Abstract
Objective
To perform a systematic review and meta-analysis evaluating the efficacy of middle meningeal artery embolization in terms of both clinical and radiographic outcomes, when performed with different embolic agents.
Methods
A systematic literature review and meta-analysis was performed to evaluate the impact of embolic agents on outcomes for middle meningeal artery (MMA) embolization. The use of polyvinyl alcohol (PVA) with or without (±) coils, N-butyl cyanoacrylate (n-BCA) ± coils, and Onyx alone were separately evaluated. Primary outcome measures were recurrence, the need for surgical rescue and in-hospital periprocedural complications.
Results
Thirty-one studies were identified with a total of 1,134 patients, with 786 receiving PVA, 167 receiving n-BCA, and 181 patients receiving Onyx. There was no difference in the recurrence rate (5.5% for PVA, 4.5% for n-BCA, and 6.5% for Onyx, with P=0.71) or need for surgical rescue (5.0% for PVA, 4.0% for n-BCA, and 6.9% for Onyx, with P=0.89) based on the embolic agent. Procedural complications also did not differ between embolic agents (1.8% for PVA, 3.6% for n-BCA, and 1.6% for Onyx, with P=0.48).
Chronic subdural hematomas (SDH) are considered a sentinel health event with a 1-year mortality rate of over 30% [10,27]. Despite surgical treatment, there is a high recurrence rate and preventing recurrence remains challenging [1,16,31,37]. Middle meningeal artery (MMA) embolization for the treatment of chronic SDH was first described by Mandai et al. in 2000 as an adjunct for a patient with persistent recurrence of a chronic SDH [24]. It has since gained popularity as both a prophylactic and therapeutic technique, particularly in patients requiring antiplatelet or anticoagulant therapy [14,18,22].
Early series predominantly favored the use of polyvinyl alcohol (PVA) particles with or without (±) coils as their embolic agent, likely secondary to their extensive track record, low cost, and widespread availability [3,6,20,22,30,45]. Liquid embolic agents including n-BCA and Onyx have since increased in popularity, and many interventionalists now favor their use due to improved radiographic visualization and durability. Consequently, several recent studies have also outlined their use in MMA embolization for chronic subdural hematoma [33,47,49]. Each embolic agent is associated with a unique risk profile, and differences in efficacy between agents for cases of MMA embolization for the treatment of chronic SDH have not been delineated. The aim of this systematic review and meta-analysis is to compare rates of surgical rescue and procedural complications, based on the embolic agent, for patients who undergo MMA embolization for chronic SDH.
The authors performed a systematic review and metaanalysis to determine whether the chosen embolic agent influences the need for surgical rescue and the rate of complication. This was performed up to February of 2023, according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. The MEDLINE, SCOPUS, and Cochrane Databases were searched using the following key words and phrases: (MMA) AND (embolization), (middle meningeal artery embolization), (chronic subdural hematoma embolization), (meningeal artery) AND (embolic), (subdural hematoma) AND (meningeal artery). Duplicate studies were excluded. Study designs were analyzed, and randomized trials, observational studies, and case series with at least three cases were included. Case reports or case series of two cases were excluded. After this list was compiled, initial screening was performed using the titles and abstracts of each article. All articles that cleared the initial screen were independently read in their entirety and evaluated by the first and second author (NE and DS), with any disagreements settled by consulting the senior author (Fig. 1). The data was collected and analyzed by two authors to ensure accuracy and studies were only included if they reported at least one of the two outcome measures described below.
All cases of middle meningeal artery embolization for the treatment of chronic SDH using Onyx and n-BCA, performed by the senior author (MB) between January 2020 and February 2023, were retrospectively identified from a prospectively maintained database at our institution, the University of Rochester Medical Center, and also included in the meta-analysis.
Clinical outcomes of embolization using PVA with or without (±) coils, N-butyl cyanoacrylate (n-BCA) ± coils, and Onyx alone were separately evaluated. Outcome measures included recurrence, need for surgical rescue and in hospital periprocedural complications.
A random-effects model was utilized to calculate pooled proportions for each clinical outcome variable based on the embolization agent, where each individual study was weighted as an inverse of its estimated variance to account for inter-study variations (DerSimonian and Laird method). Corresponding forest plots with subgroups stratified by embolization agent were subsequently generated along with their respective 95% confidence interval (CI) for each embolic subgroup. Furthermore, a mixed-effects meta-regression was completed to evaluate for potential differences between each embolic agent. All statistical analysis and forest plots were completed using OpenMeta[Analyst] from Brown University, based on R statistical software (http://www.cebm.browm.edu/openmeta/). For all statistical analyses, a P value of <0.05 was used to indicate statistical significance.
Interstudy heterogeneity of the included studies within the pooled analysis was also completed using the Cochrane Q statistic and described with the I2 measure and its corresponding P value. An I2 value of >50% was used to indicate moderate interstudy heterogeneity while a value of >75% was used to indicate considerable heterogeneity. Also, a P value of >0.10 was used as the threshold for nonsignificant heterogeneity.
From our prospectively maintained database, we identified 48 patients who underwent embolization of their middle meningeal artery for the purposes of treatment of chronic SDH by the senior author (MB), between January of 2020 and February of 2023. Of the 32 patients who underwent Onyx embolization, 3 had radiographic recurrence, 4 required surgical rescue, while one had a stroke after the embolization procedure. Of the 16 patients who underwent n-BCA embolization, 1 recurred on follow up CT, 1 required surgical rescue, while 2 had procedural related complications.
