Abstract
Background
Resistance training for leg muscles is recommended for patients with postural tachycardia syndrome (POTS). However, no study has characterized the relationships between orthostatic symptoms, heart rate (HR) increase, and the mass of the lower leg muscle in patients with POTS. We sought to determine the relationships between the mass of the lower leg muscle, HR increase during the head-up tilt (HUT) test, and orthostatic symptoms in patients with POTS.
Methods
We prospectively enrolled 42 patients with POTS who were older than 16 years. The muscle mass was estimated using bioelectrical impedance analysis. We used the International Physical Activity Questionnaire-Short Form to measure self-reported physical activity. All patients were asked to complete the Korean version of the Orthostatic Grading Scale (KOGS).
Results
The HR increased during the HUT test by 38.7±7.88 beats/minutes. Both the HR increase during the HUT test and the total KOGS score were negatively correlated with the total metabolic equivalent of the task. The leg circumference and muscle mass were not correlated with the HR increase during the HUT test or the KOGS score.
Postural tachycardia syndrome (POTS) is defined as an increase in heart rate (HR) of ≥30 beats/minutes or an absolute HR of ≥120 beats/minutes within 10 minutes during standing or the head-up tilt (HUT) test. POTS is the leading cause of orthostatic intolerance (OI) in young patients. Sympathetic denervation, hyperadrenergic state, venous pooling, hypovolemia, and deconditioning have been suggested as causes of POTS. Because deconditioning and venous pooling are the main pathophysiologies of POTS, physical reconditioning with regular exercise is considered to be the cornerstone of treatment for POTS.
A structured exercise program featuring endurance (aerobic) reconditioning with resistance (strength) training for the leg muscles is recommended for patients with POTS. The skeletal muscle pumps represent the primary defense against the pooling of blood in the lower extremities in humans.1 Because contractions of the leg and gluteal muscles increase interstitial pressure and push venous blood back to the heart in the presence of venous pooling,2 it is reasonable to assume that the leg circumference and muscle mass are related to HR increases or orthostatic symptoms in POTS patients. A previous study of POTS patients found that a low calf blood flow was associated with calf size and impairment of the ejection fraction of the skeletal muscle pump while standing.3
However, endurance training is prioritized for the treatment of POTS because this increases the blood and plasma volumes, increases cardiac size and mass, and improves orthostatic tolerance.4 Moreover, a study found that the leg muscle mass does not appear to be a significant determinant of tolerance to increased venous pooling in healthy controls.5 This means that there is currently an inadequate understanding of the role of lower leg muscles on OI in patients with POTS. We therefore sought to determine the relationships between the circumference and muscle mass of the lower leg, the HR increase during the HUT test, and orthostatic symptoms in patients with POTS.
We prospectively enrolled patients with POTS older than 16 years from May 2018 to September 2019. All patients underwent standardized autonomic function tests. The quantitative sudomotor axon reflex test, HUT test, Valsalva maneuver, and HR response to deep breathing were assessed to evaluate sympathetic and parasympathetic autonomic function. Beat-to-beat blood pressure (BP) and HR responses were measured noninvasively using the Finometer device (Finapres Medical Systems, Amsterdam, The Netherlands). The HUT test protocol included 10 minutes in the supine position and 20 minutes of tilting at 70 degrees. POTS is defined as an increase in HR of ≥30 beats/minutes within 10 minutes of moving from a supine position to standing or during the HUT test in the absence of orthostatic hypotension, or an absolute HR of ≥120 beats/minutes. The required increment in HR is ≥40 beats/minutes for individuals aged 12 to 19 years. In addition to BP and HR monitoring using the Finometer device, BP and HR were measured at baseline and every minute during the HUT test using a manual sphygmomanometer (Tycos, Skaneateles Falls, NY, USA). All autonomic function tests were performed under standardized environmental conditions (i.e., quiet, an ambient temperature of 23-25°C, and humidity of approximately 50%). No coffee, food, or nicotine was permitted for 6 hours before the measurements. Patients stopped taking medications before the test. We excluded patients who experienced acute illness. All patients were asked to complete the Korean version of the Orthostatic Grading Scale (KOGS), which is a validated tool for screening patients with orthostatic dizziness during daily life in South Korea.6
We measured the body weight and height and the circumferences of the thigh, waist, calf, and hip. Body fat mass, body weight, and standing height were measured with subjects dressed in light clothing and barefoot. Body mass index was calculated as body weight divided by height squared. The thigh circumference was measured at a position 15 cm proximal to the superior pole of the patella.7 The muscle mass (bone-free lean-tissue mass) was estimated using bioelectrical impedance analysis with a body composition analyzer (InBody 720; Biospace, Seoul, Korea). The Korean version of the International Physical Activity Questionnaire8-Short Form was performed to measure self-reported physical activity within the previous 7 days.
