Abstract
Objective
This study aimed to determine the clinical advantage of spindle-view intracytoplasmic sperm injection (SVICSI; a novel technology) over conventional intracytoplasmic sperm injection (cICSI) in patients with poor ovarian response (POR) and previous implantation failure.
Methods
The study included 37 patients who underwent SVICSI followed by fresh embryo transfer (FET) at a single fertility clinic from January to December 2022, 58 patients who underwent cICSI followed by FET at the same fertility clinic from January to December 2021 as a control group. All study participants met the Bologna criteria for POR and had at least three or more previous failed embryo transfers.
Results
The number of blastocyst transfers was significantly higher in the SVICSI group than in the cICSI group. A good-quality cleavage embryo rate, blastocyst rate, and good-quality blastocyst rate were also significantly higher in the SVICSI group than in the cICSI group. There were no significant differences in the rates of fertilization, implantation, clinical pregnancy, or clinical abortion between the two groups.
In 1992, the first healthy baby conceived through intracytoplasmic sperm injection (ICSI), a groundbreaking technique where a single sperm is directly injected into an oocyte, was born [1]. Since then, ICSI has become the primary treatment for male factor infertility and is also used in cases of in vitro fertilization (IVF) cycle failure. As of 2012, in the United States, ICSI accounted for 93.3% of all assisted reproductive technology (ART) procedures for male factor infertility and 66.9% of all procedures for nonmale factor infertility [2]. However, despite its widespread use and success, there are still some limitations associated with the conventional ICSI (cICSI) procedure. When the ICSI procedure was initially introduced in clinical practice, a sperm was injected at the 6 o’clock or 12 o’clock position relative to the first polar body (PB) to ensure that the pipette was as far away as possible from the meiotic spindle (MS), which was presumed to be located just beneath the PB [3,4]. However, the MS is not directly visible in living oocytes, and the use of a microinjection pipette during ICSI can cause physical and chemical disruptions that may alter or damage the spindle or other important organelles inside the mature oocyte [5–8]. The correct positioning and preservation of the MS are crucial, as damage to the spindle during ICSI can lead to chromosomal abnormalities during embryo development and increase the risk of aneuploidy and subsequent implantation failure [9].
Spindle-view ICSI (SVICSI) is a recently developed technique to overcome the limitations of cICSI and enable a more accurate and effective ART procedure [10]. A polarized light microscope (PLM) is a new approach that allows for noninvasive visualization of the MS in living oocytes without causing damage [9,11]. It is expected that if the observed spindle position is first referenced using PLM and then precise pipetting is done at the appropriate location, it can prevent spindle damage, ultimately leading to increased rates of fertilization and normal embryonic development.
Recent advances in the dynamic views of living cells using birefringence imaging has also allowed researchers to investigate the relationship between oocyte morphology and clinical outcomes. Studies have reported on the importance of the MS for a successful outcome in ART [9,12–15]. Some studies have even reported better fertilization rates and embryo quality with SVICSI compared to cICSI [6].
The ovarian response to ovarian stimulation plays a crucial role in the success of IVF and is a significant factor influencing prognosis. Patients with poor ovarian response (POR) exhibit an inadequate response to ovarian stimulation, leading to higher risks of cycle cancellation and difficulty in achieving pregnancy through IVF [16]. The European Society of Human Reproduction and Embryology published the Bologna criteria in 2011 to standardize the definition of POR. These criteria include an advanced age, a previous poor response during ART, a low antral follicle count (AFC), and decreased anti-Müllerian hormone (AMH) levels [16]. Various treatment options are available to increase clinical outcomes in patients with POR, but the effectiveness of these treatment options is conflicting among studies. Existing evidence shows that patients with POR consistently show very low pregnancy rates, even when women undergo natural-cycle IVF or ovarian stimulation using widely accepted treatment protocols (e.g., the short agonist protocol). In addition, the introduction of new treatments such as corifollitropin alpha followed by recombinant follicle-stimulating hormone (FSH), have not improved the pregnancy rates [17,18].