Using the MEDLINE, SCOPUS, and Cochrane Library databases, we identified a total of 3,860 articles. This consisted of 1,957 unique articles after removing duplicates. These were screened and 1,766 were excluded based on the abstract or title, leaving 191 articles. The full text of each article was read and 31 of these articles fit the criteria and were therefore included in the analysis (Fig. 1) [2-4,6,8,9,12,13,15,19-23,25,28-30,32-35,38-42,45,47,49,50]. Each of the articles were reviewed independently by two authors (NE and DS) to ensure that they fit the inclusion criteria. Manuscripts reporting on the use of coils alone, Squid, Embospheres, absolute alcohol, and gelatin sponge were identified but not included as they were limited to 10 or fewer total cases for each agent, and therefore of insufficient volume to analyze in the meta-analysis. Articles were only included if they utilized the same embolic agent in >95% of cases.
From 31 studies combined with our institutional experience, there was a total of 1,134 patients, with 786 receiving PVA ± coils, 167 receiving n-BCA ± coils, and 181 patients receiving Onyx alone (Table 1). Pooled rates of recurrence following embolization were 5.5% (95% CI: 3.3% to 7.7%) for PVA ± coils, 4.5% (CI: 1.4% to 7.6%) for n-BCA ± coils, and 6.5% (CI: 2.8% to 10.3%) for Onyx embolization (Fig. 2). Pooled rates of need for surgical rescue following embolization were 5.0% (CI: 3.1% to 6.8%) for PVA ± coils, 4.0% (CI: 1.1% to 6.9%) for n-BCA ± coils, and 6.9% (CI: 1.8% to 11.9%) for Onyx embolization (Fig. 3). However, meta-regression indicated that there was no significant difference in the embolic agents and the recurrence rate (P=0.71) or rate of surgical rescue (P=0.89). For procedural related complication rates, there was a pooled composite rate of 1.8% (CI: 0.9% to 2.8%) for PVA ± coils, 3.6% (CI: 0.8% to 6.4%) for n-BCA ± coils, and 1.6% (CI: 0% to 3.4%) for Onyx embolization (Fig. 4). Similarly, meta-regression indicated no significant difference between PVA based, n-BCA based and Onyx embolization procedural related complications (P=0.4).
There was generally low, non-significant statistical interstudy heterogeneity within most of the subgroup and overall analyses. There was low, non-significant interstudy heterogeneity found within each subgroup for the complication analysis. The calculated I2 value for Onyx, n-BCA based, and PVA based embolization were all 0% (P value all >0.56). Likewise, for the overall complication analysis, there was low, non-significant heterogeneity between all of the included studies within the analysis (I2=0%, P=0.953). Similarly, for the surgical rescue analysis, there was low, non-significant heterogeneity within the PVA based subgroup (I2=27%, P=0.120), the n-BCA based embolic agent subgroup (I2=0%, P=0.964), and the Onyx subgroup (I2=45%, P=0.101). However, overall heterogeneity for the surgical rescue analysis was considerable, though not significant (I2=93%, P=0.310). Regarding the recurrence analysis, there was low, but significant interstudy heterogeneity within the PVA subgroup (I2=32%, P=0.09), but low, non-significant in the n-BCA subgroup (I2=0%, P=0.99), and high, non-significant heterogeneity in the Onyx subgroup analysis (I2=73%, P=0.4). Lastly, low, non-significant statistical heterogeneity was seen in the overall quantitative analysis for recurrence (I2=31%, P=0.415).
This was a systematic review and meta-analysis evaluating the impact of embolic agent on outcomes of MMA embolization for chronic SDH, incorporating our institutional experience with Onyx and n-BCA. We found no difference in the recurrence rate (5.5% for PVA, 4.5% for n-BCA, and 6.5% for Onyx, with P=0.71) or need for surgical rescue (5.0% for PVA, 4.0% for n-BCA, and 6.9% for Onyx, with P=0.89) based on the embolic agent utilized. Similarly, procedural complications did not differ between embolic agents (1.8% for PVA, 3.6% for n-BCA, and 1.6% for Onyx, with P=0.48). PVA particles were the most reported embolic agent, though several recent publications reporting the use of Onyx suggests an increasing trend towards its utilization.
There is mounting evidence to support MMA embolization as a primary treatment for chronic subdural hematoma. Conventional surgical treatment of chronic subdural hematoma is heterogeneous and may utilize a variety of techniques including SEPS (Subdural Evacuating Port System) placement, craniotomy or burr hole with or without subdural or subgaleal drain placement. Regardless of technique, conventional surgery is associated with substantial recurrence rates ranging up to 37% [1,16,31,37]. MMA embolization for treatment of chronic subdural hematoma was first described by Mandai et al. in 2000. Since then, several studies have demonstrated its effectiveness at reducing hematoma recurrence and need for surgical rescue, while maintaining relatively low complication rates [5,14,18,22]. The present meta-analysis shows a 4.7% (CI: 3.4% to 6.0%) recurrence rate, 4.4% (CI: 3.1% to 5.6%) need for surgical rescue and a 1.9% (CI: 1.1% to 2.7%) complication rate associated with MMA embolization. As MMA embolization for treatment of chronic subdural hematoma remains in its infancy, there are several prospective randomized controlled trials ongoing to determine the ideal timing of MMA embolization in relationship to conventional surgical evacuation, the role of surgical adjunctive therapy, which embolic agents to use, the best techniques for embolization and the role of antiplatelet and anticoagulation therapy (NCT04270955, NCT04750200, NCT04742920, NCT04816591, NCT04372147, NCT04511572, NCT04402632, NCT04410146).
Many of the early studies describing MMA embolization for the treatment of chronic subdural hematoma, used polyvinyl alcohol (PVA) particle embolization with or without coil embolization. The principle advantages of PVA particles are track record and cost. PVA particles were first used in 1974, come in a variety of sizes 45-1,180 μm, and function by aggregating and lodging distally within the vasculature [46]. While liquid embolic agents require distal penetration and mandate the use of separate microcatheters for each branch, PVA particles can be injected from a more proximal position and allow for microcatheter repositioning without the need for a new catheter. Avoiding distal MMA branch penetration may provide benefits in regard to overall risk profile. Additionally, use of large particles may prevent penetration of dangerous external-internal carotid artery anastomoses that are important for the neurointerventionalist to avoid. One downside is technical challenge: Particles are transported by anterograde blood flow and are not radio-opaque, making it difficult to determine the degree of distal penetration and proximal reflux after navigating a microcatheter through a tortuous middle meningeal artery. Particles are also known to reabsorb and degrade overtime, resulting in increased rates of recanalization and concerns about durability when compared to liquid embolic agents [7,44,48]. Despite this oft-voiced concern, there have been no reports of late SDH recurrence attributable to MMA recanalization after PVA embolization.
