Abstract
In an 8-year period at two medical center, 138 patients underwent uterine artery embolization, and 11 of them were diagnosed with uterine necrosis. Among them, three were successfully conceived. However, one of them developed an arteriovenous malformation after an artificial abortion, and another experienced complications, including placenta previa and placenta accreta spectrum, which resulted in early preterm delivery and recurrent postpartum hemorrhage, necessitating subtotal hysterectomy. Therefore, it is crucial to prepare for potential adverse pregnancy outcomes in subsequent pregnancies for patients with a history of uterine necrosis.
Postpartum hemorrhage (PPH) is the leading cause of increased maternal morbidity and mortality. The primary treatment is a conservative approach, including bimanual uterine compression, uterine massage, uterine packing, intrauterine balloon tamponade, fluid resuscitation, transfusion of packed red blood cells, and administration of uterotonic agents [1,2]. Other surgical treatments include uterine compression sutures and uterine artery ligation, with hysterectomy being the definitive treatment [2].
In recent years, uterine artery embolization (UAE) has been implemented as a conservative treatment for patients with hemodynamically stable PPH who wish to preserve their uterus and maintain fertility. Complications of UAE include uterine necrosis, placenta accreta spectrum (PAS), recurrent PPH, infection, and amenorrhea [3–5]. Currently, there are no definitive diagnostic standard guidelines for uterine necrosis; however, it is suspected when patients present with fever, lower abdominal pain, and foul-odorous vaginal discharge. Uterine necrosis is a serious complication of UAE in the treatment of PPH. Therefore, we aimed to identify patients with uterine necrosis who underwent UAE for PPH and investigate subsequent pregnancy complications. We identified 11 patients who were diagnosed with uterine necrosis after UAE, three of whom were confirmed to have spontaneous pregnancies and reported the outcome of their pregnancy.
This retrospective observational study used the medical records of two medical centers from January 2014 to December 2021. We studied 138 patients who underwent UAE for PPH; among them, 11 were diagnosed with uterine necrosis. The incidence of uterine necrosis after UAE at the two medical centers was 7.97%. We investigated subsequent three pregnancies in the 11 patients with confirmed uterine necrosis. Symptoms of uterine necrosis include vaginal discharge, abdominal pain, fever, and amenorrhea. The average period from UAE to uterine necrosis was 87.91±87.66 days (range, 8–280) and the majority of uterine necrosis was confirmed through enhanced computed tomography (CT) of the abdomen and pelvis with uterine wall disruption and intrauterine free bubbles. Spontaneous pregnancy was confirmed 54, 42, and 6 months after UAE. In the third case, the gestational sac was confirmed using ultrasound at 5 weeks of gestation, but was lost to follow-up.
The first patient was a 35-year-old multiparous woman who was transferred to our hospital because of PPH after a normal vaginal delivery at a gestational age of 39 weeks. Upon arrival, active vaginal bleeding and uterine atony were identified on enhanced pelvic CT (Fig. 1A, B), and emergency UAE was performed using Gelfoam (Next Biomedical, Incheon, Korea). Fifty days after UAE, the patient visited the emergency department because of vaginal bleeding. Remnant placental expulsion was observed on vaginal dressing. Pathological examination of the remnant placenta revealed degenerative and necrotic chorionic villi and decidua, with neutrophils and focal calcification. The menstrual cycle recovered 20 months after UAE; however, the recovery was very short. Approximately 4.5 years after the implementation of the UAE, the fetal heartbeat was confirmed at 8 weeks of gestation; however, artificial abortion was performed for personal reasons. Subsequently, abnormal vaginal bleeding was observed, and the patient was diagnosed with a uterine arteriovenous malformation (AVM) on ultrasonography and CT (Fig. 1C–E) of the abdomen and pelvis. The patient did not undergo any treatment for the AVM and is currently under follow-up without other interventions.
The second patient was a 30-year-old primiparous woman referred for uterine atony and vaginal bleeding after a cesarean section at 37 weeks and 5 days of gestation. UAE using Gelfoam (Next Biomedical) was performed 240 minutes after cesarean section. Sixty days later, foul-odor vaginal discharge and necrotic tissue were observed, with necrotic tissue showing multiple abscess formations. The pathology report of the necrotic tissue revealed a totally necrotic tissue with multiple abscesses.