Considering the relatively low number of oocytes obtained after ART in patients with POR, treatment options that improve the fertilization rate of retrieved oocytes and the quality of fertilized embryos is important for these patients because it can help improve ART outcomes in pregnancy. SVICSI could be a promising alternative treatment option for these patients. However, given that the SVICSI is a new technology, there is limited research and data on the technology. Moreover, only few studies have examined outcomes beyond fertilization and cleavage stages [5,6], and limited data exists on clinical pregnancy rates and live birth rates, especially in Korea.
Therefore, this study was conducted to compare the clinical outcomes related to ART between patients who underwent SVICSI or cICSI to determine the potential clinical advantage of SVICSI over cICSI in patients with POR with previous implantation failure.
We selected patients with POR who met at least two of the following three criteria [16,17]: 1) advanced maternal age (≥40 years) or any other risk factor for POR; 2) a previous POR (cancelled cycles or ≤3 oocytes retrieved with a conventional protocol); and 3) an abnormal ovarian reserve test (AFC <7 follicles or AMH level <1.1 ng/mL). Two episodes of POR after maximal stimulation are also sufficient to define a patient as poor responder in the absence of advanced maternal age or an abnormal ovarian reserve test. Among patients with POR, those who had at least three episodes of previous failed embryo transfer (implantation failure) were included in this study. This retrospective study was approved by the Institutional Review Board of a fertility hospital (HR-2023-46-02), which waived the obligation to provide informed patient consent for the study.
Participants who underwent SVICSI for the first time and had fresh embryo transfers (FETs) between January and December 2022 were included in the SVICSI group. The cICSI group included patients who met the same criteria and underwent cICSI followed by FETs between January and December 2021. In both the groups, patients who underwent all embryo cryopreservation after oocyte retrieval were excluded from the study. A total of 37 patients in the SVICSI group and 58 patients in the cICSI group were included in the study.
Oocyte retrieval was conducted in two ways: either naturally or following controlled ovarian hyperstimulation. Hyperstimulation was achieved using recombinant FSH such as Gonal-F® (Serono, Istanbul, Turkey), Follitrope® (LG Life Science, Seoul, Korea) or Menotrophin (IVF-M HP®, LG Life Science). The stimulation protocols encompassed either a long protocol involving a gonadotropin-releasing hormone (GnRH) agonist or a short protocol utilizing a GnRH antagonist [19]. When the cohort of ovarian follicles reached maturity, final oocyte maturation was triggered using recombinant human chorionic gonadotropin (Ovidrel®, Merck KGaA, Darmstadt, Germany) or GnRH agonist (Decapeptyl®, Ferring, Malmo, Sweden). In order to increase the fertilization rate of all the retrieved oocytes, only ICSI was used as the IVF method for oocytes collection from all the participants.
Basal serum AMH levels were measured using the AMH Gen II assay (Beckman Coulter Inc., Brea, CA, USA), and basal serum FSH levels were measured using an Elecsys FSH assay (Roche Diagnostics Corp., Indianapolis, IN, USA). In compliance with the Declaration of Helsinki guidelines, blood samples were obtained from all the patients. The serum AMH levels showed intra- and inter-assay coefficients of variation of <5.0%.
In SVICSI, PB-aligned oocytes were transferred into 6 μL of warm ICSI media (MRC#ICSI, MARIA Research Center, Seoul, Korea) during microinjection. A sperm cell was placed in a 4 μL polyvinylpyrrolidone solution (SAGE BioPharma, Trumball, CT, USA) in the center of a glass-bottom dish (Matsunami, Osaka, Japan) immediately before sperm injection; and oocytes were placed under an inverted microscope (IX73, Olympus, Tokyo, Japan) with a heated stage at 37.0°C±0.5°C and observed at 400× magnification. Oocytes were imaged using an Olympus IX73 (Olympus) inverted PLM in combination with a IX73-ICSI components computerized image analysis system. For SVICSI, a sterile, disposable glass-bottom dish during spindle localization and microinjection were required for the IX73-ICSI components were used. For enhanced visualization of the spindle and the PB, the oocytes were rotated by using a holding and injection pipette. Additionally, the maximum portion of the spindle-shaped area within the oocyte was captured. After imaging, ICSI was conducted with the spindle in the 0 o’clock position. Following microinjection and imaging, oocytes from both the groups were transferred to individually equilibrated 1 mL culture media (MRC#ID16, MARIA Research Center) in a sterile plastic dish in an incubator supplied with 5% CO2 at 37°C. If the spindle was not visible in the oocyte, ICSI needle was inserted at the 3 o’clock position with the PB in the 0 o’clock position.