Ethylene vinyl alcohol copolymer (Onyx) is the least-studied liquid embolic agent in MMA embolization for SDH, but has some advantages in terms of ease of use. First, Onyx consists of ethylene vinyl alcohol prepared with the solvent dimethyl sulfoxide (DMSO) and tantalum powder and is straightforward to visualize with fluoroscopy. This improved visualization allows for more control and better precision when embolizing distal branches. Onyx is also nonadhesive, lowering the likelihood of catheter adherence seen with other liquid embolic agents. While PVA particles require blood flow for their delivery, Onyx can be pushed from a wedged position allowing for deep penetration into small distal branches of the MMA. This is generally thought to be advantageous, although care is necessary to avoid pushing Onyx into undesired territories. Onyx also results in a permanent cast which does not reabsorb and is therefore thought to have better durability.
A notable disadvantage of Onyx is its cost, particularly compared to PVA particles. At our institution, Contour PVA particles cost approximately $200, compared to $2000 for Onyx and $4000 for n-BCA. This cost may be prohibitive in certain centers worldwide and this should be taken into consideration. Onyx also requires the utilization of specific DMSO-compatible microcatheters and syringes, which may further increase overall costs. Additionally, DMSO is caustic to the microvasculature and can cause vasospasm and significant discomfort. This may necessitate administration of lidocaine or the use of general anesthesia when performing MMA embolization. Rapid polymerization may also result in proximal “stump” embolization rather than distal arteriolar penetration [22].
N-butyl-2 cyanoacrylate (n-BCA) is a synthetic glue that is combined with ethiodized oil, and tantalum powder to achieve titratable viscosity and visualization. The inclusion of tantalum powder again allows improved visualization compared to PVA particles. Ethiodol acts to slow the polymerization of n-BCA and adjustments between the ratio of n-BCA and ethiodol allow for titration of the polymerization rate to the procedure or surgeon preference. Concomitant administration of 5% dextrose in water (D5W) can also be used to facilitate deeper penetration of n-BCA which is of particular benefit in cases of proximal MMA tortuosity [2,23].
Although the titratability of the n-BCA polymerization rate can be advantageous, it requires expertise and can therefore be a disadvantage and result in inconsistency for interventionalists without substantial experience. As a synthetic glue, n-BCA can also result in adherence of the catheter to the vessel wall following injection. This increases the risk for potential complications upon catheter removal, such as vessel rupture and associated intracranial hemorrhage. Notably, this is less of a concern in dural branches of the external carotid artery (ECA) as opposed to subarachnoid vessels. Although n-BCA is also thought to provide durable, permanent vessel occlusion, particularly in comparison to PVA particles, it may be associated with a lower permanent occlusion rate than Onyx [11]. It is also more expensive than both PVA particles and Onyx.
The main limitations of this meta-analysis are related to the retrospective nature of its constituent studies. This includes significant variability in embolization technique. For instance, we could not account for the number of branches embolized, as Catapano et al. have previously demonstrated improved hematoma resolution with embolization of both anterior and posterior branches compared to a single branch [5]. Additional variables (procedure length, status of antiplatelet or anticoagulant medications) and outcome measures (length of stay, modified Rankin Score (mRS) upon follow up, subdural hematoma volume) were not uniformly reported and therefore could not be pooled for analysis. Much of the available literature on MMA embolization has included multiple different embolic agents [17,18,26,43]. As this meta-analysis requires at least 95% of cases to use the same embolic agent, we also do not fully capture the published experience of MMA embolization.
It is possible that the presented meta-analysis is underpowered to detect differences between embolic agents given the average included study size is approximately 25 patients, and the small observed effect size for each variable/agent ranging from 1.6% to 5.5%. This is further limited by the expected publication bias of the included studies, which consist predominantly of retrospective single center studies. The overall number of complications reported within this meta-analysis is relatively low. Whether this is because the procedure is safe or due to underreporting consistent with a publication bias is unclear. Given that statistical heterogeneity can reflect various forms of publication bias between studies and data sets, and that various degrees of heterogeneity were observed, this further suggests possible bias within the included studies should be considered. However, while this may be a limitation of this analysis, it is also a limitation of the current state of the literature from which the presented data is derived and suggests the need for larger, randomized studies of which many are ongoing. Finally, there are likely numerous variables that contribute to the durability and efficacy of MMA embolization outside of embolic agent, which may be currently unknown. For example, it has been shown that distal penetration of embolic material plays a role in the timing of chronic SDH improvement [36]. Future studies are likely to elucidate additional variables that function to alter the clinical outcomes of MMA embolization.
Based on the current literature, the rate of periprocedural complication, rate of radiographic recurrence, and need for surgical rescue following MMA embolization for chronic subdural hematoma do not appear to be influenced by the type of embolic agent utilized. This procedure remains novel within neurointerventional surgery, and many specifics including the ideal time-frame and patient population are yet to be solidified. Therefore, further validation of these findings with ongoing larger prospective studies and randomized controlled trials should be pursued.
ACKNOWLEDGMENTS
This work has been presented as an oral poster at the Congress of Neurological Surgeons Annual Meeting in San Francisco, CA, in October of 2022.
REFERENCES
1. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014; Mar. 259(3):449–57.
2. Al-Mufti F, Kaur G, Amuluru K, Cooper JB, Dakay K, El-Ghanem M, et al. Middle meningeal artery embolization using combined particle embolization and n-BCA with the dextrose 5% in water push technique for chronic subdural hematomas: A prospective safety and feasibility study. AJNR Am J Neuroradiol. 2021; May. 42(5):916–20.
3. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018; Mar. 286(3):992–9.