The metabolic equivalent of task (METs) and sedentary behavior were calculated according to standard instructions (http://www.ipaq.ki.se). Physical activity was quantified by measuring the amount of time spent performing vigorous physical activity, moderate physical activity, walking, and sedentary activity during the previous 7 days and converting it to the METs in minutes per week to derive a continuous score and a categorical score. The total physical activity score for the continuous score was calculated as the sum of walking METs, moderate-activity METs, and vigorous-activity METs, with less than 10 minutes of physical activity considered to be no physical activity.
The three measures for quantifying the continuous score were calculated as follows: 1) walking MET=3.3 (MET level)× time walked (minutes)×days. 2) Moderate-activity MET=4.0 (MET level)×minutes of moderate-intensity activity×days. 3) Vigorous-activity MET=8.0 (MET level)×minutes of vigorous-intensity activity×days.
The three categorical scores were calculated as follows: category 1 (inactive) was the lowest level of physical activity and included subjects not included in categories 2 and 3, who were considered to be insufficiently active. Category 2 (minimally active) included those who engaged in vigorous physical activity for ≥20 minutes/days on ≥3 days/weeks, moderate physical activity for ≥30 minutes/days on ≥5 days/ weeks, or a combination of walking, moderate activity, and vigorous physical activity equaling 600 METs (minutes/ weeks) on ≥5 days/weeks. Category 3 (health-promoting physical activity) encompassed vigorous physical activity equaling 1,500 METs (minutes/weeks) on ≥3 days/weeks, or a combination of walking, moderate activity, and vigorous physical activity equaling 3,000 METs (minutes/weeks) on ≥7 days/weeks.
The Korean Anxiety Sensitivity Index-Revised (ASI-R)9 and the Beck Depression Inventory (BDI) were used to assess anxiety and depression in all patients. Spearman’s correlation coefficients were used to quantify the correlations between parameters. We used partial correlation analysis to examine the correlation between variables after adjusting sex. The AMOS software version 21.0 (AMOS, Chicago, IN, USA) was used for the path analysis of the direct effects of exercise amount and leg muscle mass on the HR increase and orthostatic dizziness, and the Sobel test was used for the indirect effect. Before performing path analysis, we performed regression analysis to assess multicollinearity among the variables. The goodness of fit of the path analysis model was appropriate according to the chi-square test, goodness-of-fit index, and standardized root-mean-square residual. In addition, the total MET was used after log transformation due to the considerable variance identified when performing the path analysis.
All experiments complied with the tenets of the Declaration of Helsinki, and the study protocol was reviewed and approved by the Institutional Review Board of the Keimyung University Dongsan Hospital.
Forty-two patients with POTS aged 24.9±10.35 years (mean± standard deviation) were enrolled, 40% of whom were male. One patient had a history of hypertension and one had diabetes. The HR increase during the HUT test was 38.7±7.88 beats/minutes. The total KOGS score was 7.93±3.27. Twenty-one patients showed mild adrenergic or sudomotor dysfunction. The ASI-R and BDI scores were 32.6±21.38 and 16.4±9.02, respectively.
The HR increase during the HUT test was negatively correlated with age (r=-0.43, p=0.00). The ASI-R score was positively correlated with the total KOGS score (r=0.35, p=0.02). The walking METs were negatively correlated with the total KOGS score (r=-0.38, p=0.01). The HR increase during the HUT test and the total KOGS score were both negatively correlated with the total METs (r=-0.44, p=0.00; r=-0.33, p=0.04; respectively) (Fig. 1). The circumferences of the thigh and calf as well as the mass of the leg muscle were not correlated with the HR increase during the HUT test or the KOGS score (Table 1).
The amount of physical activity exerted a significant direct effect of -0.32 on the total KOGS score (p=0.041), whereas the indirect effect of 0.051 mediated by leg muscle mass was not significant (p=0.350). The direct effect of the amount of physical activity of -0.28 on the HR increase during the HUT test was not statistically significant (p=0.074), as was the indirect effect of -0.023 mediated by leg muscle mass (p=0.644) (Fig. 2). There was almost no multicollinearity among the variables in the path model, with a variance inflation factor value of 1.27 for log (total MET).
