Abstract
Intracranial non-galenic pial arteriovenous fistula (PAVF) is an extremely rare vascular malformation, where one or more pial arteries feeds directly into a cortical vein without any intervening nidus. Though occasionally they can be asymptomatic, neurological symptoms such as headache, seizure, or focal neurological deficit are more common presenting features. Life threatening or fatal hemorrhage is not uncommon, hence needed to be treated more often than not. Spontaneous occlusion of PAVF is reported only four times before. We report a 49-year-old gentleman, who was diagnosed to have a PAVF, possibly secondary to trauma. He presented 5 months and 22 days from initial digital subtraction angiography (DSA) for treatment, and follow-up angiogram showed complete obliteration. He denied any significant event, medication or alternate treatment during this period. His clinical symptoms were stable as well. We postulate iodinated contrast medium induced vasculopathy as a possible cause, which has been described for other vascular pathologies, but never for PAVF.
Pial arteriovenous fistula (PAVF) is an extremely rare condition, accounting for less than 2% intracranial vascular malformations. They can be congenital or results from iatrogenic or traumatic injury. Considering the fact that direct arteriovenous shunt results in high venous blood flow and varix formation with the subsequent risk of haemorrhage, these pathologies need to be treated on most occasions, either by endovascular or microsurgical approach. We encountered a 49-year-old gentleman with complex right middle cranial fossa PAVF with feeders from a single right orbitofrontal middle cerebral artery (MCA) branch with an aneurysm, direct shunting into the basal vein of Rosenthal (BVR) which harboured a venous varix at the origin. When patient returned for treatment, baseline angiogram showed complete occlusion of PAVF. We postulate contrast-induced vasculopathy as a possible cause. To the best of our knowledge, spontaneous occlusion of PAVF was documented only four times in English literature, prior to this case.
A 49-year-old male with no medical co-morbidities was referred to our department for evaluation of a right MCA aneurysm and an AVF. He suffered a motor vehicle accident 31 years prior, with head trauma which required extended hospitalization and multiple craniofacial surgeries, the details of which were unknown. He made a complete recovery except for residual right facial weakness. He currently presented to an external facility solely for consultation regarding facial reconstructive surgery. Magnetic resonance imaging (MRI) of the brain was performed as part of the evaluation and he was subsequently referred. Neurological review of symptoms was negative for headache, seizure, tinnitus or focal neurological deficits. On examination, he demonstrated lower motor neuron right facial paresis without any other deficit.
Non-contrast MRI brain showed postoperative changes of right frontal craniotomy and sequelae of prior head trauma including gliosis maximum in the right frontal lobe, but also present in the left frontal and left parieto-occipital lobes (Fig. 1A). Multiple flow voids were seen in the right middle cranial fossa, along the right MCA past the bifurcation and an enlarged right BVR was also noted (Fig. 1B, C). No obvious tangle of vessels suggestive of a nidus was seen. A non-contrast time-of-flight MR angiogram showed a frontal MCA branch aneurysm shunting into a venous varix continuing into the BVR (Fig. 1D).
Findings were suspicious for an arteriovenous fistula, and the patient was subjected to a catheter digital subtraction angiography (DSA) using Omnipaque, to completely define the angioarchitecture of the vascular malformation. OmnipaqueⓇ 300 (GE Healthcare, CO/Cork, Ireland) is a low osmolar, non-ionic contrast medium containing 647 mg of the active substance Iohexol per milliliter.
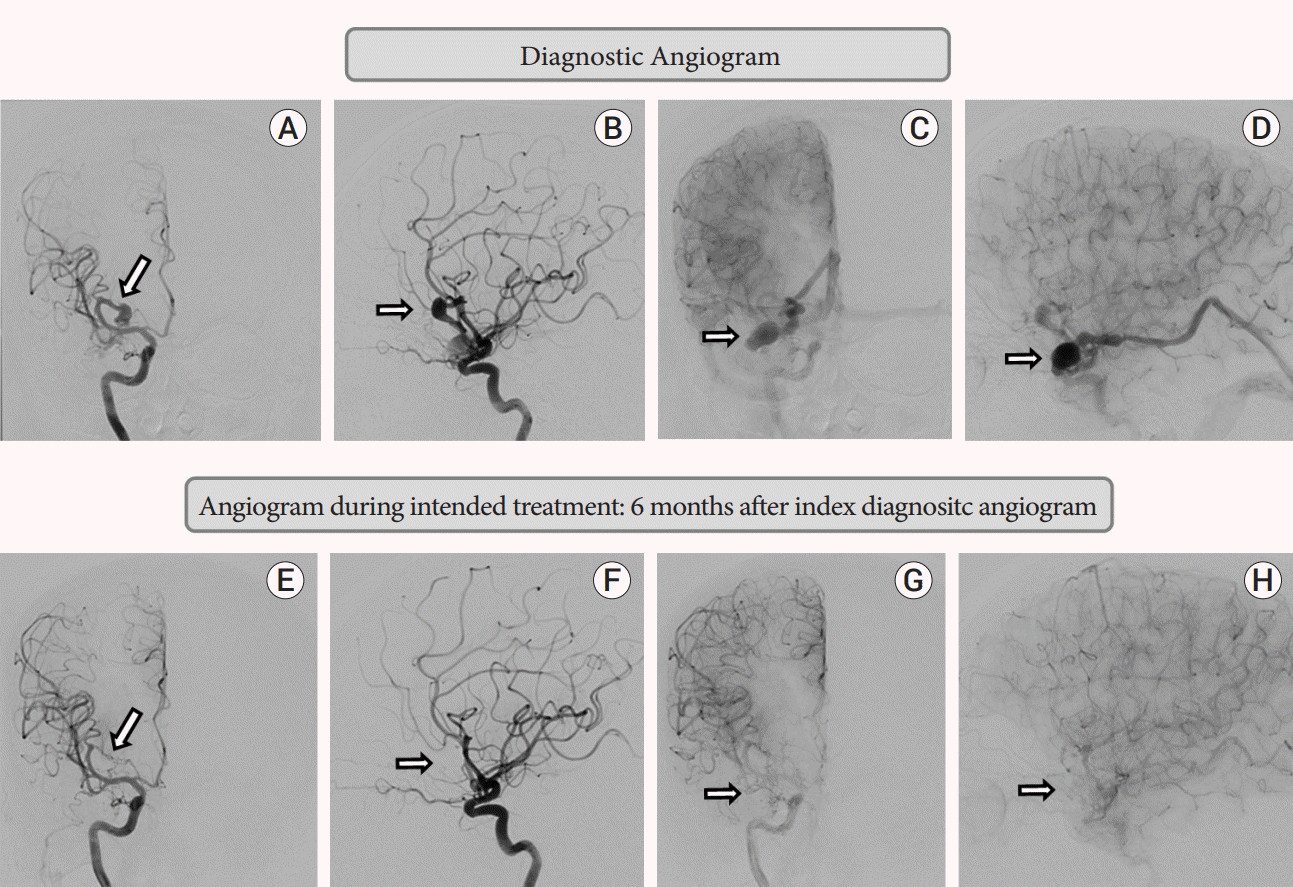