4. Carpenter A, Rock M, Dowlati E, Miller C, Mai JC, Liu AH, et al. Middle meningeal artery embolization with subdural evacuating port system for primary management of chronic subdural hematomas. Neurosurg Rev. 2022; Feb. 45(1):439–49.

5. Catapano JS, Ducruet AF, Nguyen CL, Baranoski JF, Cole TS, Majmundar N, et al. Middle meningeal artery embolization for chronic subdural hematoma: An institutional technical analysis. J Neurointerv Surg. 2021; Jul. 13(7):657–60.

6. Chihara H, Imamura H, Ogura T, Adachi H, Imai Y, Sakai N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. 2014; May. 1(1):1–5.

7. Davidson GS, Terbrugge KG. Histologic long-term follow-up after embolization with polyvinyl alcohol particles. AJNR Am J Neuroradiol. 1995; Apr. 16(4 Suppl):843–6.
8. Dofuku S, Sato D, Nakamura R, Ogawa S, Torazawa S, Sato M, et al. Sequential middle meningeal artery embolization after burr hole surgery for recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo). 2023; Jan. 63(1):17–22.

9. Dowlati E, Chesney K, Carpenter AB, Rock M, Patel N, Mai JC, et al. Awake transradial middle meningeal artery embolization and twist drill craniostomy for chronic subdural hematomas in the elderly: Case series and technical note. J Neurosurg Sci. 2021; Jun. Online ahead of print.

10. Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: A sentinel health event. World Neurosurg. 2013; Dec. 80(6):889–92.

11. Elsenousi A, Aletich VA, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with N-butyl cyanoacrylate or Onyx: A meta-analysis. J Neurointerv Surg. 2016; Mar. 8(3):265–72.

12. Enriquez-Marulanda A, Gomez-Paz S, Salem MM, Mallick A, Motiei-Langroudi R, Arle JE, et al. Middle meningeal artery embolization versus conventional treatment of chronic subdural hematomas. Neurosurgery. 2021; Aug. 89(3):486–95.

13. Entezami P, Field NC, Dalfino JC. Outpatient management of chronic expanding subdural hematomas with endovascular embolization to minimize inpatient admissions during the COVID-19 viral pandemic. Interv Neuroradiol. 2021; Oct. 27(5):716–21.

14. Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP, et al. Middle meningeal artery embolization for chronic subdural hematoma: A systematic review and meta-analysis. J Neurointerv Surg. 2021; Oct. 13(10):951–7.

15. Ishihara H, Ishihara S, Kohyama S, Yamane F, Ogawa M, Sato A, et al. Experience in endovascular treatment of recurrent chronic subdural hematoma. Interv Neuroradiol. 2007; Mar. 13 Suppl 1(Suppl 1):141–4.

16. Ivamoto HS, Lemos HP, Atallah AN. Surgical treatments for chronic subdural hematomas: A comprehensive systematic review. World Neurosurg. 2016; Feb. 86:399–418.

17. Joyce E, Bounajem MT, Scoville J, Thomas AJ, Ogilvy CS, Riina HA, et al. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: A multiinstitutional experience of 151 cases. Neurosurg Focus. 2020; Oct. 49(4):e5.

18. Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt JK, et al. Middle meningeal artery embolization for chronic subdural hematoma: A multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021; Jan. 88(2):268–77.
19. Khorasanizadeh M, Shutran M, Garcia A, Enriquez-Marulanda A, Moore J, Ogilvy CS, et al. Middle meningeal artery embolization for treatment of chronic subdural hematomas: Does selection of embolized branches affect outcomes? J Neurosurg. 2022; Nov. 1–9.

20. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017; May. 101:520–7.

21. Krothapalli N, Patel S, Fayad M, Elmashad A, Killory B, Bruno C, et al. Outcomes of particle versus liquid embolic materials used in middle meningeal artery embolization for the treatment of chronic subdural hematoma. World Neurosurg. 2023; Jan. Online ahead of print.

22. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A series of 60 cases. Neurosurgery. 2019; Dec. 85(6):801–7.

23. Majidi S, Matsoukas S, De Leacy RA, Morgenstern PF, Soni R, Shoirah H, et al. Middle meningeal artery embolization for chronic subdural hematoma using N-butyl cyanoacrylate with D5W push technique. Neurosurgery. 2022; May. 90(5):533–7.

24. Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. J Neurosurg. 2000; Oct. 93(4):686–8.

25. Matsumoto H, Hanayama H, Okada T, Sakurai Y, Minami H, Masuda A, et al. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. 2018; Mar. 49:40–47.

26. Mir O, Yaghi S, Pujara D, Burkhardt JK, Kan P, Shapiro M, et al. Safety of antithrombotic resumption in chronic subdural hematoma patients with middle meningeal artery embolization: A case control study. J Stroke Cerebrovasc Dis. 2022; Apr. 31(4):106318.

27. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: Not a benign disease. J Neurosurg. 2011; Jan. 114(1):72–6.

28. Mureb MC, Kondziolka D, Shapiro M, Raz E, Nossek E, Haynes J, et al. DynaCT enhancement of subdural membranes after middle meningeal artery embolization: Insights into pathophysiology. World Neurosurg. 2020; Jul. 139:e265–70.

29. Nakagawa I, Park HS, Kotsugi M, Wada T, Takeshima Y, Matsuda R, et al. Enhanced hematoma membrane on DynaCT images during middle meningeal artery embolization for persistently recurrent chronic subdural hematoma. World Neurosurg. 2019; Jun. 126:e473–9.

30. Ng S, Derraz I, Boetto J, Dargazanli C, Poulen G, Gascou G, et al. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: A pilot study assessing hematoma volume resorption. J Neurointerv Surg. 2020; Jul. 12(7):695–9.

31. Nia AM, Srinivasan VM, Lall RR, Kan P. Middle meningeal artery embolization for chronic subdural hematoma: A national database study of 191 patients in the United States. World Neurosurg. 2021; Sep. 153:e300–7.

32. Onyinzo C, Berlis A, Abel M, Kudernatsch M, Maurer CJ. Efficacy and mid-term outcome of middle meningeal artery embolization with or without burr hole evacuation for chronic subdural hematoma compared with burr hole evacuation alone. J Neurointerv Surg. 2022; Mar. 14(3):297–300.

33. Rajah GB, Waqas M, Dossani RH, Vakharia K, Gong AD, Rho K, et al. Transradial middle meningeal artery embolization for chronic subdural hematoma using Onyx: Case series. J Neurointerv Surg. 2020; Dec. 12(12):1214–8.

34. Rinaldo L, Cloft H, Brinjikji W. E-113 middle meningeal artery embolization for treatment of chronic subdural hematoma: A prospective institutional case series. J Neurointerv Surg. 2020; Aug. 12(Suppl 1):A90.
35. Saito H, Tanaka M, Hadeishi H. Angiogenesis in the septum and inner membrane of refractory chronic subdural hematomas: Consideration of findings after middle meningeal artery embolization with low-concentration N-butyl-2-cyanoacrylate. NMC Case Rep J. 2019; Sep. 6(4):105–10.
36. Samarage HM, Kim WJ, Zarrin D, Goel K, Wang ACH, Johnson J, et al. The “Bright Falx” sign-midline embolic penetration is associated with faster resolution of chronic subdural hematoma after middle meningeal artery embolization: A case series. Neurosurgery. 2022; Sep. 91(3):389–98.

37. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burrhole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009; Sep. 374(9695):1067–73.

38. Sarma P, Garg M, Prem P, Gupta R. Embolization of the middle meningeal artery for the treatment of chronic subdural hematoma: A path less travelled so far. J Neurosci Rural Pract. 2022; Aug. 13(3):471–5.

39. Schwarz J, Carnevale JA, Goldberg JL, Ramos AD, Link TW, Knopman J. Perioperative prophylactic middle meningeal artery embolization for chronic subdural hematoma: A series of 44 cases. J Neurosurg. 2021; May. 135(6):1627–35.

40. Scoville JP, Joyce E, A, Tonetti D, Bounajem MT, Thomas A, Ogilvy CS, et al. Radiographic and clinical outcomes with particle or liquid embolic agents for middle meningeal artery embolization of nonacute subdural hematomas. Interv Neuroradiol. 2022; Jun. 15910199221104631.

41. Seok JH, Kim JH, Kwon TH, Byun J, Yoon WK. Middle meningeal artery embolization for chronic subdural hematoma in elderly patients at high risk of surgical treatment. J Cerebrovasc Endovasc Neurosurg. 2022; Oct. Online ahead of print.

42. Shehabeldin M, Amllay A, Jabre R, Chen CJ, Schunemann V, Herial NA, et al. Onyx versus particles for middle meningeal artery embolization in chronic subdural hematoma. Neurosurgery. 2022; Dec. Online ahead of print.

43. Shotar E, Meyblum L, Premat K, Lenck S, Degos V, Grand T, et al. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. J Neurointerv Surg. 2020; Dec. 12(12):1209–13.

44. Sorimachi T, Koike T, Takeuchi S, Minakawa T, Hiroshi A, Nishimaki K, et al. Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: Angiographic reappearance and complications. AJNR Am J Neuroradiol. 1999; Aug. 20(7):1323–8.
45. Tempaku A, Yamauchi S, Ikeda H, Tsubota N, Furukawa H, Maeda D, et al. Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: Five cases and a review of the literature. Interv Neuroradiol. 2015; Jun. 21(3):366–71.

46. Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol. 2008; Sep. 25(3):204–15.

47. Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: Systematic review and case series. World Neurosurg. 2019; Jun. 126:228–36.