Skeletal muscle pumps defend against venous pooling in the lower extremities. This study found that the leg circumference and muscle mass were not related to HR increases or orthostatic symptoms in POTS patients. However, POTS patients who reported performing more physical activity tended to exhibit smaller increases in the HR during the HUT test as well as milder orthostatic symptoms.
Numerous studies have investigated the role of skeletal muscle pumps in OI. One study found that a smaller calf circumference was related to a smaller volume of blood ejected by the skeletal muscle pump during voluntary muscle contraction, especially in patients with a low calf blood flow while supine.3 Those authors suggested that a lower resting blood flow in some patients with POTS can worsen OI through impairment of the skeletal muscle pumps. However, other studies have produced contradictory findings, such as the muscle pumps possibly not playing a fundamental role in increasing venous return, venous preload, or cardiac output during exercise, presumably due to local vasodilation.10 The leg muscle mass was found to not be a critical determinant of a low body negative pressure tolerance in healthy controls.5 Therefore, even leg crossing and muscle tensing can be used to prevent orthostatic symptoms and vasovagal syncope. The present results indicate that whether leg muscle is a principal factor for OI in all POTS patients remains inconclusive. This result is not necessarily surprising considering the heterogeneous pathophysiology of POTS and arterial and cardiopulmonary baroreflexes, which are involved in controlling arterial pressure.
We found that physical activity exerted a significant direct effect on orthostatic symptoms, while the indirect effect mediated by leg muscle mass was not significant. We can therefore assume that the impact of physical activity is due to factors other than increasing leg muscle mass. Numerous studies have also investigated the use of physical training for POTS. This intervention expands the blood volume and plasma volume,11 increases the left ventricular mass and end-diastolic volume,12 improves autonomic circulatory control and arterial-cardiac baroreflex function,13 and improves orthostatic tolerance.12
Our study had some limitations. First, we did not observe the effects of physical activity on OI both before and after training or exercise. It is therefore not possible to draw any conclusion about whether the HR increase in patients with POTS is related to less physical activity. Second, because the number of patients was relatively small, the possible contribution of the mass of low leg muscle on OI in patients with POTS should be investigated in larger samples. Third, the disease duration can interfere with daily activity, and so a longer disease duration may impact the MET and/or leg muscle mass. We did not adjust for the disease duration and so this may have also affected the reported results.
Patients who reported performing more physical activity experienced milder orthostatic symptoms. However, the leg circumference and muscle mass were not related to orthostatic symptoms in the patients with POTS. Cardiac remodeling or blood volume increase may be responsible for improvement in POTS after physical activity.
Notes
REFERENCES
2. Wang Y, Marshall RJ, Shepherd JT. The effect of changes in posture and of graded exercise on stroke volume in man. J Clin Invest. 1960; 39:1051–1061.

3. Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. 2004; 286:H1216–H1222.

4. Fu Q, Levine BD. Exercise in the postural orthostatic tachycardia syndrome. Auton Neurosci. 2015; 188:86–89.

5. Lawler LA, Halliwill JR, Summer JM, Joyner MJ, Mulvagh SL. Leg mass and lower body negative pressure tolerance in men and women. J Appl Physiol (1985). 1998; 85:1471–1475.
6. Kim HA, Lee H, Park KJ, Lim JG. Autonomic dysfunction in patients with orthostatic dizziness: validation of orthostatic grading scale and comparison of Valsalva maneuver and head-up tilt testing results. J Neurol Sci. 2013; 325:61–66.

7. Chen BB, Shih TT, Hsu CY, Yu CW, Wei SY, Chen CY, et al. Thigh muscle volume predicted by anthropometric measurements and correlated with physical function in the older adults. J Nutr Health Aging. 2011; 15:433–438.

8. Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007; 28:532–541.
9. Lim YJ, Yu BH, Kim JH. Korean anxiety sensitivity index-revised: its factor structure, reliability, and validity in clinical and nonclinical samples. Depress Anxiety. 2007; 24:331–341.

10. Casey DP, Hart EC. Cardiovascular function in humans during exercise: role of the muscle pump. J Physiol. 2008; 586:5045–5046.

11. Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968; 38:VII1–VII78.
Fig. 1.
Correlation between the amount of physical activity and orthostatic intolerance. MET, metabolic equivalent; HR, heart rate; HUT, head-up tilt; KOGS, Korean version of the Orthostatic Grading Scale.