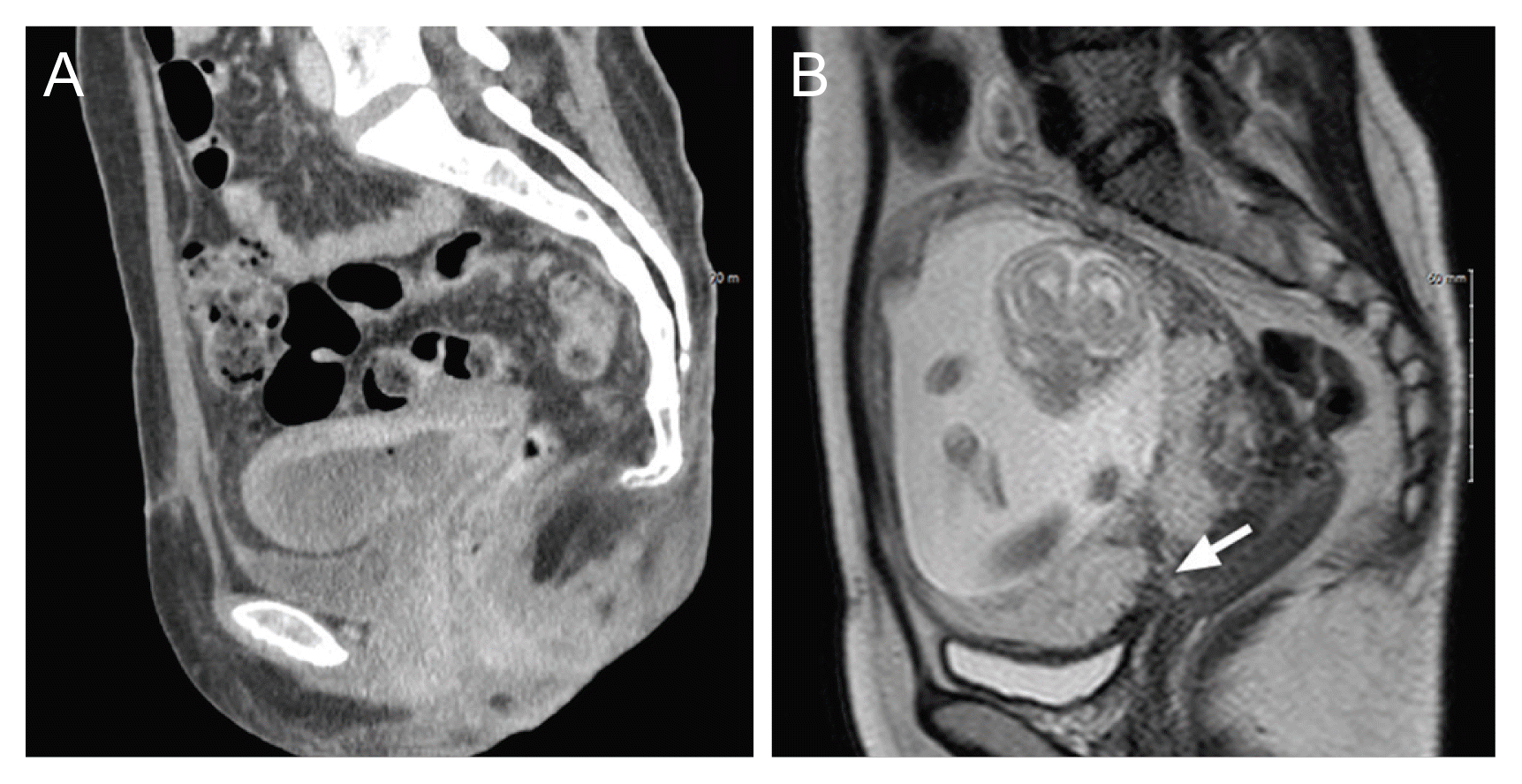
Enhanced abdominal pelvic CT revealed partial uterine necrosis in the anterior and fundal walls (Fig. 2A). After 3.5 years, she visited the hospital to confirm her pregnancy. At the gestational age of 17 weeks, non-enhanced magnetic resonance imaging was performed, and placenta previa and PAS were identified (Fig. 2B). At 29 weeks of gestation, emergency cesarean section was performed because of recurrent fetal distress and oligohydramnios. Placental increta were observed at the uterine necrosis site, and placenta percreta was confirmed as a bladder component in approximately 5% of the placenta. Cesarean subtotal hysterectomy was performed because of the presence of uterine atony, and the estimated blood loss was 7,000 mL. Gross findings of the uterus showed placental invasion with a disrupted uterine serosa. Microscopy revealed extensive infiltration of the myometrium by trophoblasts and loss of the decidual layer. No evidence of uterine necrosis was found upon histopathological examination.
