Abstract
Cervical cancer continues to pose a challenge to the health of Thai women, as the second most common cancer after breast cancer. Since high-risk human papillomavirus (HPV) types are the main cause for cervical cancer, cervical cancer screening and HPV vaccination are necessary to reduce the incidence of this disease. At present, the World Health Organization hopes to reduce the incidence of cervical cancer to 4 or less cases per 100,000 women-years using 90%-70%-90% intervention by 2030. The first intervention involves vaccinating 90% of women aged 15 years with the HPV vaccine. The second intervention involves screening 70% of women between the ages of 35 and 45 years using a high-performance screening test. The third intervention involves detecting cervical lesions in 90% of affected women to enable diagnosis and treatment. In this context, this study reviews trends in the incidence and mortality rates of cervical cancer in Thailand, in addition to providing an up-to-date overview of the causes and necessary risk factors for cervical cancer, as well as reporting on cervical screening and HPV vaccination rates and cervical cancer during the coronavirus disease 2019 (COVID-19) pandemic. This study may prove useful for the formulation of policy aimed at eliminating cervical cancer in Thailand, such as the implementation of a free HPV vaccine service and providing at-home kits for cervical screening through clinics and pharmacies. In addition, this review also highlights the need for further research on the effects of the COVID-19 pandemic on cervical cancer screening rates in Thailand.
Globally, cervical cancer is the fourth most common cancer in women [1]. In 2020, The Global Cancer Observatory estimated that there were 604,127 new cases and 341,831 deaths due to cervical cancer, with an age-standardized incidence rate (ASR) of 13.3 cases per 100,000 women per year and a mortality rate of 7.2 cases per 100,000 women per year. The highest incidence and mortality rates of all cases were found in Asia (more than 58%), whereas the highest age-standardized incidence and mortality rates were observed in Africa (40 cases and 28.6 deaths per 100,000 women per year, respectively) [2].
In Thailand, cervical cancer remains the second most common cancer after breast cancer. There were an estimated 13.8% new cervical cancer cases in 2021 [3]. The world ASR was 11.1 cases per 100,000 women per year, whereas the mortality rate was 6.8 deaths per 100,000 women per year. The highest incidence rate of cervical cancer was observed in Central and East of Thailand with an ASR of 14.4 cases per 100,000 women per year, followed by North (ASR=13.4), North-East (ASR=9.5), and South (ASR=8.4) [3].
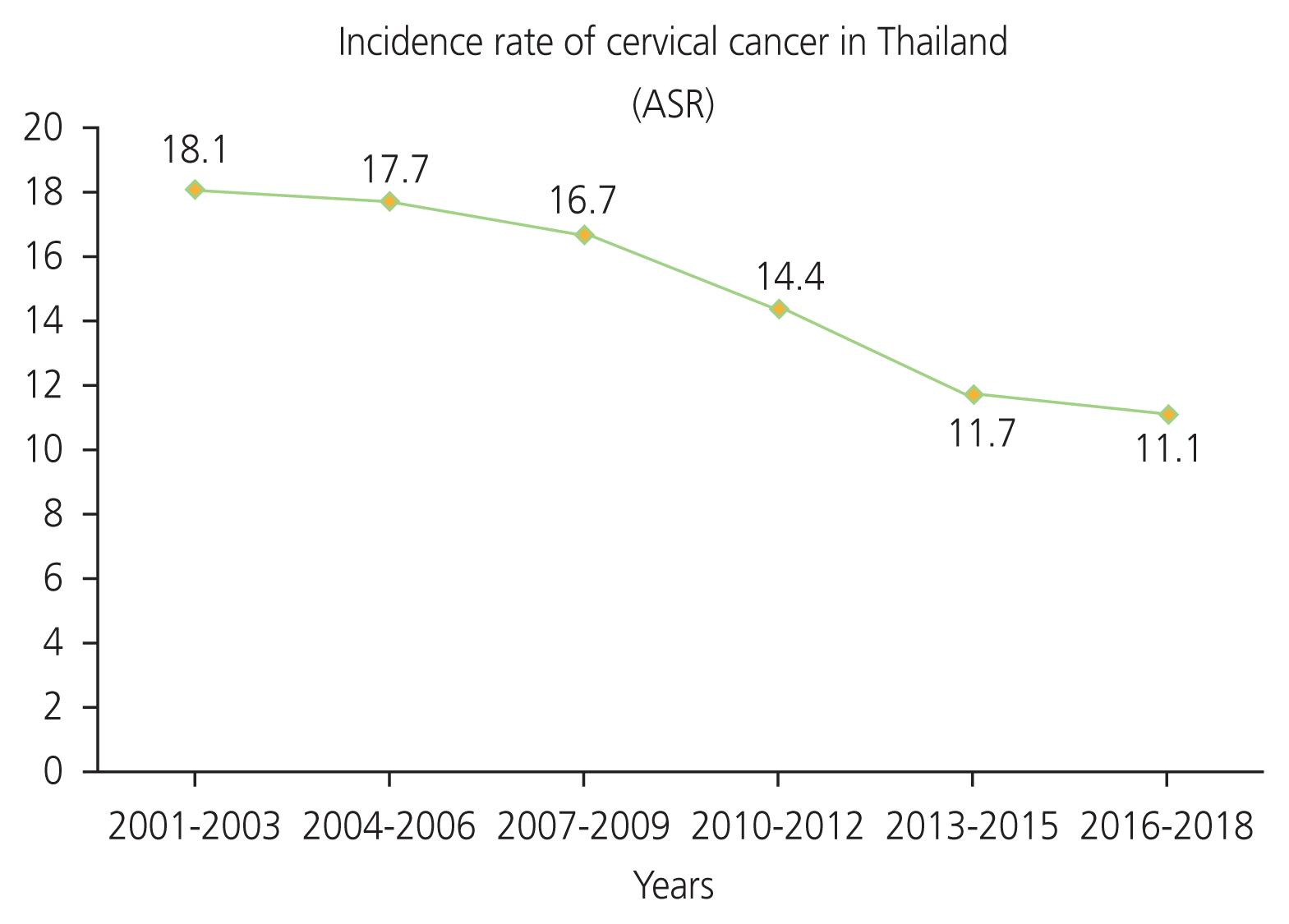
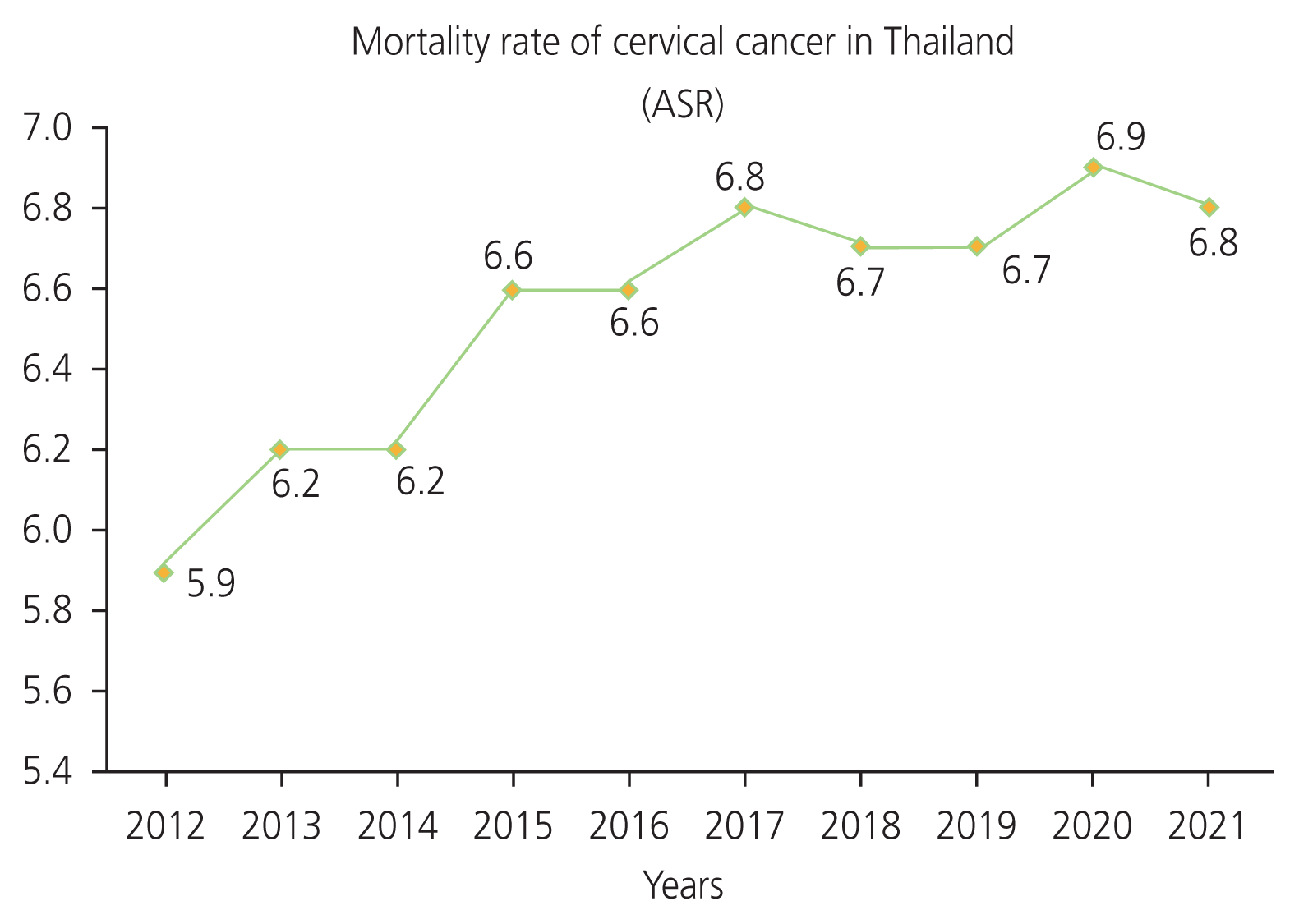
This study used the data from database of National Cancer Institute and Ministry of Public Health of Thailand. Ten years worth of retrospective data of age-standardized incidence and mortality rate (ASRs) of cervical cancer per 100,000 women per year in Thailand was collected. An analysis of trends in the estimated incidence and mortality rates of cervical cancer was conducted in Microsoft Office Professional Plus 2019 (Microsoft Corporation, Redmond, WA, USA). The results showed that the estimated incidence rate of cervical cancer decreased in Thailand. The mean annual ASR per 100,000 women of 2001 to 2003 was 18.1, whereas the latest mean annual ASR from 2016 to 2018 was 11.1 (Fig. 1) [4–9]. In contrast, the estimated mortality rate of cervical cancer showed an increasing trend. The mean ASR increased from 5.6 deaths per 100,000 women in 2012 to 6.8 deaths per 100,000 women in 2021 (Fig. 2) [10–14].
It has been known for over two decades that human papillomavirus (HPV) infection is a major cause of cervical cancer in Thailand, accounting for 86–95% of cervical cancer cases [15–17], as is the case elsewhere in the world [18]. The most common HPV types are 16 and 18, followed by other subtypes, such as 58, 52, 45, 33, 35, 59, 31, and 39 [15,19]. Although HPV infection is the main cause for cervical cancer, it can be naturally cleared by the host immune system after infection and may take a long period of time (around 10–20 years) to develop into cervical cancer. As a result, only persistent high risk HPV infection can lead to cervical cancer [20–24]. The main mechanism of cervical carcinogenesis commences with high-risk HPV infected cervical epithelium through micro-abrasions. Then, the capsid protein L1 of HPV interacts with tissue-specific heparin sulfate proteoglycan receptor and enter the host’s cells by clathrin- or caveolamediated endocytosis [25,26]. After access to epithelial cells, the HPV genome undergoes replication and integration into the host’s genome to produce viral E6 and E7 protein, which play important roles in cervical carcinogenesis by inducing genome instability and DNA damage. Briefly, E6 and E7 interfere with tumor suppressor 53 (P53) gene and retinoblastoma gene, respectively leading to the prevention of the DNA repair and apoptosis of infected cells, as well as the promotion of cell proliferation, resulting in aberrant proliferation and malignant progression [26].