This demonstrated a complex right middle cranial fossa AVF with the predominant component being a pial fistula from a single right orbitofrontal MCA branch with direct shunting in to the BVR which harboured a venous varix at the origin, and ectatic segments. A fusiform aneurysm of the orbitofrontal feeder measuring 11 mm in largest diameter was present. There was a minor dural component with the petrous branch of middle meningeal artery shunting to the same venous varix at the origin of the right BVR (Fig. 2A-D).
Given young age, presence of a large pial shunt with the deep venous system, and associated feeding artery aneurysm, treatment with endovascular embolization was advised to mitigate the risk of future intracerebral hemorrhage. Plan was for transarterial balloon assisted liquid embolic embolization to occlude both the aneurysm and the fistulous connection, with transvenous access as backup if above not completely achieved from the transarterial route. Patient agreed for treatment but requested a delayed date.
He presented 5 months and 22 days from initial DSA for treatment. Baseline angiography showed spontaneous complete occlusion of the pial AVF, and the feeding artery orbitofrontal aneurysm (Fig. 2E-H). The minor dural supply from the middle meningeal artery had also ceased. The patient denied any interval neurological symptoms and his examination was stable. He also denied any new interval events, medications, or alternate treatments.
The patient continued to remain clinically stable thereafter. Three-month follow-up non-contrast MRI brain (Fig. 1E-G) and MR angiogram with contrast (Fig. 1H) showed no new parenchymal changes and stable complete occlusion of the aneurysm and the AVF. One-year follow-up diagnostic angiogram showed stable occlusion of aneurysm and arteriovenous fistula.
Nongalenic PAVF is a rare vascular malformation (less than 2% of all intracranial vascular malformations) in which one or more pial arteries feeds directly into a cortical vein without any intervening nidus [3]. PAVF is distinct from dural arteriovenous fistula (DAVF). In PAVF, there is abnormal direct communication or shunting between pial arteries that would normally supply the brain tissue, whereas in DAVF, vessels that normally would not supply the brain tissue are involved. On both pathologies, they feed into veins that normally drain the brain tissue. On most occasions, PAVF consists of one or more arterial feeders and usually a single venous channel. The direct arteriovenous shunt results in high venous blood flow and varix formation with the subsequent risk of haemorrhage [7]. On rare occasions, DAVF and PAVF can exist together. While the exact reasons remain unknown, PAVF can be congenital or result from iatrogenic or traumatic injury [4]. PAVF can rarely present without symptoms, but mostly present with wide range of neurological symptoms including potentially catastrophic to fatal events secondary to rupture causing intracranial hemorrhage. Considering high morbidity and mortality secondary to rupture, fistula disconnection using either microsurgery or endovascular embolization is generally advised over observation.
Spontaneous occlusion of a PAVF is an extremely rare phenomenon. The occurrence of spontaneous occlusion may be higher than DAVF, as PAVF is associated with high venous blood flow. The present case highlights the spontaneous occlusion of a large PAVF after a diagnostic DSA. Given the history of head trauma 3 decades prior, with imaging evidence of trauma sequalae, it is likely that the PAVF developed after and secondary to the traumatic event. Although we cannot be sure, it is also likely that development of the AVF was not acute since he was completely asymptomatic, suggesting a chronic pathology, possibly years. Against this background, the only modifying factor from initial diagnosis to follow-up angiography demonstrating spontaneous occlusion was the administration of intra-arterial iodinated contrast media (CM). We postulate that the iodinated CM induced thrombosis of the fistula, and explore potential mechanisms for the same.