Fig. 1 shows the living human metaphase II oocytes imaged by using the inverted PLM according to the angles of deviation of the MS with regard to the position of the PB. In Fig. 1, the MS was located between 0 o’clock and 6 o’clock relative to the PB in metaphase II oocytes. Fig. 2 illustrates the distribution of MS positions according to angles of deviation with regard to the position of the PB, and it shows that the proportion of MSs located at 0 o’clock was the highest at 35.1% (65/185).
In cICSI, mature oocytes (defined by the presence of the first PB) were injected 4–6 hours after egg retrieval, with the first PB either at the 0 o’clock or 6 o’clock position. At the 3 o’clock position, the ICSI needle was inserted. One spermatozoon was then aspirated into the cytoplasm, drawn back into the pipette, and ultimately expelled back into the oocyte.
The rates of fertilization, cleavage, good-quality cleavage embryos, blastocysts, good-quality blastocysts, implantation, clinical pregnancy, and clinical abortion were compared between the two groups. The fertilization rate was calculated as the percentage transformation of microinjected oocytes into two pronuclei. The cleavage rate was defined as the total number of day 3 embryos divided by the total number of fertilized oocytes. A good-quality cleavage embryo was defined as an embryo with more than five regular blastomeres with less than 10% fragmentation on day 3. A good-quality cleavage embryo rate was defined as the number of good-quality cleavage embryos on day 3 divided by the total number of fertilized oocytes. The blastocyst rate was defined as the number of embryos that reached the blastocyst stage divided by the total number of fertilized oocytes. A good-quality blastocyst was defined as a blastocyst with an expansion grade ≥3 and both inner cell mass and trophectoderm grade A or B on day 5 or 6 according to the Gardner and Schoolcraft grading system [20] grading system. A good-quality blastocyst rate was defined as the number of good-quality blastocysts divided by the total number of fertilized oocytes.
To calculate the implantation rate, the number of gestational sacs confirmed through transvaginal ultrasonography were divided by the number of embryos that were transferred. A clinical pregnancy was considered confirmed when a gestational sac was visible during transvaginal ultrasound examination. The clinical pregnancy rate was calculated by dividing the total number of cases where at least one gestational sac was evident by the overall number of transfer cycles. The clinical abortion rate was determined by dividing the total number of clinical pregnancy losses, including ectopic pregnancies occurring before the 20th week of gestation, by the overall number of clinical pregnancies [19].
Data are expressed as mean±standard deviation. Statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA). An unpaired t-test was used to compare the means of variables between the SVICSI group and the cICSI group, and a chi-square test or Fisher’s exact test was used to compare the frequencies of the categorical variables between the two groups. P-values <0.05 were considered significant for all analyses.
Comparisons of the clinical parameters were compared between the SVICSI group and the cICSI group are shown in Table 1. The percentage of blastocyst transfer was higher in the SVICSI than in the cICSI group (35.1% vs. 8.6%; P=0.001). The number of previous failed embryo transfers was significantly higher in the SVICSI than in the cICSI group (6.89±3.22 vs. 5.33±2.47; P=0.009). The other variables were not significantly different between the two groups.
Comparisons of the laboratory and clinical outcomes are shown in Table 2. The good-quality cleavage embryo rate was significantly higher in the SVICSI than in the cICSI group (68.6% and 54.1%, respectively; P=0.007). The blastocyst rate (36.4% and 16.3%, respectively; P<0.001) and the good-quality blastocyst rate (21.4% and 4.3%, respectively; P<0.001) were also significantly higher in the SVICSI than in the cICSI group. However, there were no significant differences in the fertilization rate, cleavage rate, implantation rate, clinical pregnancy rate, or clinical abortion rate between the two groups. The clinical pregnancy rate seemed to be higher in the SVICSI group than in the cICSI group, but the difference was not statistically significant (18.9% vs. 13.8%, P=0.504).
An increase in maternal age at first pregnancy has led to an age-related decline in fertility, resulting in a higher demand for and frequency of IVF procedures [21]. Therefore, markers that can predict the developmental potential of oocytes has become one of the most extensively researched and crucial aspects in ART [22]. In particular, efforts are made to improve embryo quality and increase pregnancy rates in women of advanced maternal age with POR [23]. The morphology of oocytes is used to assess oocyte quality [8,24]. Oocyte morphology can be evaluated using noninvasive PLM, based on which MS can be classified as normal or abnormal [25]. MS normality is significant for predicting the potential of a specific oocyte to result in a successful pregnancy. Several studies have established