The transformation zone of the cervix uteri is a common target area for HPV infection. Normally, the cervix uteri can be divided into three zones, including endocervix, transformation zone, and ectocervix. The endocervix is located in the inner part of the cervix, consists of columnar epithelium, and has a functional role in producing mucus. The transformation zone is “immature metaplastic squamous epithelium”. Lastly, the ectocervix is located in the outer part of the cervix and contains squamous epithelium [27]. Around the 20-24-weeks fetal stage, a border between endo- and ectocervix, called the squamocolumnar junction, develops. It then undergoes metaplasia throughout a female’s life, particularly during puberty, pregnancy, and menopause [28]. Hence, this transformation zone has the highest susceptibility to HPV carcinogenesis because it has CK17/p63-expressing subcolumnar reserve cells that undergo proliferation [29,30].
Although HPV is a necessary factor of cervical cancer, not all HPV-infected patients develop cervical cancer. Previous studies have reported that about 4.7–14% of cervical cancer patients exhibited as HPV negative [15–17]. Several studies have evaluated possible risk factors for developing cervical cancer, with factors associated with an am increased risk of cervical cancer in Thailand including age at first sexual intercourse (≤16 years), interval between menarche and first sexual intercourse (≤6 years), multiple sexual partners (≥1), multiple parities (≥3), age at first delivery (≤18 years), multiple pregnancies (≥3), prolonged used of oral contraceptives, and exposure to cigarette smoke [17,31,32].
In addition, genetic factors were also observed to increase the risk of cervical cancer in Thailand. Phuthong et al. [33] reported that carriers of the mutant TT genotype of Fok1 and the mutant TC or CC genotype of Taq1 of vitamin D receptor (VDR) were associated with a 2-fold increased risk of cervical cancer when compared with the wild-type CC and TT genotypes, respectively. Similar to a Chinese study, they found that the mutant genotype of Fok1 was associated with an increased risk for CIN2+ (CIN2, CIN3, and cervical cancer) [34]. However, studies regarding the association between Taq1 polymorphism and cervical cancer risk are currently lacking. Wongpratate et al. [35] found that the heterozygous CA genotype of cytochrome P450 1A1 (CYP1A1)-m4 may be a risk factor for cervical cancer as it has an incidence of 31% among cervical cancer patients while being absent among healthy controls. At present, no studies regarding a potential association between CYP1A1-m4 polymorphism and cervical cancer risk have been found. Nevertheless, other subtypes of CYP1A1 polymorphisms (i.e. CYP1A1-m1 and CYP1A1-m2) showed a significantly increased risk of cervical cancer among Asians and Caucasians, suggesting that CYP1A1 polymorphism may be implicated in cervical cancer susceptibility [36,37]. Moreover, polymorphism in the glutathione S-transferase (GST) gene was reported, with the null genotype of GSTT1 resulting in a 3- and 4-fold increased risk of cervical cancer among cervical cancer patients who had a smoking partner and who used oral contraceptives, respectively. Furthemore, the heterozygous and null genotypes of GSTM1 were associated with a 2.6- and 1.7-fold increased risk of cervical cancer when compared with the respective wild-type (present) genotype [38]. Similarly, a meta-analysis reported that the null genotype of GSTM1 was associated with an increased risk of cervical cancer, particularly in the Indian and Chinese populations [39]. Moreover, the null genotypes of GSTT1 and GSTM1 were associated with a 10 -and 7-fold increased risk of cervical cancer among passive smokers in Indian individuals [40]. In addition, MDR1 haplotype was associated with an increased risk of cervical cancer in Thailand [41]. However, no study regarding a potential association between MDR1 polymorphism and the risk of cervical cancer has been reported. These data indicate that these risk factors may be implicated in cervical cancer development in Thai women.
Cervical cancer screening programs continue to be the best way to reduce the incidence rate of cervical cancer. Previous studies have reported a high screening rate in Thailand, with an overall screening coverage of over 70%, including 10,762,081 target women in 2005–2014. Screening methods include a Papanicolaou smear for women aged 30–60 years and visual inspection with acetic acid for women aged 35–45 years [42]. Another option is the use of a self-swab kit for HPV testing, which can help women who are afraid of or embarrassed about cervical screening [43].