PPH is a life-threatening condition and a common cause of maternal morbidity and mortality. The success rate of UAE for PPH ranges from 61% to 100% [6–9]. UAE is applicable to patients who desire future pregnancy and maintain a hemodynamically stable state [2].
A systematic review studied short- and long-term adverse outcomes after UAE and concluded that UAE does not appear to adversely affect the menstrual cycle, fertility, and subsequent pregnancies but may affect placentation [5]. PAS was significantly more common in the post-UAE group, suggesting that uterine necrosis occurred due to damage to the myometrium and endometrium, followed by decreased uterine blood flow after UAE [10–12].
The diagnostic criteria for uterine necrosis were the presence of myometrial gas, absence of myometrial uptake following contrast material injection, peripheral uptake, and uterine elongation [13]. The surgical specimen obtained after hysterectomy showed two well-differentiated myometrial areas: a brownish color change in the uterine wall and detached mucosa, and granulation tissue. Microscopic examination revealed ischemic necrosis of myometrial fibers and vascular structures [13].
One study identified 17 patients with UAE followed by PPH, of which four completed subsequent deliveries, and all of them experienced recurrent PPH [6]. Another study found that PAS was significantly more common in the post-UAE group and suggested that uterine necrosis occurred because of damage to the myometrium and endometrium, followed by decreased uterine blood flow after UAE [10]. In this study, compared to our previous study, the incidence of uterine necrosis after UAE were 7.97% and 9.4%, respectively [1]. This may be because the procedure was performed carefully considering the risk of uterine necrosis.
Uterine necrosis following UAE for PPH was divided into total and partial categories [13]. Partial uterine necrosis showed zonal ischemic myometrium, endometrial atrophy, and intrauterine adhesions; thus, the uterus was preserved with conservative treatment [13]. Conservative management may be successful; however, surgical intervention is required in some patients. The cause of uterine necrosis after UAE remains unclear.
In this study, there were subsequent spontaneous pregnancies in three of the 11 cases (27.3%). Unfortunately, two of these cases were lost to follow-up in early pregnancy, whereas the other case clearly showed PAS combined with placenta previa. When uterine necrosis occurs, necrotic tissue is absorbed or expelled and defective deformations may appear in the uterus. Uterine defects are thought to increase the risk of pregnancy-associated complications in subsequent pregnancies.
The major strength of our study is that it is the first to investigate the period from uterine necrosis to future pregnancy and subsequent pregnancy outcomes. We can inform these patients about the increased risk of PAS in the next pregnancy after uterine necrosis and the need to be prepared for postpartum bleeding.
Our study has several limitations. First, this was a retrospective study with a small sample size. However, uterine necrosis is rare, and there have been no reports of subsequent pregnancies. A more robust study such as a large-scale prospective review is required to further investigate UAE in future pregnancies.
Uterine necrosis after UAE is a rare complication, and standard treatment and prognosis have not yet been established. If uterine necrosis occurs after UAE in PPH, it may cause serious pregnancy outcome complications; therefore, more intensive management and preparation for delivery and caution regarding the risk of the next pregnancy are required.