48. Wikholm G. Occlusion of cerebral arteriovenous malformations with N-butyl cyano-acrylate is permanent. AJNR Am J Neuroradiol. 1995; Mar. 16(3):479–82.
Fig. 1.
PRISMA flow diagram. This diagram demonstrates the systematic method used for the identification, screening, and inclusion of articles that met criteria for inclusion within the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis
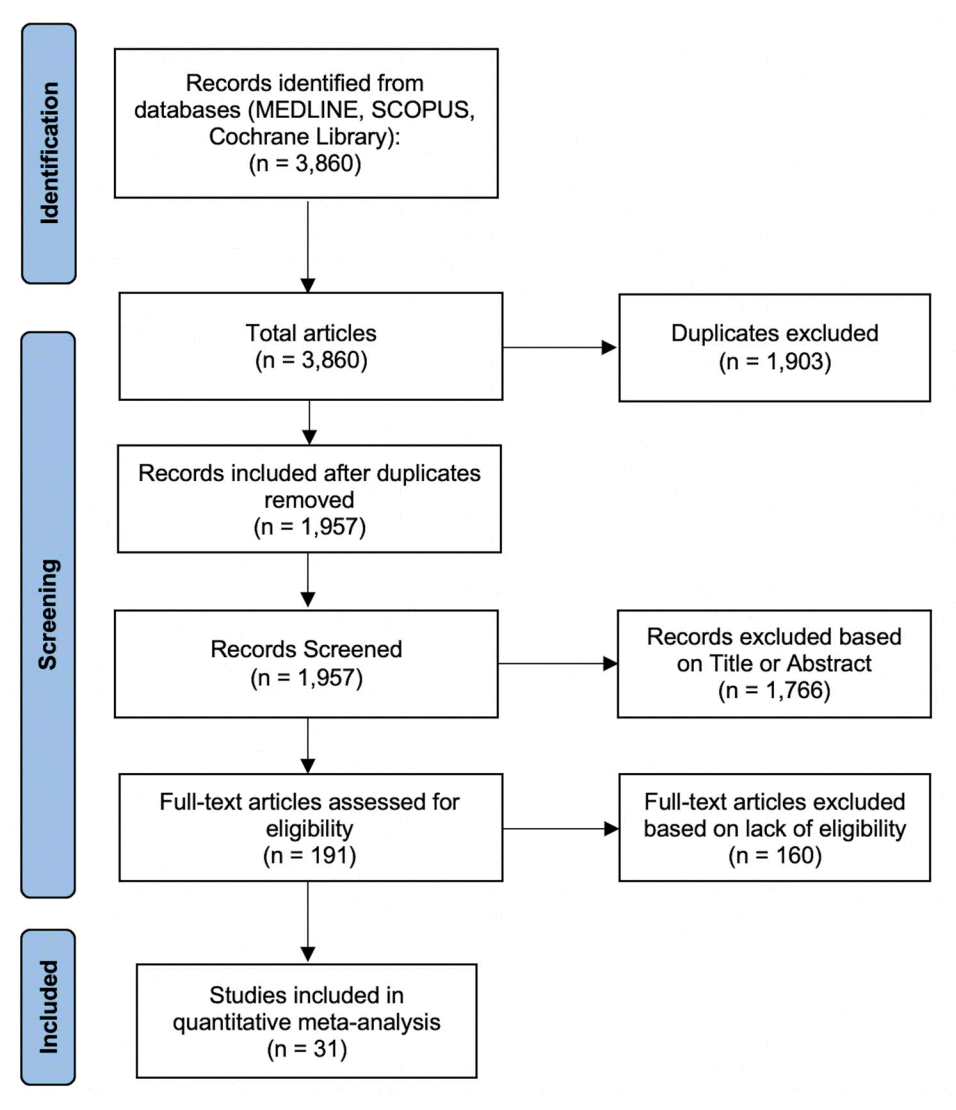
Fig. 2.
Rate of recurrence. Forest plot demonstrating the results from the meta-analysis of rates of recurrence following MMA embolization using PVA particles ± coils, n-BCA ± coils, and Onyx. MMA, middle meningeal artery; PVA, polyvinyl alcohol; n-BCA, N-butyl cyanoacrylate
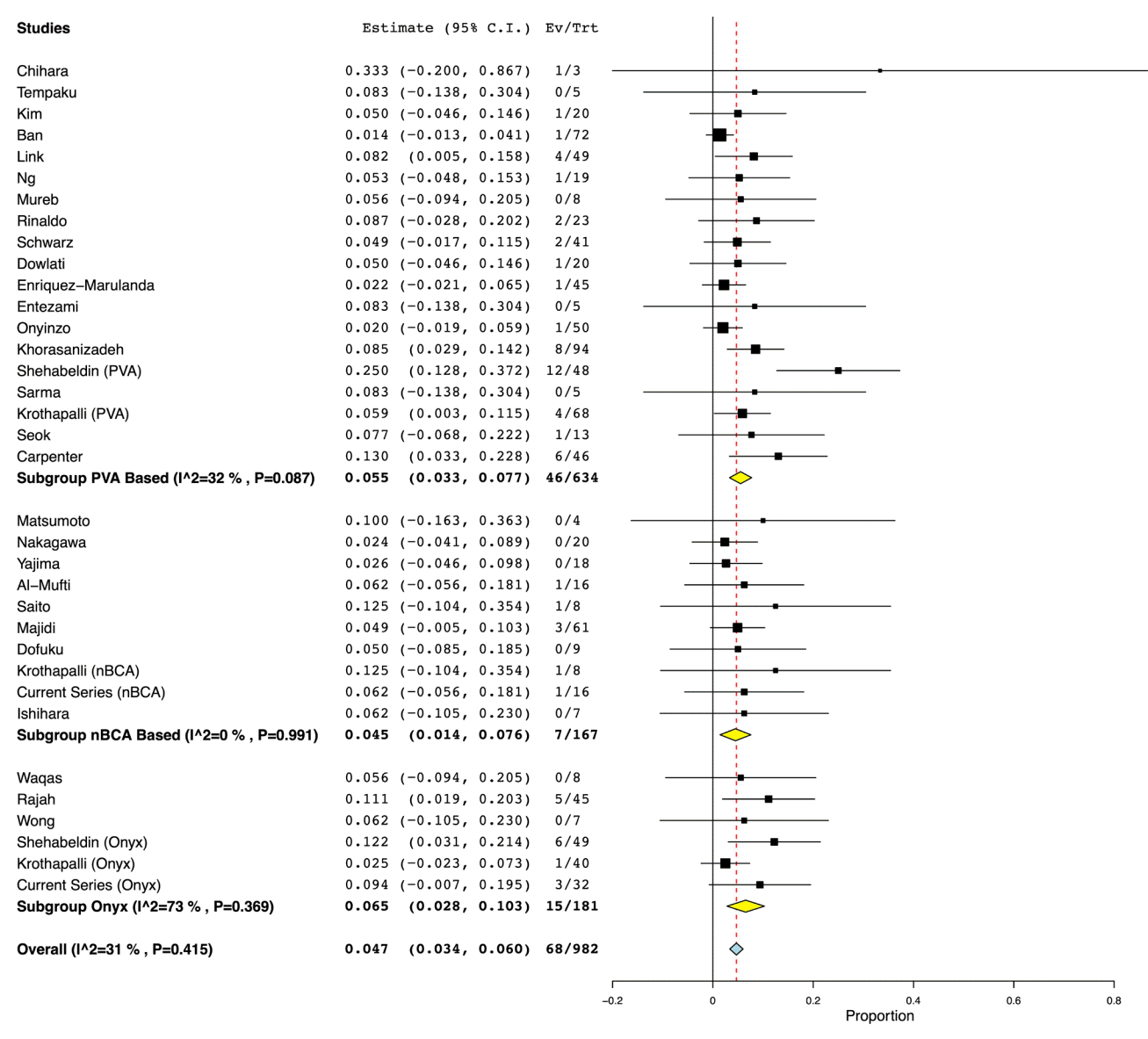
Fig. 3.