Moreover, another way to prevent cervical cancer is vaccination for HPV. The HPV vaccine has been found to be highly cost-effective in reducing the rates of cervical cancer [44]. However, some regions of Thailand have very low vaccination rates, accounting for 1.2–3.3% of participants [45–47]. Nevertheless, the HPV vaccine has been well accepted among participants in Phra Nakhon Si Ayutthaya province (91%) when administering the vaccine in grade five students to assess HPV vaccine acceptability [48]. Moreover, Ong et al. [49] reported that HPV vaccination coverage (at least one dose) was 80% of the target aged group (11–12 years). Studies on the intention to get HPV vaccine found that there was high rates of intention among Thai women (30–84%) [47,50,51]. Nonetheless, reasons for not being vaccinated including the cost of the vaccine, poor knowledge regarding HPV infection and cervical cancer, the perceived efficacy and safety of vaccine, as well as perception of low-risk behaviors [46,47,50,51]. Therefore, cultivating positive attitudes to HPV vaccination and vaccine safety among individuals by imparting knowledge and increasing awareness about HPV infection and cervical cancer should take into consideration. Furthermore, providing HPV vaccines free of charge for the target age group should be considered as part of national school health program and/or a public health strategy in Thailand [49,52,53].
The COVID-19 pandemic was triggered by the global spread of the COVID-19 virus, which itself causes respiratory illness. At the time of the pandemic, global measures adopted to manage this disease included social distancing and self-isolation to prevent disease transmission. As a result, COVID-19 had an effects on day-to-day life, work, and businesses, as well as the health care services [54].
In this regard, cervical screening, an important health care service for cervical cancer prevention, was also affected, decreasing during the pandemic. Recently, one study reported that an estimated 1,149,727 pap smears were missed during COVID-19 pandemic in Brazil [55]. Similar findings were reported in England, with the number of samples in 2020 being 43–91% lower than before (2018) the COVID-19 pandemic [56]. Furthemore, a decrease of 11% in cervical cancer screening in 2020 was reported in the United States when compared with before (2018) COVID-19 [57]. In Thailand, no studies were found regarding trends in cervical cancer screening during the COVID-19 pandemic [58]. In fact, only one study reported on the self-collection of specimens for cervical cancer screening to increase cervical screening rate during the recovery phase of COVID-19 pandemic [59].
At present, the WHO [60] plans to reduce the incidence of cervical cancer to 4 or fewer cases per 100,000 women per year by 2030 using the so-called 90%-70%-90% intervention. The first intervention involves vaccinating 90% of women at the age of 15 years with the HPV vaccine. The second intervention involves screening 70% of women between the ages of 35 and 45 years for cervical cancer using a high-performance screening test. The third intervention involves the detection of cervical lesions in 90% of affected women to ensure early diagnosis and treatment. In Thailand, a high rate of cervical screening (over 70%) was found to be effective in reducing the overall incidence of cervical cancer [42,61]. However, with regards to the uptake of the HPV vaccine among Thai women, rates continue to be very low in some areas [45–47]. Despite this, at the time of writing, HPV vaccination coverage was 80% among the target group of young women aged 11–12 years [49].
Our study aims to provide an up to date review on the trends in the incidence and mortality rates of cervical cancer in Thailand, in addition to updating the causes and necessary risk factors for cervical cancer, as well as reporting on cervical screening and HPV vaccination rates and on cervical cancer during the COVID-19 pandemic in Thailand.
As a result, we found that cervical cancer remains the second most common cancer in Thailand [3]. Analyzing estimated incidence and mortality rates in past 10 years, the data showed that the incidence rate has decreased, whereas the mortality rate is increasing [4–9,10–14]. According to the WHO strategy, cervical cancer screening and HPV vaccination have been crucial in reducing the incidence and mortality rates of cervical cancer [60]. In Thailand, we found high rates of cervical screening among women. Cervical screening coverage among Thai women aged 30–60 years was found to account for 67% in the past 5 years and 77% historically. When compared with other Asian National Cancer Centers Alliance member countries, 16 out of 21 countries had a screening coverage below 50%, including Bangladesh, Brunei, Cambodia, Chaina, India, Indonesia, Iran, Laos, Malasia, Mongolia, Myanmar, Nepal, Pakistan, Philippines, Sri Lanka, and Vietnam, with only five out of 21 countries having a screening coverage above 50% (i.e., Bhutan, Japan, Sout Korea, Singapore, and Thailand) [49]. Thus, a decline in the incidence rate of cervical cancer in Thailand may be due to a high rate of cervical screening, which conforms with the strategy implemented by WHO. The HPV vaccine