Similar to our case, only 4 earlier cases have described spontaneous occlusion of unruptured PAVF after diagnostic cerebral angiography. Santosh et al. described spontaneous occlusion of a PAVF documented on follow-up DSA 10 days after initial angiography [11]. Satow et al. described 2 cases where spontaneous PAVF occlusion was documented on follow-up DSA 17 and 11 days after initial angiography respectively [12]. In the above 3 cases, likely initiation of thrombosis was marked by clear clinical change including new severe headache, cessation of headache, or cessation of tinnitus occurring 7 days, immediately after, and 2 days after initial angiography respectively. In our case, patient was initially asymptomatic and did not report any new or acute symptoms, suggesting slow, progressive thrombosis. In a case series of 15 patients with PAVF, a single patient showed spontaneous occlusion of a PAVF on 4-month follow-up DSA after initial angiography [7]. The report does not suggest any change of clinical features during this period. Other reports of spontaneous PAVF occlusion occurred in relation to prior surgical/endovascular interventions [5,8] or after PAVF rupture [6].
Plausible explanation for PAVF occlusion discussed in above cases is the use of intra-arterial iodinated CM. Insights into possible pathophysiological mechanisms of this phenomenon may be gleaned from reviewing the problem of iodinated CM induced acute kidney injury (CI-AKI) after vascular angiography or intervention. Vasculopathy with functionally impaired endothelial cells is a hallmark of CI-AKI [13]. CM increases the production of reactive oxygen species, the expression of vascular cell adhesion molecule-1, and the secretion of inflammatory factors (MCP-1, TNF-alpha, and IL-6) in endothelial cells [1,10]. CM also inhibit vasodilator (Nitric Oxide and Prostacyclin) production and promote vasoconstrictor (Endothelin-1) generation in endothelial cells contributing to vasoconstrictor response in renal microcirculation [2,15]. After CM injection in rats, the plasma levels of endothelial cell markers (plasminogen activator inhibitor-1 and von Willebrand factor) significantly increase [9].
Furthermore, CM also induce the release of circulating endothelial microparticles in patients with cardiovascular disease after angiography [14]. This constellation of above factors contributes to endothelial toxicity and results in its impaired role in maintaining vascular homeostasis including vascular inflammation, vascular tone and thrombosis.
We clearly acknowledge the limitation of postulating the role of iodinated CM in inducing spontaneous occlusion of unruptured PAVFs, when drawing conclusion from a single case. Almost all PAVFs undergo preoperative diagnostic DSA, and it is unclear why only a small fraction should spontaneously occlude. It is difficult to comment on the timing of such occlusion in absence of any clinical symptoms. The role of other factors including baseline comorbidities, concurrent hypercoagulable state, angioarchitecture of the PAVF or drainage patterns are unknown.
Spontaneous occlusion is certainly a rarity and can’t be predicted. But interventionist/surgeon may be pleasantly surprised when it happens. A prior knowledge of this fact can be helpful during the intervention.
PAVFs are rare vascular malformations with unknown natural history, but often associated with high morbidity and mortality. Spontaneous occlusion of a PAVF is an exquisitely rare phenomenon, but can occur after cerebral angiography. This may be related to the use of iodinated CM inducing thrombosis. CM related endothelial injury could be a potential pathophysiological mechanism for the phenomenon of PAVF spontaneous occlusion.
REFERENCES
1. Chang CF, Liu XM, Peyton KJ, Durante W. Heme oxygenase-1 counteracts contrast media-induced endothelial cell dysfunction. Biochem Pharmacol. 2014; Jan. 87(2):303–11.