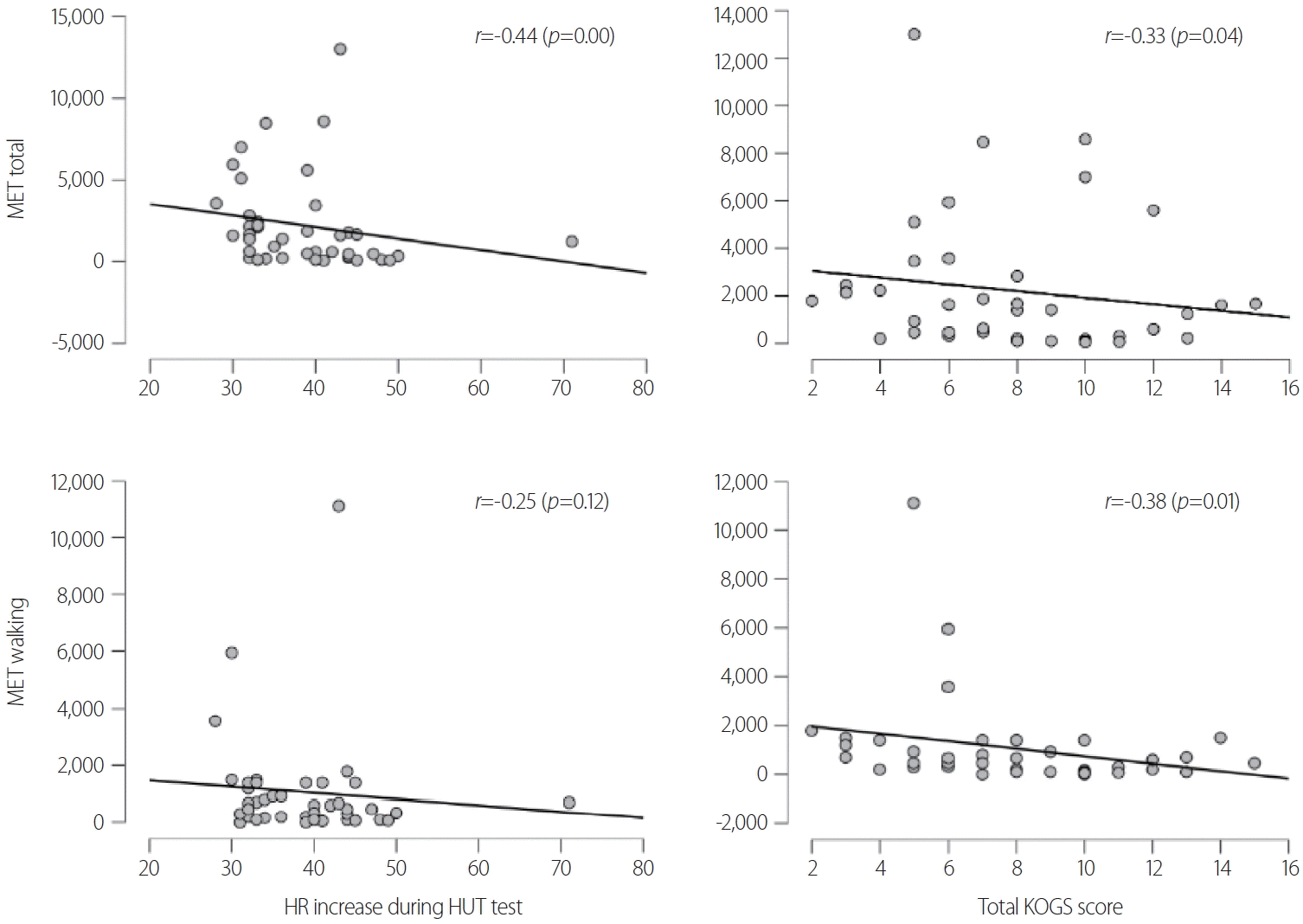
Fig. 2.
Results from a path analysis model of the effect of physical activity on orthostatic intolerance. Physical activity exerted a significant direct effect on the total KOGS score, while the indirect effect mediated by leg muscle mass was not significant. The direct effect of the amount of physical activity on HR increase during the HUT test was not significant, as was the indirect effect mediated by leg muscle mass. MET, metabolic equivalent; KOGS, Korean version of the orthostatic grading scale; HR, heart rate; HUT, head-up tilt; GFI, goodness of fit index; AGFI, adjusted goodness of fit index; SRMR, standardized root mean residual.