was included in the national immunization program in Thailand for girls aged 11 and 12 years in 2017 [62]. There was also a high HPV vaccination coverage, accounting for 80% of the target aged group. Furthermore, more recently, the Ministry of Public Health of Thailand launched a comprehensive cancer policy “Quick Win” in November 2023. The goal of this policy is to vaccinate 1 million doses of HPV in 100 days among girls aged 11–20 years to reduce incidence and mortality rates of cervical cancer [63]. This initiative is indicative of proactive public management and implies ongoing efforts aimed at the management of cervical cancer in Thailand. In addition, both high cervical cancer screening rates and the ongoing management of HPV vaccination in Thailand are indicative of strong preventive initiatives to eliminate cervical cancer. However, despite these efforts, the mortality rate of cervical cancer continues to increase [10–14]. Previous studies in tertiary hospitals in Northeast Thailand and Bangkok found that stages III and IV (advance stage) of the disease had the highest incidence rates of all the cervical cancer stages. Data regarding the survival rates of cervical cancer have reported that stage I has a 10-year survival rate of approximately 88%, whereas the 5-year survival rate of stage III and IV is below 50%. Moreover, older age is associated with a decrease in the survival rate in Thailand; patients aged 60 or older had a 5-year survival rate below 40%, which was similar to in Korea [64–66]. These findings indicate that cervical cancer screening may help to reduce the incidence of new cervical cancer cases, leading to a reduction in the overall incidence rate of cervical cancer [42,61,67–69]. However, at present, the majority of patients are diagnosed in the advance stage of the disease, leading to a decreased survival rate. This suggests that the mortality rate may remain high in Thailand. For these reasons, proactive cervical cancer screening followed by the excision of pre-cancerous lesions or the treatment for cervical cancer at an early stage may help to reduce the mortality rates of cervical cancer in Thailand [70].
In terms of the causes and necessary risk factors for cervical cancer in Thailand, HPV infection, especially type 16 and 18, remains the main cause of cervical cancer among Thai women, as in other countries [15–18]. Moreover, many studies have reported an association between risk factors and an increased risk of cervical cancer in Thai women, including exposure to, certain risk factors and genetic polymorphisms, such as VDR, CYP1A1, MDR, and GST [17,31–41]. Finally, the COVID-19 pandemic resulted in an interruption to cervical cancer screening globally [55–58,68]. However, little has been reported in this regard in Thailand [59].
Although the incidence rate of cervical cancer in Thailand has dropped from an ASR of 18.1 to 11.1 cases per 100,000 women [4–9], it is far from the goal of the recent strategy launched by WHO [60]. The present study suggests that continuous standard screening, proactive services for cervical cancer screening, the provision of HPV vaccines for girls and boys on the large scale, an improvement in knowledge and awareness of cervical cancer and the HPV vaccine, avoiding risk factors, and early treatment for cervical cancer remain key measures necessary for the elimination of cervical cancer in Thailand [18,49,60].
This review provides an overview of the current status of cervical cancer prevention and outcomes for cervical cancer management in Thailand, based on the 90%-70%-90% intervention strategy proposed by WHO. In addition, the review provides an update on progress in HPV vaccination. However, the main focus of this study was cervical cancer prevention. Thus, information regarding cervical cancer treatment is outside of the scope of this work.
In conclusion, this study demonstrates that the incidence rate of cervical cancer in Thailand has decreased, highlighting efforts being made towards the implementation of the WHO’s cervical cancer screening strategy as potential reasons for this. Moreover, HPV vaccination was found to show high rates of vaccine coverage in target age groups, which will help to reduce the incidence rates of cervical cancer in the future. However, despite these promising findings, the mortality rate continues to show an increasing trend. In this respect, early treatment for pre-cancerous or early stage of cervical cancer may help to improve survival rate. In summary, this study may be useful for the formulation of policies aimed at eliminating cervical cancer in Thailand, with initiatives including free HPV vaccine services (recommended for women aged 26 or below) and the provision of at-home kits for cervical cancer screening through clinics and pharmacies. Lastly, this review highlights the need for further research on the effects of COVID-19 pandemic on the rates of cervical cancer screening in Thailand.
References
1. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021; 155(Suppl 1):28–44.