that a higher rate of normal MS within the oocytes is associated with better fertilization rates and embryo development [9,26,27]. Although the reasons for limited embryo development in oocytes with abnormal MS are not yet fully understood, Wang et al. [9] argued that one of the contributing factors could be an increased risk of damage to the MS during ICSI due to inaccuracies in predicting spindle positions (with the PB). If MS normality indeed affects the outcomes of IVF procedures, it is crucial to prevent any potential damage to the MS during the procedure. Based on this theory, a novel ICSI technique called SVICSI has emerged. SVICSI aims to minimize the risk of damaging the MS during injection by referencing the observed spindle’s position for precise pipette insertion. The expectation is that by avoiding potential MS damage during pipette insertion, SVICSI could lead to higher fertilization rates and normal embryo development rates.
Our study aimed to compare the outcomes of SVICSI and cICSI in patients with POR with previous implantation failure. The results of our study showed that a good-quality cleavage embryo rate (68.6% vs. 54.1%), the blastocyst rate (36.4% vs. 16.3%), and the good-quality blastocyst rate (21.4% vs. 4.3%) were significantly higher in the SVICSI than in the cICSI group. However, despite significantly better quality of embryos generated during ART in the SVICSI group (compared with the cICSI group), there were no statistically significant differences observed in the implantation and clinical pregnancy rates between the two groups. Considering that better embryo quality and the higher blastocyst rate are expected to lead to higher implantation rates and clinical pregnancy rates in general, these findings are hard to explain. Several possible factors could underlie these findings. First, SVICSI was conducted in a sample size of 37 patients and cICSI in 58 patients, which made it difficult to reach a sufficient sample size to verify our findings. Further, the patients who underwent SVICSI had a significantly higher number of previous failed embryo transfers (with a mean of 6.89±3.22) compared to the patients who underwent cICSI (with a mean of 5.33±2.47 failed embryo transfers). This retrospective study was conducted on patients admitted at a single fertility hospital. At this fertility hospital, the introduction and application of SVICSI was started in 2022. The control cICSI group comprised of patients who underwent the procedure in 2021. Thus, it appears that participants in the SVICSI group may have experienced more implantation failures than those in the cICSI group, which may have negatively affected the implantation and pregnancy outcomes in the SVICSI group. As mentioned above, the application of SVICSI was limited to patients with POR who previously had failed embryo transfers due to the additional time, labor, and cost incurred for performing this procedure in this hospital. Therefore, the control group was naturally selected from among patients with the same criteria who underwent cICSI in 2021 when SVICSI had not yet been introduced.
All the study patients met the Bologna criteria for POR. However, by selecting a different protocol to obtain more oocytes from patients and applying superovulation using a higher or maximum dose of gonadotropins, a relatively large number of eggs were obtained compared to previous attempts (6.81±5.18 in SVICSI group and 6.66±6.08 in cICSI group, respectively).
Asa et al. [6] compared fertilization rates and embryo cleavage rates between a control group and a spindle-aligned group with oocytes randomly assigned to both groups. In this study, the spindles were aligned in the 6 o’clock and 12 o’clock directions in the spindle-aligned group. The results showed that the spindle-aligned group had higher fertilization and embryo development rates than the control group. Another study by Woodward et al. [5] evaluated spindle imaging before ICSI procedures and blindly assessed spindle positions for the impact of the spindle positions on oocyte quality and embryo development, and concluded that the spindle position did not influence oocyte quality or embryo development. The highest rates of successful fertilization and good-quality embryos were observed when the spindles were located at or near the 3, 4, 8, and 9 o’clock positions. Thus, even when the injection was given in close proximity to the MS, it did not affect the fertilization rate or early embryo development. However, the previous studies showed that normal fertilization was more frequently observed in oocytes where MS was present. Studies have focused only on embryo quality and development stages, but not on other clinical outcomes such as implantation rates and clinical pregnancy rates, which are crucial for assessing the ultimate success of IVF procedures.