Notes
References
1. Kim MJ, Kim IJ, Kim S, Park IY. Postpartum hemorrhage with uterine artery embolization: the risk of complications of uterine artery embolization. Minim Invasive Ther Allied Technol. 2022; 31:276–83.
2. Brown M, Hong M Jr, Lindquist J. Uterine artery embolization for primary postpartum hemorrhage. Tech Vasc Interv Radiol. 2021; 24:100727.

3. Inoue S, Masuyama H, Hiramatsu Y. Efficacy of transarterial embolisation in the management of post-partum haemorrhage and its impact on subsequent pregnancies. Aust N Z J Obstet Gynaecol. 2014; 54:541–5.
4. Poggi SH, Yaeger A, Wahdan Y, Ghidini A. Outcome of pregnancies after pelvic artery embolization for postpartum hemorrhage: retrospective cohort study. Am J Obstet Gynecol. 2015; 213:576.e1–5.
5. Matsuzaki S, Lee M, Nagase Y, Jitsumori M, Matsuzaki S, Maeda M, et al. A systematic review and meta-analysis of obstetric and maternal outcomes after prior uterine artery embolization. Sci Rep. 2021; 11:16914.
6. Salomon LJ, deTayrac R, Castaigne-Meary V, Audibert F, Musset D, Ciorascu R, et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe post-partum haemorrhage. A cohort study. Hum Reprod. 2003; 18:849–52.
7. Boulleret C, Chahid T, Gallot D, Mofid R, Tran Hai D, Ravel A, et al. Hypogastric arterial selective and superselective embolization for severe postpartum hemorrhage: a retrospective review of 36 cases. Cardiovasc Intervent Radiol. 2004; 27:344–8.
8. Cheong JY, Kong TW, Son JH, Won JH, Yang JI, Kim HS. Outcome of pelvic arterial embolization for postpartum hemorrhage: a retrospective review of 117 cases. Obstet Gynecol Sci. 2014; 57:17–27.
9. Holub Z, Mara M, Kuzel D, Jabor A, Maskova J, Eim J. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008; 90:1886–91.
10. Jitsumori M, Matsuzaki S, Endo M, Hara T, Tomimatsu T, Matsuzaki S, et al. Obstetric outcomes of pregnancy after uterine artery embolization. Int J Womens Health. 2020; 12:151–8.
11. Piñas Carrillo A, Chandraharan E. Placenta accreta spectrum: risk factors, diagnosis and management with special reference to the triple P procedure. Womens Health (Lond). 2019; 15:1745506519878081.
12. Lee JY, Hwang JY, Lee HA, Lee DH, Lee SK. Uterine necrosis after partial obstruction of the uterine artery via selective embolization in postpartum hemorrhage: a case report. Korean J Obstet Gynecol. 2009; 52:576–80.
13. Poujade O, Ceccaldi PF, Davitian C, Amate P, Chatel P, Khater C, et al. Uterine necrosis following pelvic arterial embolization for post-partum hemorrhage: review of the literature. Eur J Obstet Gynecol Reprod Biol. 2013; 170:309–14.
Fig. 1
Abdomen pelvis CT and transvaginal sonography in case 1. (A) Axial plane and (B) coronal plane of CT before UAE. The leakage of the contrast medium and hematoma are visible (white arrow). (C) After dilatation and curettage at 8 weeks gestation, the patient was referred again for amenorrhea. We performed enhanced abdomen-pelvis CT and AVM was diagnosed. (D) Sagittal plane of transvaginal sonography. Dilated vessels is observed. (E) When color Doppler was applied, massive blood flow in uterine body and fundus is observed. CT, computed tomography; UAE, uterine artery embolization; AVM, arteriovenous malformation.