2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023; 11:e197–206.

3. National Cancer Institute. Hospital-based cancer registry 2021 [Internet]. Bangkok (TH): National Cancer Institute;c2022. [cited 2023 Jun 1]. Available from: https://www.nci.go.th/e_book/hosbased_2564/index.html.
4. Ministry of Public Health. Cancer in Thailand Vol. V 2001–2003 [Internet]. Bangkok (TH): National Cancer Institute;c2010. [cited 2023 Mar 2]. Available from: https://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/Book%20Cancer%20In%20Thailand%202010%20for%20Web.pdf.
5. Ministry of Public Health. Cancer in Thailand Vol. VI 2004–2006 [Internet]. Bangkok (BK): National Cancer Institute;c2012. [cited 2023 Mar 2]. Available from: https://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/Cancer%20in%20thailand.pdf.
6. Ministry of Public Health. Cancer in Thailand. Vol VII. 2007–2009 [Internet]. Bangkok (TH): National Cancer Institute;c2013. [cited 2023 Mar 2]. Available from: https://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/Cancer%20in%20thailand_VII.pdf.
7. Ministry of Public Health. Cancer in Thailand Vol. VIII 2010–2012 [Internet]. Bangkok (TH): National Cancer Institute;c2015. [cited 2023 Mar 2]. Available from: https://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/Cancer%20in%20Thailand8.pdf.
8. Ministry of Public Health. Cancer in Thailand Vol IX 2013–2015 [Internet]. Bangkok (TH): National Cancer Institute;c2018. [cited 2023 Mar 2]. Available from: https://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/In%20Cancer%20in%20Thailand%20IX%20OK.pdf.
9. Ministry of Public Health. Cancer in Thailand Vol. X 2016–2018 [Internet]. Bangkok (TH): National Cancer Institute;c2021. [cited 2023 Mar 2]. Available from: https://www.nci.go.th/e_book/cit_x/index.html.
10. Ministry of Public Health. Public health statistics A.D. 2012 [Internet]. Nonthaburi (TH): Ministry of Public Health;c2013. [cited 2023 Apr 25]. Available from: https://spd.moph.go.th/wp-content/uploads/2022/11/Hstatistic55.pdf.
11. Ministry of Public Health. Public health statistics AD. 2015. 1st ed. Nonthaburi: Ministry of Public Health;2016.
12. Ministry of Public Health. Public health statistics A.D. 2019 [Internet]. Nonthaburi (TH): Ministry of Public Health;c2020. [cited 2023 Apr 25]. Available from: https://spd.moph.go.th/wp-content/uploads/2022/08/สถิติสาธรารณสุข.pdf.
13. Ministry of Public Health. Public health statistics A.D. 2020 [Internet]. Nonthaburi (TH): Ministry of Public Health;c2021. [cited 2023 Apr 25]. Available from: https://anyflip.com/tseqz/dxml.
14. Ministry of Public Health. Public health statistics A.D. 2021 [Internet]. Nonthaburi (TH): Ministry of Public Health;c2022. [cited 2023 Apr 25]. Available from: https://spd.moph.go.th/wp-content/uploads/2022/11/Hstatistic64.pdf.
15. Chichareon S, Herrero R, Muñoz N, Bosch FX, Jacobs MV, Deacon J, et al. Risk factors for cervical cancer in Thailand: a case-control study. J Natl Cancer Inst. 1998; 90:50–7.