There is a lack of research comparing the critical clinical ART outcomes such as pregnancy rates and conception rates, between SVICSI and cICSI procedures. Oh et al. [28] reported that patients who underwent SVICSI had significantly higher clinical pregnancy rates than those who underwent cICSI among patients over 40 years old (21.1% vs. 6.7%; P<0.001). These findings are contradictory to our study findings. The fertilization rate in SVICSI and cICSI procedures (66.7% vs. 61.9%) and good-quality embryo rate (59.2% vs. 56.0%) were not significantly higher in their study, which is also contrary to our study findings. One significant point in the study by Oh et al. [28] was that in patients aged over 40 years, the position of the MS was more than 30 degrees different compared to that of the PB. In other words, the MS showed a higher degree of misalignment (≥30°) in patients over 40 years old. This suggests that directly confirming the position of the MS with PLM and conducting SVICSI could be advantageous and SVICSI could become more beneficial with increasing maternal age at first pregnancy. On the other hand, for patients aged under 40 years, there was no difference in the embryo quality, fertilization rate, or pregnancy rate between the two ICSI methods. It is important to note that in the study by Oh et al. [28], unlike our present study, they did not specifically target patients with three or more episodes of failed embryo transfer.
Mahfoudh et al. [29] conducted a study to analyze the clinical pregnancy rate as a function of the pre-ICSI oocyte spindle angle. They stated that embryos resulting from oocytes with pre-ICSI spindle angles between 0° and 29° were associated with better blastocyst rate, pregnancy rate, live birth rate, and miscarriage rate when compared to oocytes that had no visible spindle. That study also showed that MS of oocytes located between 0° and 29°, in relation to the first PB, is a positive predictor for blastocyst development. These findings are partially consistent with that reported by Oh et al. [28] which showed better clinical outcomes when SVICSI was performed in patients aged over 40 years due to a higher degree of MS misalignment (≥30°) in patients aged over 40 years. However, as already mentioned, our study was a retrospective study conducted on patients at a single fertility clinic, and at this institution, embryo transfers were performed without distinguishing each embryo according to the angle difference between PB and MS or based on visibility of MS, so we could not conduct a subgroup analysis for analyzing the impact of these factors on the clinical outcomes.
This study has several strengths. First, although there are numerous studies on the relationship among oocyte morphology, fertilization rates, and embryo quality, there is very little research on the impact of SVICSI (a novel technique based on this theory) on the ultimate ART-related outcomes, such as implantation and pregnancy rates. In this study, we focused not only on the morphology of oocytes but also on the utility of SVICSI technology on the ultimate outcomes of ART, and we compared not only the fertilization rates and embryo quality but also the overall pregnancy rate.
Several limitations of this study need to be acknowledged. First, this was a retrospective study and potential confounding bias could be present. Second, the participants in our study were limited to women who met the criteria for POR and had three or more failed embryo transfers. Therefore, it may be challenging to directly apply our study results to other general patient populations undergoing ART. Third, this study focused on patients with POR, and did not categorize the patients by age or conduct a subgroup analysis. Furthermore, we did not compare the clinical outcomes based on the orientation of the PB and the visibility of the MS in the present study. Several studies have analyzed differences in clinical outcomes depending on the visibility of MS [1,29]. However, a subgroup analysis could not be performed in our patient cohort because transfers were performed without distinguishing embryos based on the visibility of MS. In particular, in cases where MS was not visible in the oocytes, IVF of these oocytes was performed similar to the cICSI method, which could have affected our results.
In conclusion, this study showed that compared to cICSI, the novel SVICSI technology is likely to be related to better embryo quality during ART. However, in our study, no significant differences were found in terms of the fertilization rate, cleavage rate, implantation rate, clinical pregnancy rate and clinical abortion rate between the two procedures. Further research in a larger patient population and for a longer duration is needed to clarify the impact of SVICSI not only on embryo quality and stage but also on implantation and pregnancy rates.
Notes
Ethical approval
This retrospective study was approved by the lnstitutional Review Board of the Maria Fertility Hospital (IRB No. HR-2023- 46-02).
References
1. Matsunaga R, Horiuchi T. ICSI with the assistance of meiotic spindle imaging for the production of high quality embryos. J Mamm Ova Res. 2015; 32:3–10.

2. Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015; 313:255–63.

3. Lanzendorf S, Maloney M, Ackerman S, Acosta A, Hodgen G. Fertilizing potential of acrosome-defective sperm following microsurgical injection into eggs. Gamete Res. 1988; 19:329–37.

4. Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, et al. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993; 8:1061–6.

5. Woodward BJ, Montgomery SJ, Hartshorne GM, Campbell KH, Kennedy R. Spindle position assessment prior to ICSI does not benefit fertilization or early embryo quality. Reprod Biomed Online. 2008; 16:232–8.

6. Asa E, Tabatabaee R, Farrokhi A, Nejatbakhsh R. Relationship between meiotic spindles visualization and intracytoplasmic sperm injection outcomes in human oocytes. Anat Cell Biol. 2017; 50:26–32.

7. Eichenlaub-Ritter U, Shen Y, Tinneberg HR. Manipulation of the oocyte: possible damage to the spindle apparatus. Reprod Biomed Online. 2002; 5:117–24.

8. Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997; 12:1750–5.

9. Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001; 75:348–53.

10. Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001; 16:1464–8.

11. Petersen CG, Oliveira JB, Mauri AL, Massaro FC, Baruffi RL, Pontes A, et al. Relationship between visualization of meiotic spindle in human oocytes and ICSI outcomes: a meta-analysis. Reprod Biomed Online. 2009; 18:235–43.

12. Cooke S, Tyler JP, Driscoll GL. Meiotic spindle location and identification and its effect on embryonic cleavage plane and early development. Hum Reprod. 2003; 18:2397–405.

13. Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003; 18:817–20.

14. Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003; 18:1289–93.

15. Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, et al. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004; 19:649–54.

16. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011; 26:1616–24.

17. Polyzos NP, Blockeel C, Verpoest W, De Vos M, Stoop D, Vloeberghs V, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod. 2012; 27:3481–6.

18. Polyzos NP, DeVos M, Humaidan P, Stoop D, Ortega-Hrepich C, Devroey P, et al. Corifollitropin alfa followed by rFSH in a GnRH antagonist protocol for poor ovarian responder patients: an observational pilot study. Fertil Steril. 2013; 99:422–6.
19. Chun S, Seo JE, Rim YJ, Joo JH, Lee YC, Koo YH. Efficacy of hyaluronan-rich transfer medium on implantation and pregnancy rates in fresh and frozen-thawed blastocyst transfers in Korean women with previous implantation failure. Obstet Gynecol Sci. 2016; 59:201–7.

20. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000; 73:1155–8.

21. Liu C, Li Y, Jiang H, Liu Y, Song X. The clinical outcomes of fresh versus frozen embryos transfer in women ≥40 years with poor ovarian response. Obstet Gynecol Sci. 2021; 64:284–92.

22. Younis JS, Laufer N. Oocyte donation is an independent risk factor for pregnancy complications: the implications for women of advanced age. J Womens Health (Larchmt). 2015; 24:127–30.

23. Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod. 2014; 29:1842–5.

24. Khalili MA, Mojibian M, Sultan AM. Role of oocyte morphology on fertilization and embryo formation in assisted reproductive techniques. Middle East Fertil Soc J. 2005; 10:72–7.
25. Omidi M, Khalili MA, Nahangi H, Ashourzadeh S, Rahimipour M. Does women’s age influence zona pellucida birefringence of metaphase II oocytes in in-vitro maturation program? Iran J Reprod Med. 2013; 11:823–8.
26. Nguyen TT, Doan HT, Quan LH. The spindle of oocytes observed by polarized light microscope can predict embryo quality. Int J Reprod Contracept Obstet Gynecol. 2019; 8:131–5.