2. Franke RP, Fuhrmann R, Hiebl B, Jung F. Influence of radiographic contrast media (Iodixanol and Iomeprol) on the endothelin-1 release from human arterial and venous endothelial cells cultured on an extracellular matrix. Clin Hemorheol Microcirc. 2012; 52(2-4):229–34.

3. Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Dowd CF, Barnwell SL. Transarterial occlusion of solitary intracerebral arteriovenous fistulas. AJNR Am J Neuroradiol. 1989; Jul-Aug. 10(4):747–52.
4. Hoh BL, Putman CM, Budzik RF, Ogilvy CS. Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery. 2001; Dec. 49(6):1351–63.

5. John S, Hussain SI, Elhammady MS, Navarro R, Zahra K. Multiple cranial dural and pial arteriovenous fistulas with occlusion of all after embolization of primary superior sagittal sinus dural fistula. World Neurosurg. 2020; Aug. 140:224–8.

6. Lyons MK, Hoxworth JM, McClendon J, Krishna CX, Patel NP. Spontaneous resolution of ruptured intracranial pial arteriovenous fistula following spinal surgery. Neuroradiol J. 2017; Apr. 30(2):175–9.

7. Medhi G, Gupta AK, Saini J, Ramalingaiah AH, Pendharkar H, Parida S. Pial arteriovenous fistula: A clinical and neuro-interventional experience of outcomes in a rare entity. Indian Journal of Radiology and Imaging. 2020; JulSep. 30(3):286–93.