16. Settheetham-Ishida W, Kanjanavirojkul N, Kularbkaew C, Ishida T. Human papillomavirus genotypes and the p53 codon 72 polymorphism in cervical cancer of Northeastern Thailand. Microbiol Immunol. 2005; 49:417–21.
17. Natphopsuk S, Settheetham-Ishida W, Sinawat S, Pientong C, Yuenyao P, Ishida T. Risk factors for cervical cancer in northeastern Thailand: detailed analyses of sexual and smoking behavior. Asian Pac J Cancer Prev. 2012; 13:5489–95.

18. World Health Organization. Comprehensive cervical cancer control: a guide to essential practice. 2nd ed. Geneva: World Health Organization;2014.
19. HPV Information Centre. Thailand: human papillomavirus and related cancers, fact sheet 2023 [Internet]. Barcelona (ES): HPV Information Centre;c2023. [cited 2023 Oct 2]. Available from: https://hpvcentre.net/statistics/reports/THA_FS.pdf.
20. Frazer I. Correlating immunity with protection for HPV infection. Int J Infect Dis. 2007; 11(Suppl 2):S10–6.

22. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010; 117:S5–10.

23. Panatto D, Amicizia D, Trucchi C, Casabona F, Lai PL, Bonanni P, et al. Sexual behaviour and risk factors for the acquisition of human papillomavirus infections in young people in Italy: suggestions for future vaccination policies. BMC Public Health. 2012; 12:623.

24. Bouvard V, Wentzensen N, Mackie A, Berkhof J, Brotherton J, Giorgi-Rossi P, et al. The IARC perspective on cervical cancer screening. N Engl J Med. 2021; 385:1908–18.

25. Song D, Li H, Li H, Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol Lett. 2015; 10:600–6.

26. Gupta SM, Mania-Pramanik J. Molecular mechanisms in progression of HPV-associated cervical carcinogenesis. J Biomed Sci. 2019; 26:28.
27. Regauer S, Reich O. The origin of human papillomavirus (HPV) - induced cervical squamous cancer. Curr Opin Virol. 2021; 51:111–18.