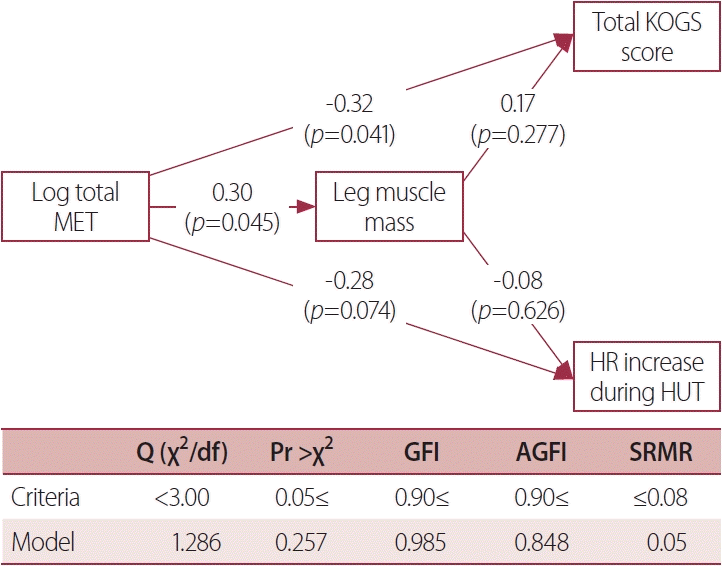
Table 1.
Correlations between physical parameters, heart rate increases during the head-up tilt test, and scores on orthostatic grading scales
|
Spearman’s correlation |
Partial correlation after adjusting sex |
|||||||
|---|---|---|---|---|---|---|---|---|
|
HR increase during HUT test |
KOGS total score |
HR increase during HUT test |
KOGS total score |
|||||
| r | p | r | p | r | p | r | p | |
| Age | -0.43 | 0.00a | -0.21 | 0.18 | -0.41 | 0.01a | -0.21 | 0.20 |
| ASI-R | -0.22 | 0.17 | 0.35 | 0.02a | -0.30 | 0.06 | 0.36 | 0.02a |
| BDI | -0.01 | 0.94 | 0.27 | 0.08 | -0.06 | 0.70 | 0.27 | 0.08 |
| Hight | -0.10 | 0.52 | 0.04 | 0.81 | 0.09 | 0.57 | 0.10 | 0.53 |
| Weight | -0.15 | 0.35 | 0.12 | 0.46 | -0.02 | 0.92 | 0.18 | 0.25 |
| BMI | -0.14 | 0.37 | 0.23 | 0.15 | -0.07 | 0.67 | 0.26 | 0.10 |
| Waist size | -0.18 | 0.25 | 0.17 | 0.29 | -0.08 | 0.64 | 0.23 | 0.15 |
| Thigh circumference | ||||||||
| Left | -0.08 | 0.60 | 0.06 | 0.70 | -0.06 | 0.73 | 0.07 | 0.67 |
| Right | -0.07 | 0.66 | 0.03 | 0.86 | -0.05 | 0.78 | 0.03 | 0.84 |
| Mean | -0.09 | 0.59 | 0.04 | 0.79 | -0.06 | 0.73 | 0.05 | 0.76 |
| Calf circumference | ||||||||
| Left | -0.14 | 0.39 | 0.13 | 0.41 | -0.02 | 0.91 | 0.18 | 0.25 |
| Right | -0.13 | 0.41 | -0.02 | 0.89 | 0.00 | 1.00 | 0.00 | 0.99 |
| Mean | -0.15 | 0.33 | 0.04 | 0.80 | -0.03 | 0.86 | 0.08 | 0.62 |
| Leg mass | ||||||||
| Left | -0.21 | 0.18 | 0.01 | 0.94 | -0.06 | 0.71 | 0.08 | 0.63 |
| Right | -0.16 | 0.32 | 0.04 | 0.79 | 0.04 | 0.79 | 0.14 | 0.40 |
| Mean | -0.18 | 0.25 | 0.02 | 0.92 | -0.02 | 0.91 | 0.08 | 0.63 |
| MET | ||||||||
| Walking | -0.25 | 0.12 | -0.38 | 0.01a | -0.25 | 0.11 | -0.39 | 0.01a |
| Moderate | -0.21 | 0.19 | 0.07 | 0.66 | -0.15 | 0.36 | 0.09 | 0.59 |
| Severe | -0.12 | 0.45 | -0.12 | 0.46 | -0.02 | 0.92 | -0.12 | 0.47 |
| Total | -0.44 | 0.00 | -0.33 | 0.04a | -0.39 | 0.01a | -0.34 | 0.03a |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download