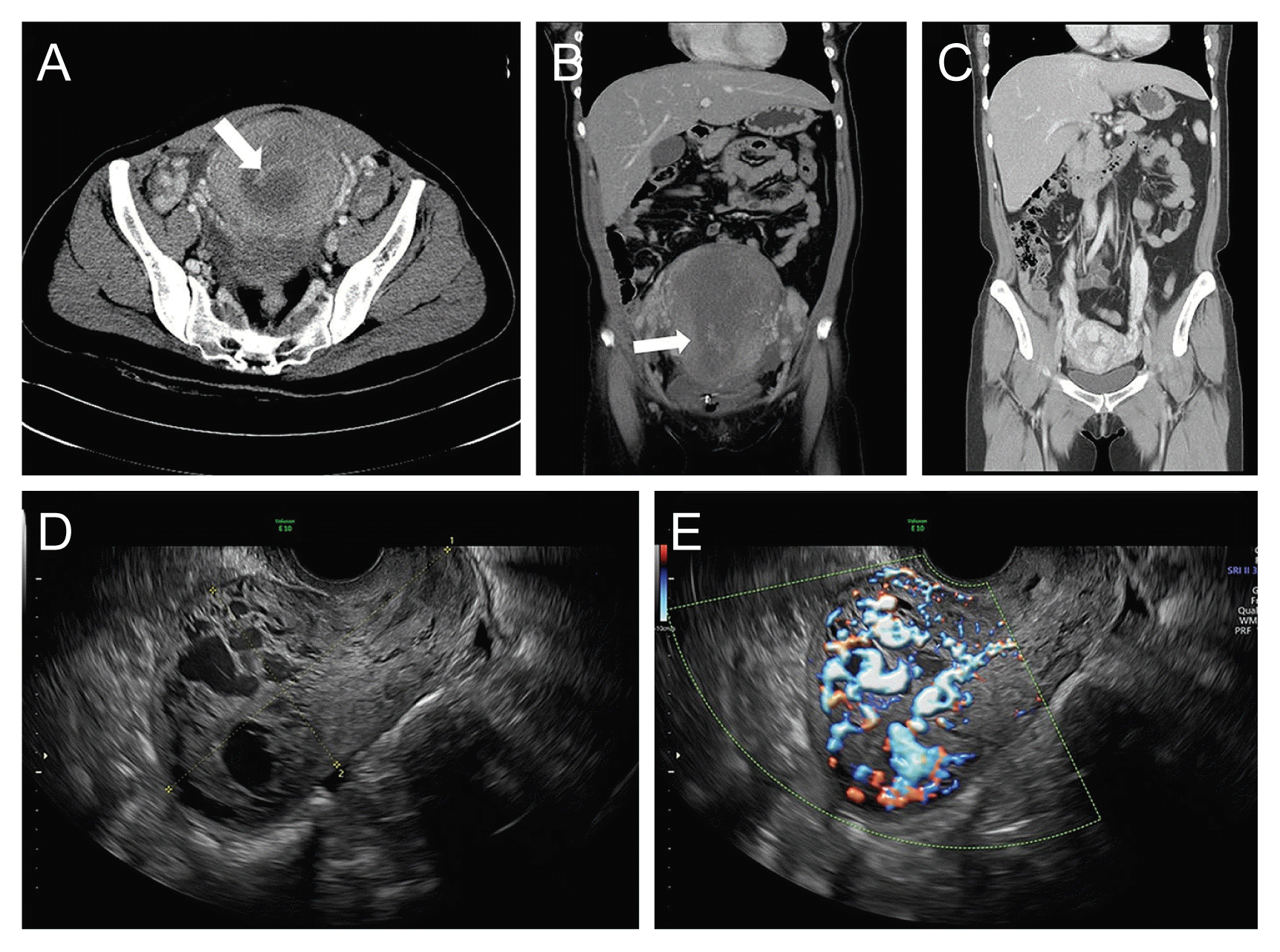
Fig. 2
Enhanced abdomen-pelvis CT at the time of uterine necrosis and non-enhanced uterus MRI at 17 weeks gestation age of case 2. (A) Axial plane of enhanced abdomen-pelvis CT. Partial uterine necrosis in the anterior and fundal wall are identified. Free air is observed in the intrauterine cavity. (B) The axial plane of non-enhanced uterus MRI. Placenta previa (white arrow) is identified. PAS was not observed, but the portion of placenta increta and percreta at the time during cesarean section on gross was indentified. CT, computed tomography; MRI, magnetic resonance imaging; PAS, placenta accreta spectrum.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download