28. Alzamil L, Nikolakopoulou K, Turco MY. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021; 28:35–51.

29. Deng H, Hillpot E, Mondal S, Khurana KK, Woodworth CD. HPV16-immortalized cells from human transformation zone and endocervix are more dysplastic than ectocervical cells in organotypic culture. Sci Rep. 2018; 8:15402.

30. Doorbar J, Griffin H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019; 7:176–9.

31. Sriamporn S, Parkin DM, Pisani P, Suwanrungruang K, Pengsaa P. Behavioural risk factors for cervical cancer from a prospective study in Khon Kaen, Northeast Thailand. Cancer Detect Prev. 2004; 28:334–9.

32. Ishida WS, Singto Y, Kanjanavirojkul N, Chatchawan U, Yuenyao P, Settheetham D, et al. Co-risk factors for HPV infection in Northeastern Thai women with cervical carcinoma. Asian Pac J Cancer Prev. 2004; 5:383–6.
33. Phuthong S, Settheetham-Ishida W, Natphopsuk S, Ishida T. Genetic polymorphisms of vitamin D receptor gene are associated with cervical cancer risk in Northeastern Thailand. Asian Pac J Cancer Prev. 2020; 21:2935–9.

34. Li D, Liu Y, Kong D, Papukashvili D, Rcheulishvili N, Zhao H, et al. Vitamin D receptor gene polymorphisms and the risk of CIN2+ in Shanxi population. Biomed Res Int. 2022; 2022:6875996.

35. Wongpratate M, Ishida W, Phuthong S, Natphopsuk S, Ishida T. Genetic polymorphisms of the human cytochrome P450 1A1 (CYP1A1) and cervical cancer susceptibility among Northeast Thai women. Asian Pac J Cancer Prev. 2020; 21:243–8.

36. Wu B, Liu K, Huang H, Yuan J, Yuan W, Wang S, et al. MspI and Ile462Val polymorphisms in CYP1A1 and overall cancer risk: a meta-analysis. PLoS One. 2013; 8:e85166.

37. Ding B, Sun W, Han S, Cai Y, Ren M, Shen Y. Cytochrome P450 1A1 gene polymorphisms and cervical cancer risk: a systematic review and meta-analysis. Medicine (Baltimore). 2018; 97:e0210.
38. Settheetham-Ishida W, Wongpratate M, Phuthong S, Natphopsuk S, Ishida T. Genetic polymorphism of glutathione S-transferase and cervical cancer susceptibility in Northeastern Thailand. APJCB. 2020; 5:35–41.

39. Ye J, Mu YY, Wang J, He XF. Individual effects of GSTM1 and GSTT1 polymorphisms on cervical or ovarian cancer risk: an updated meta-analysis. Front Genet. 2023; 13:1074570.
40. Sobti RC, Kaur S, Kaur P, Singh J, Gupta I, Jain V, et al. Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet Cytogenet. 2006; 166:117–23.

41. Phuthong S, Settheetham-Ishida W, Natphopsuk S, Settheetham D, Ishida T. Haplotype analysis of MDR1 and risk for cervical cancer in Northeastern Thailand. Asian Pac J Cancer Prev. 2017; 18:1815–9.
42. Ploysawang P, Rojanamatin J, Prapakorn S, Jamsri P, Pangmuang P, Seeda K, et al. National cervical cancer screening in Thailand. Asian Pac J Cancer Prev. 2021; 22:25–30.

43. Gottschlich A, Nuntadusit T, Zarins KR, Hada M, Chooson N, Bilheem S, et al. Barriers to cervical cancer screening and acceptability of HPV self-testing: a cross-sectional comparison between ethnic groups in Southern Thailand. BMJ Open. 2019; 9:e031957.

44. Termrungruanglert W, Khemapech N, Vasuratna A, Havanond P, Deebukkham P, Kulkarni AS, et al. The epidemiologic and economic impact of a quadrivalent human papillomavirus vaccine in Thailand. PLoS One. 2021; 16:e0245894.

45. Juntasopeepun P, Davidson PM, Suwan N, Phianmongkhol Y, Srisomboon J. Human papillomavirus vaccination intention among young women in Thailand. Asian Pac J Cancer Prev. 2011; 12:3213–9.
46. Ratanasiripong NT, Sri-Umporn S, Kathalae D, Hanklang S, Ratanasiripong P. Human papillomavirus (HPV) vaccination and factors related to intention to obtain the vaccine among young college women in Thailand. J Health Res. 2018; 32:142–51.

47. Chanprasertpinyo W, Rerkswattavorn C. Human papillomavirus (HPV) vaccine status and knowledge of students at a university in rural Thailand. Heliyon. 2020; 6:e04625.
48. Klinsupa W, Pensuk P, Thongluan J, Boonsut S, Tragoolpua R, Yoocharoen P, et al. O16.3 Hpv vaccine introduction in thailand. Sex Transm Infect. 2015; 91:A1–258.
49. Ong SK, Abe SK, Thilagaratnam S, Haruyama R, Pathak R, Jayasekara H, et al. Towards elimination of cervical cancer - human papillomavirus (HPV) vaccination and cervical cancer screening in Asian National Cancer Centers Alliance (ANCCA) member countries. Lancet Reg Health West Pac. 2023; 39:100860.