Rate of surgical rescue. Forest plot demonstrating the results from the meta-analysis of rates of surgical rescue following MMA embolization using PVA particles ± coils, n-BCA ± coils, and Onyx. MMA, middle meningeal artery; PVA, polyvinyl alcohol; n-BCA, N-butyl cyanoacrylate
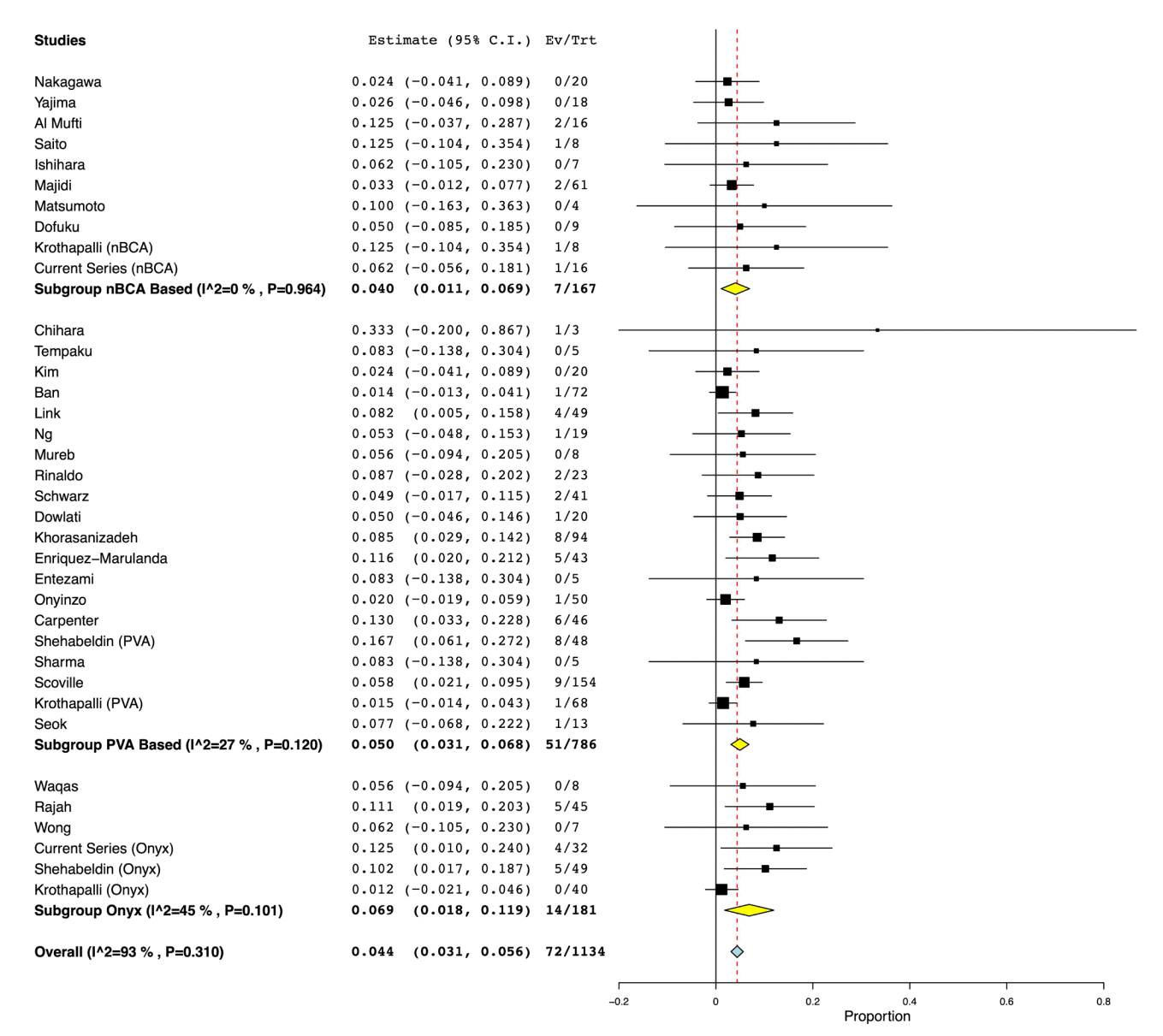
Fig. 4.
Rate of periprocedural complication. Forest plot demonstrating the results from the meta-analysis of rates of periprocedural complications following MMA embolization using PVA particles ± coils, n-BCA ± coils, and Onyx. MMA, middle meningeal artery; PVA, polyvinyl alcohol; n-BCA, N-butyl cyanoacrylate
Table 1.
Study design, embolic agent, and outcomes of included studies




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download