Fig. 1
Spindles in living human metaphase II oocytes imaged by using the inverted method according to the angles of deviation with regard to the position of the polar body. (A) 0 o’clock, (B) 1 o’clock, (C) 2 o’clock, (D) 3 o’clock, (E) 4 o’clock, (F) 5 o’clock, (G) 6 o’clock, and (H) spindle invisible. PB, polar body.
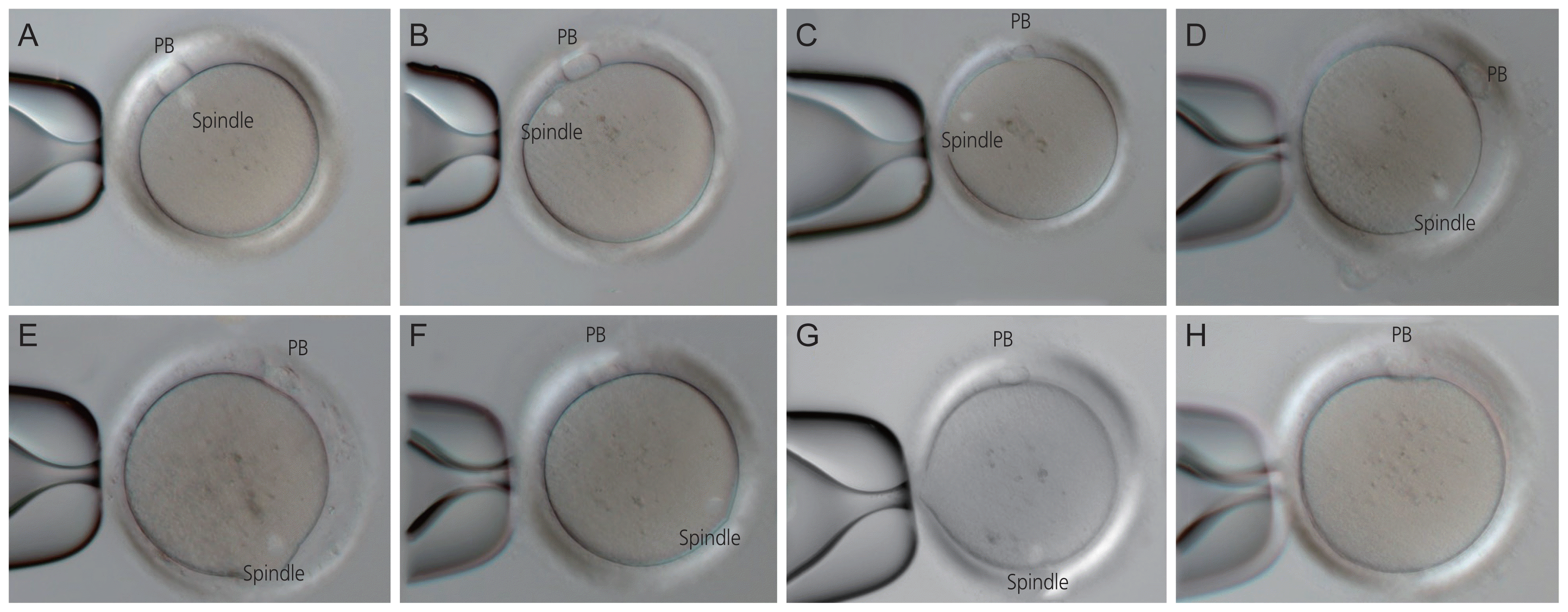
Fig. 2
Meiotic spindle distribution according to the angles of deviation based on the position of the polar body. A, 0 o’clock; B, 1 o’clock; C, 2 o’clock; D, 3 o’clock; E, 4 o’clock; F, 5 o’clock; G, 6 o’clock; and H, spindle invisible.
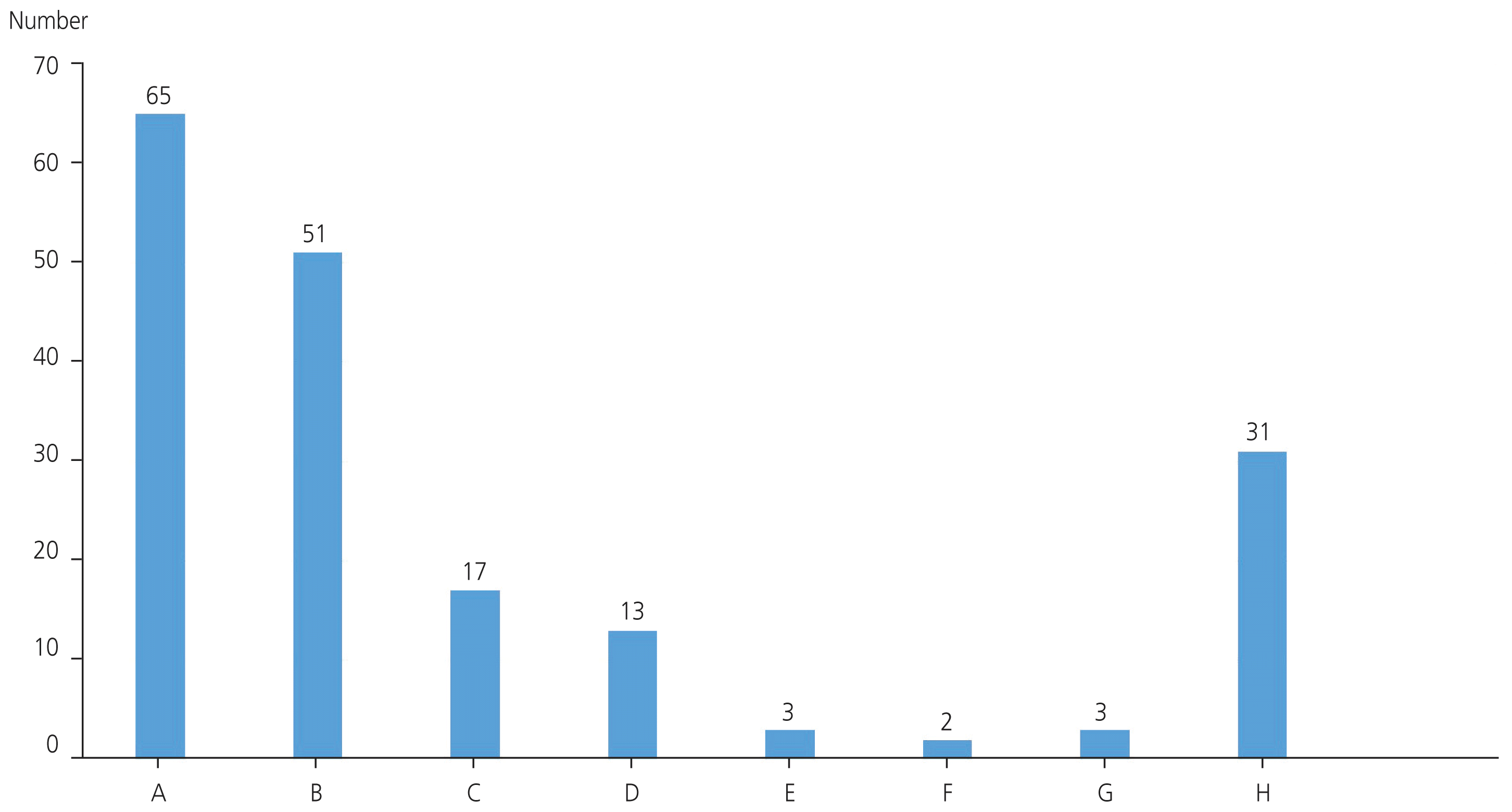
Table 1
Comparison of clinical parameters between the spindle-view intracytoplasmic sperm injection group and the conventional intra-cytoplasmic sperm injection group
| SVICSI (n=37) | cICSI (n=58) | P-value | |
|---|---|---|---|
| Age (yr) | 35.68±3.90 | 36.11±3.97 | 0.127a) |
| Parity | 0.08±0.28 | 0.09±0.28 | 0.931a) |
| Body mass index (kg/m2) | 22.80±2.86 | 22.66±3.67 | 0.836a) |
| Basal AMH levels (ng/mL) | 0.90±0.74 | 0.86±0.94 | 0.833a) |
| Basal FSH levels (mIU/mL) | 13.17±9.14 | 15.02±9.08 | 0.351a) |
| Other infertility factors | |||
| Tubal factor | 3/37 (8.1) | 5/58 (8.6) | 1.000b) |
| Stage III/IV endometriosis | 5/37 (13.5) | 8/58 (13.8) | 1.000b) |
| Male factor | 2/37 (5.4) | 1/58 (1.7) | 0.558b) |
| Number of previous failed embryo transfers | 6.89±3.22 | 5.33±2.47 | 0.009a) |