50. Santhanes D, Yong CP, Yap YY, Saw PS, Chaiyakunapruk N, Khan TM. Factors influencing intention to obtain the HPV vaccine in South East Asian and Western Pacific regions: a systematic review and meta-analysis. Sci Rep. 2018; 8:3640.

51. Kruiroongroj S, Chaikledkaew U, Thavorncharoensap M. Knowledge, acceptance, and willingness to pay for human papilloma virus (HPV) vaccination among female parents in Thailand. Asian Pac J Cancer Prev. 2014; 15:5469–74.

52. Klaipuk P, Ngoenwiwatkul Y, Tantipoj C, Phanuphak N, Khovidhunkit SP. Improved knowledge about HPV in Thai women after educational intervention. JDAT-DFCT. 2019; 69:1–7.
53. Chunuan S, Wiwattanawongsa K, Widayati A. A predictive model of human papillomavirus vaccination intention among young women in Southern Thailand. Pac Rim Int J Nurs Res. 2021; 25:298–311.
54. World Health Organization. Coronavirus disease (COVID-19) [Internet]. Geneva (CH): World Health Organization;c2023. [cited 2023 Apr 21]. Available from: https://www.who.int/news-room/fact-sheets/detail/coronavirusdisease-(covid-19.
55. Duarte MBO, Argenton JLP, Carvalheira JBC. Impact of COVID-19 in cervical and breast cancer screening and systemic treatment in São Paulo, Brazil: an interrupted time series analysis. JCO Glob Oncol. 2022; 8:e2100371.

56. Castanon A, Rebolj M, Pesola F, Pearmain P, Stubbs R. COVID-19 disruption to cervical cancer screening in England. J Med Screen. 2022; 29:203–8.

57. Fedewa SA, Star J, Bandi P, Minihan A, Han X, Yabroff KR, et al. Changes in cancer screening in the US during the COVID-19 pandemic. JAMA Netw Open. 2022; 5:e2215490.

58. Lucas E, Murillo R, Arrossi S, Bárcena M, Chami Y, Nessa A, et al. Quantification of impact of COVID-19 pandemic on cancer screening programmes - a case study from Argentina, Bangladesh, Colombia, Morocco, Sri Lanka, and Thailand. Elife. 2023; 12:e86527.
59. Eamratsameekool W, Phumiressunthon K, Sukprasert L, Pukdeesamai P. Comparison of self-to provider-collected cervical screening with HPV DNA test at roi Et province, Thailand during COVID-19 pandemic. J Med Assoc Thai. 2023; 106:8–13.

60. World Health Organization. Regional implementation framework for elimination of cervical cancer as a public health problem: 2021–2030 [Internet]. New Delhi (IN): World Health Organization;c2021. [cited 2023 Apr 21]. Available from: https://www.who.int/publications/i/item/9789290228875.
61. Sripan P, Chitapanarux I, Fidler-Benaoudia MM, Miranda-Filho A, Bardot A, Pongnikorn D, et al. Impact of universal health care and screening on incidence and survival of Thai women with cervical cancer: a population-based study of the Chiang Mai province. Cancer Epidemiol. 2019; 63:101594.

62. Ngamphaiboon N. PSY7-5 current status of HPV vaccination and HPV-associated head and neck cancer in Thailand. Ann Oncol. 2022; 33:S422.

63. Ministry of Public Health. Policy driving plan, Ministry of Public Health [Internet]. Bangkok (TH): Ministry of Public Health;c2023. [cited 2023 Jan 15]. Available from: https://drive.google.com/file/d/1o1g4T8FWygleLTI8RlLu5vde1hKJzvy-/view.
64. Wannasin R, Likitdee N, Kelly M, Thinkhamrop K. Survival after diagnosis of cervical cancer patients at a tertiary referral hospital in Northeast Thailand. Asian Pac J Cancer Prev. 2023; 24:1759–67.

65. Bangsomboon P, Kittisiam T, Chaowawanit W. Survival rate of cervical cancer patients according to the 2018 FIGO staging system: a tertiary hospital based study, Vajira Hospital, Bangkok. Thai J Obstet Gynaecol. 2022; 30:60–7.
66. Yun BS, Park EH, Ha J, Lee JY, Lee KH, Lee TS, et al. Incidence and survival of gynecologic cancer including cervical, uterine, ovarian, vaginal, vulvar cancer and gestational trophoblastic neoplasia in Korea, 1999–2019: Korea Central Cancer Registry. Obstet Gynecol Sci. 2023; 66:545–61.

67. Landy R, Sasieni PD, Mathews C, Wiggins CL, Robertson M, McDonald YJ, et al. Impact of screening on cervical cancer incidence: a population-based case-control study in the United States. Int J Cancer. 2020; 147:887–96.
68. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea central cancer registry. Obstet Gynecol Sci. 2021; 64:444–53.

69. eClinicalMedicine. Global strategy to eliminate cervical cancer as a public health problem: are we on track? eClinicalMedicine. 2023; 55:101842.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download