8. Peeters SM, Colby GP, Guivatchian E, Sun MZ, Tateshima S, Wang AC. Spontaneous resolution of dural and pial arteriovenous fistulae arising after superficial temporal artery to middle cerebral artery bypass for moyamoya disease. World Neurosurg. 2020; Oct. 142:404–7.

9. Ren L, Wang P, Wang Z, Liu Y, Lv S. Hypotonic contrast media is more toxic than isotonic contrast media on endothelial cells in vivo and in vitro. Mol Med Rep. 2017; Oct. 16(4):4334–40.
10. Ronda N, Potì F, Palmisano A, Gatti R, Orlandini G, Maggiore U, et al. Effects of the radiocontrast agent iodixanol on endothelial cell morphology and function. Vascul Pharmacol. 2013; Jan. 58(1-2):39–47.

11. Santosh C, Teasdale E, Molyneux A. Spontaneous closure of an intracranial middle cerebral arteriovenous fistula. Neuroradiology. 1991; 33(1):65–6.

12. Satow T, Suzuki M, Komuro T, Ogawa M, Kobayashi A, Nishida S. Spontaneous resolution of cerebral pial arteriovenous fistula after angiography: Report of two cases. World Neurosurg. 2017; Jul. 103:954.

13. Scoditti E, Massaro M, Montinari MR. Endothelial safety of radiological contrast media: Why being concerned. Vascul Pharmacol. 2013 ; Jan. 58(1-2):48–53.