| Total gonadotropin dosage | 2,789.19±1,277.43 | 2,617.93±1,206.76 | 0.511a) |
| Endometrial thickness on the trigger day (mm) | 8.78±1.69 | 8.38±1.41 | 0.228a) |
| Retrieved oocytes | 6.81±5.18 | 6.66±6.08 | 0.898a) |
| Transferred embryos | 1.76±0.68 | 1.90±0.79 | 0.377a) |
| Superovulation methods | 1.000b) | ||
| Natural | 2/37 (5.4) | 3/58 (5.2) | |
| Controlled ovarian hyperstimulation | 35/37 (94.6) | 55/58 (94.8) | |
| Premature LH surge prevention | 0.413c) | ||
| GnRH agonist | 11/37 (29.7) | 22/58 (37.9) | |
| GnRH antagonist | 26/37 (70.3) | 22/58 (62.1) | |
| Percentage of blastocyst transfer | 13/37 (35.1) | 5/58 (8.6) | 0.001c) |
Table 2
Comparison of laboratory and clinical outcomes between the spindle-view intracytoplasmic sperm injection group and the conventional intracytoplasmic sperm injection group
| SVICSI | cICSI | P-value | |
|---|---|---|---|
| Fertilization rate | 140/185 (75.7) | 209/266 (78.6) | 0.470a) |
| Cleavage rate | 137/140 (97.9) | 208/209 (99.5) | 0.306b) |
| Good-quality cleavage embryo rate | 96/140 (68.6) | 113/209 (54.1) | 0.007a) |
| Blastocyst rate | 51/140 (36.4) | 34/209 (16.3) | <0.001a) |
| Good-quality blastocyst rate | 30/ 140 (21.4) | 9/ 209 (4.3) | <0.001a) |
| Implantation rate | 7/66 (10.6) | 9/110 (8.2) | 0.588a) |
| Clinical pregnancy rate | 7/37 (18.9) | 8/58 (13.8) | 0.504a) |
| Clinical abortion rate | 3/7 (42.9) | 2/8 (25.0) | 0.608b) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download