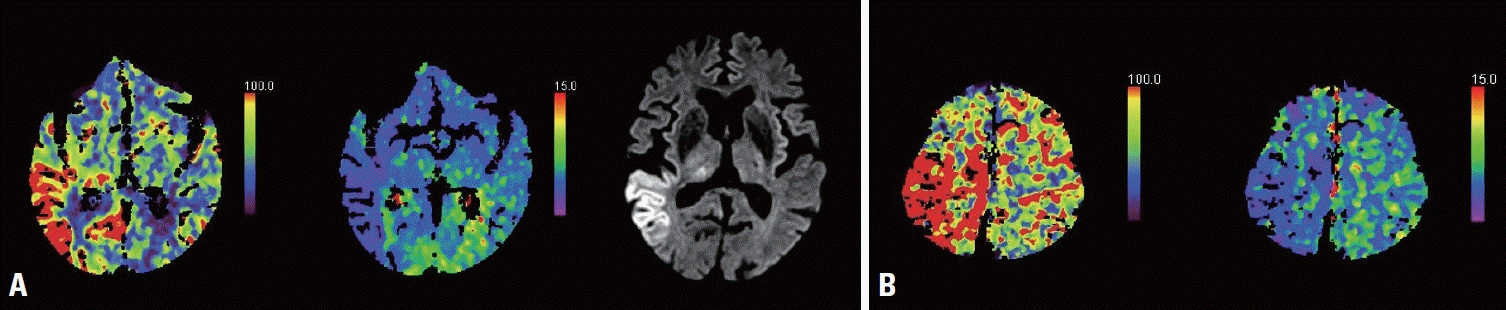
Fig. 3.
EEG and neuroimaging findings in NCSE. CTP (+), HPP in CTP; DWI (+), signal changes present in DWI. EEG, electroencephalogram; ESz, electrographic seizure; CTP, computed tomography perfusion; DWI, diffusion-weighted imaging; NCSE, nonconvulsive status epilepticus.
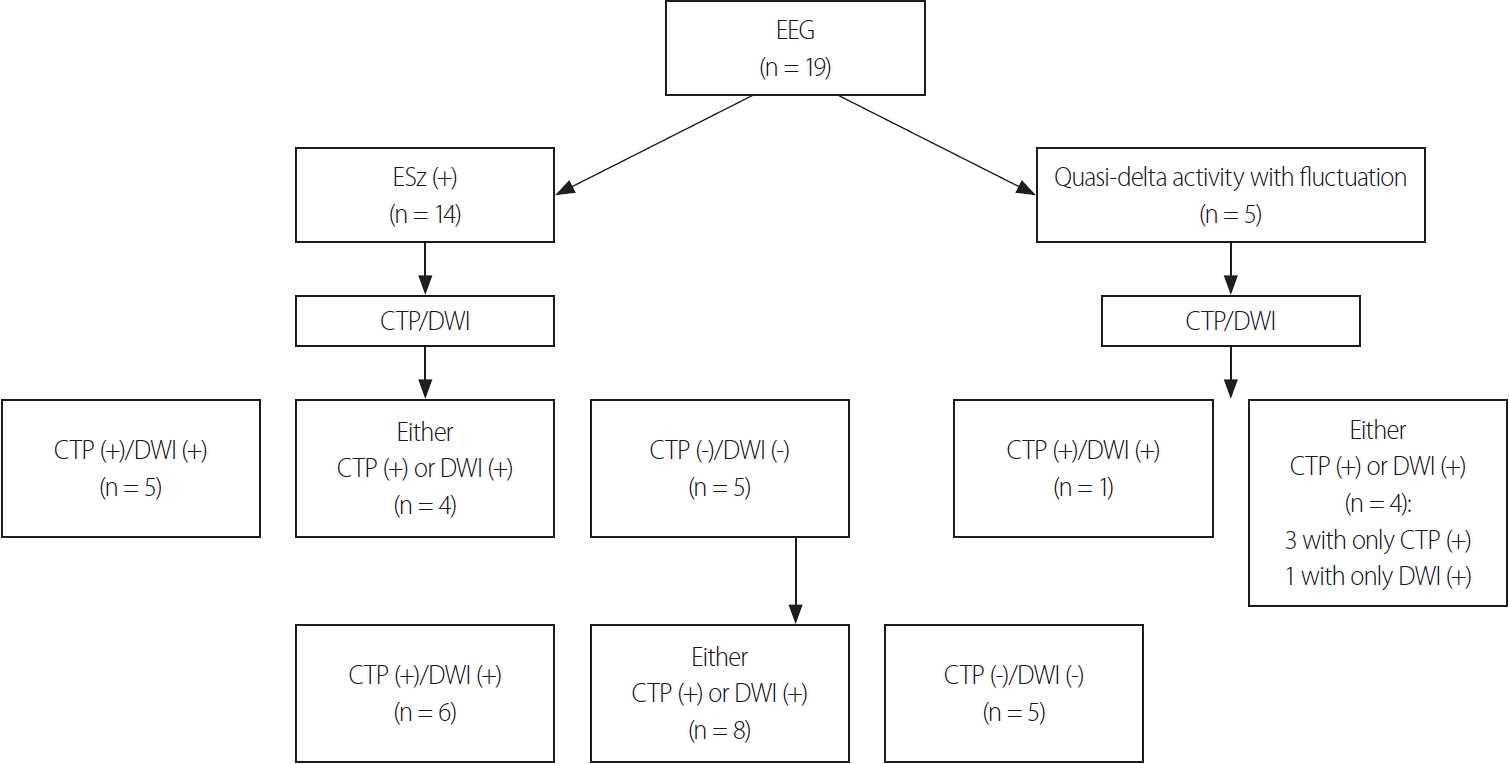
Table 1.
Demographics
Table 2.
Diagnostic yield change by adding neuroimaging modality
Table 3.
Clinical variables and responses to antiseizure medications
Values are presented as mean ± standard deviation, median (interquartile range), number (%) unless otherwise indicated.
NCSE, nonconvulsive status epilepticus; Sz, seizure; EEG, electroencephalogram; DWI, diffusion-weighted imaging; CTP, computed tomography perfusion; HPP, hyperperfusion pattern; GCS, Glasgow coma scale; CPC, cerebral performance category.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download