14. Zhang B, Zhang Y, Liu B, Fang L, Li Y, Meng S. Iso-osmolar iodixanol induces less increase in circulating endothelial microparticles in vivo and less endothelial apoptosis in vitro compared with low-osmolar iohexol. Contrast Media Mol Imaging. 2018; Apr. 2018:8303609.
Fig. 1.
MRI brain before diagnostic angiogram: A-D; MRI after second angiogram: E-H. (A) T2 FLAIR sequence shows right frontal, left frontal and left parieto-occipital signal changes, likely sequelae of previous head trauma. (B) T2 sequence shows venous varix. (C) T2 sequence shows right MCA aneurysm and prominent ectatic basal vein of Rosenthal. (D) MRA sequence shows MCA aneurysm from right orbitofrontal MCA. (E) T2 FLAIR sequence shows right frontal, left frontal and left parieto-occipital signal changes remaining same as pre-procedure suggesting no fresh changes. (F) T2 sequence shows venous varix is completely obliterated. (G) T2 sequence shows right MCA aneurysm and prominent ectatic basal vein of Rosenthal completely occluded. (H) MRA sequence shows complete occlusion of MCA aneurysm. MRI, Magnetic resonance imaging; MCA, middle cerebral artery
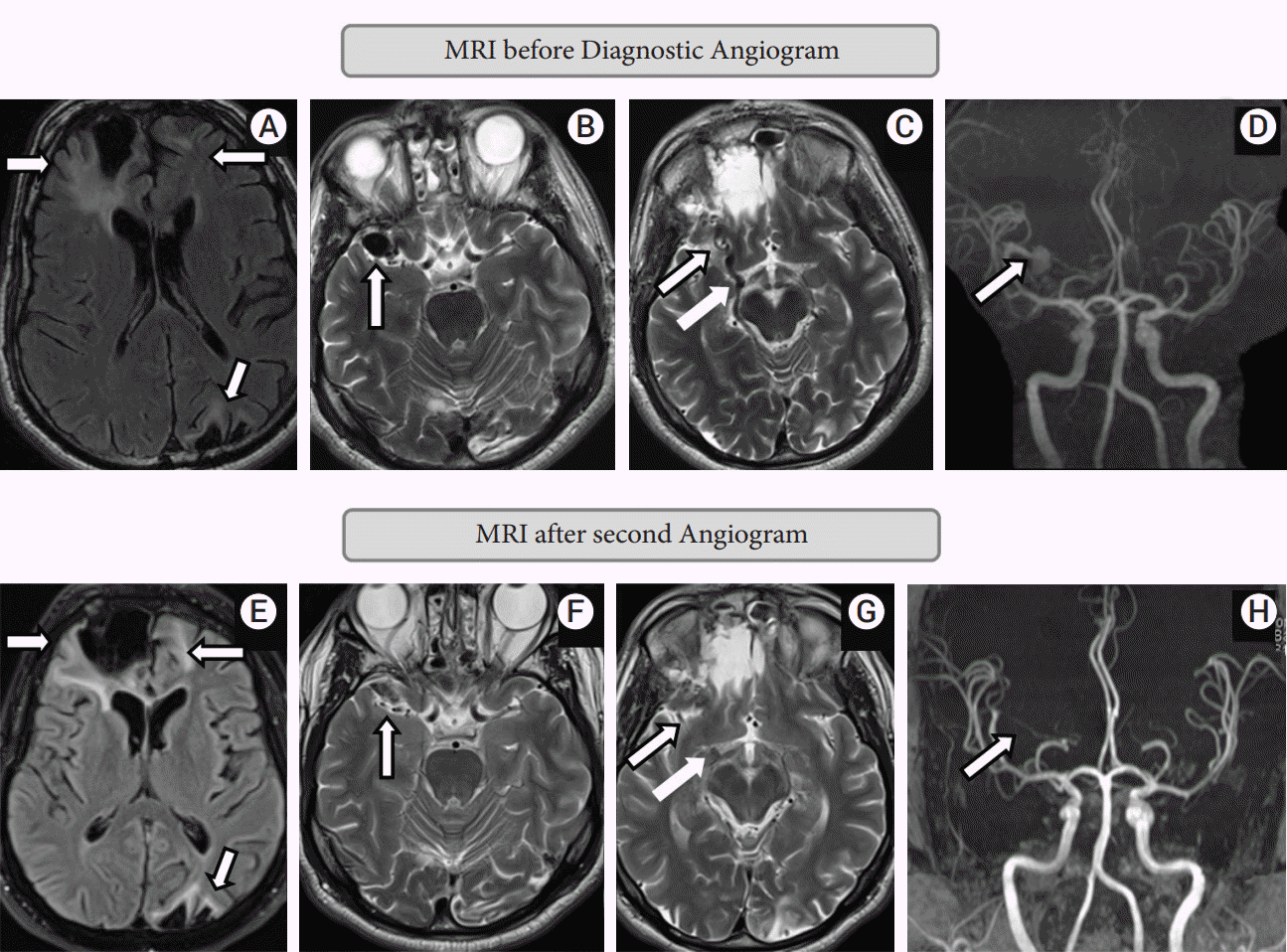
Fig. 2.
Diagnostic angiogram: A-D; Angiogram during intended treatment: 6 months after index diagnostic angiogram (second angiogram): E-H. (A) Right ICA injection: arterial phase: antero-posterior projection: fusiform aneurysm from right orbitofrontal branch of middle cerebral artery. (B) Right ICA injection: arterial phase: lateral projection: showing same as A. (C) Right ICA injection: Venous phase: antero-posterior projection: venous varix at the start of basal vein of Rosenthal with prominent ectatic course of vein. (D) Right ICA injection: Venous phase: lateral projection: showing venous varix and ectatic basal vein of Rosenthal. (E) Right ICA injection: arterial phase: antero-posterior projection: complete occlusion of fusiform aneurysm. (F) Right ICA injection: arterial phase: lateral projection: no residual aneurysm. (G) Right ICA injection: Venous phase: antero-posterior projection: complete occlusion of venous varix and basal vein of Rosenthal. (H) Right ICA injection: Venous phase: lateral projection: showing complete occlusion of venous varix and basal vein of Rosenthal. ICA, internal carotid artery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download