Abstract
Objective
Congenital intracranial pial arteriovenous fistula (PAVF) is a rare cerebral vascular pathology characterized by a direct shunt between one or more pial feeding arteries and a cortical draining vein. Transarterial endovascular embolization (TAE) is widely considered first line therapy. Curative TAE may not be achievable in the multihole variant due to the potential to harbor innumerable small feeding arteries. Transvenous embolization (TVE) may be considered to target the final common outlet of the lesion. Here, we present a series of four patients with complex multi-hole congenital PAVF treated with staged TAE followed by TVE.
Methods
A retrospective review was conducted on patients who underwent treatment for congenital, multi-hole PAVFs treated by a combined TAE/TVE approach at our institution since 2013.
Results
We identified four patients with multi-hole PAVF treated by a combined TAE/TVE. Median age was 5.2 (0-14.7) years. Median follow-up of 8 (1-15) months by catheter angiography and 38 (23-53) months by MRI/MRA was obtained. TVE achieved complete occlusion in three patients that proved durable on radiographic follow-up and demonstrated excellent clinical outcomes with a modified Rankin Score (mRS) of 0 or 1. Complete occlusion of the draining vein was not achieved by TVE in one case. This patient is graded as pediatric mRS=5 three years post-procedure.
Congenital intracranial pial arteriovenous fistula (PAVF), also known as non-Galenic arteriovenous fistula, is a rare cerebrovascular pathology characterized by a direct shunt between one or more pial feeding arteries and a cortical draining vein. This entity lacks a true “nidus,” a feature differentiating it from arteriovenous malformations (AVM). The draining vein is cortical without involvement of dural sinus, distinguishing it from dural AVF. There have been approximately 200 cases reported in the literature since 1970 [7,8,14], predominately representing the more common single-hole variant, where a single artery makes a fistulous connection to the draining vein. The multi-hole variant, where multiple arteries connect to the draining vein, is more rare, complex, and has a higher overall flow; thus, it’s associated with earlier presentation and follows a more aggressive natural history [7].
Congenital PAVFs typically present in childhood and are frequently associated with congenital diseases [25]. The most common presentations include heart failure, seizure, headache, hemorrhage, mass effect, and/or ischemia due to steal [14,25]. Untreated, PAVF are associated with poor outcomes with reported mortality rates of 63% [23].
Curative treatment is recommended to protect from the sequelae of high flow shunts including the risk of hemorrhage, venous hypertension, and “melting brain” syndrome [7,12,15]. Transarterial endovascular embolization (TAE) is first line for its minimally invasive nature and ability to reach deep and eloquent regions of the brain if required [7,14]. In 2020, Jin et al. [8] demonstrated an endovascular cure rate of 77% overall, but only 56% in cases with multiple feeders. Curative TAE may not be achievable in the multi-hole variant due to the potential to harbor innumerable small caliber, tortuous feeding arteries. In this setting, transvenous embolization (TVE) may be considered to target the final common outlet of the lesion. The success of TVE in PAVF has been demonstrated in case reports or isolated cases [2,4,13,18,20,22].
Here, we present a series of four patients with complex multi-hole congenital PAVF treated with staged TAE followed by TVE with intent to cure. Our experience represents the largest case series in the literature and demonstrates promise for this challenging disease.
A retrospective review was conducted on patients who underwent treatment for congenital, multi-hole PAVFs treated by a combined TAE/TVE approach at our institution since 2013. Data including demographic data, medical records, imaging studies, procedural data, periprocedural complications, and angiographic studies were reviewed. The Institutional Review Board (IRB) committee reviewed and approved the protocols, informed consent, and other documents included in this study.
Herein, we describe our general approach and technical considerations, which are summarized in Fig. 1. A case specific example (case 2) is presented in Fig. 2. Figs. 3-5 demonstrate the lesions at presentation, at the lowest flow state, and after curative TVE in cases 1-3, respectively. Case 4 is summarized in Figs. 6-8.
The initial management, often under emergent circumstances, follows transarterial strategies. The early goal is temporization. On occasion, the point of hemorrhage can be identified from careful review of non-invasive imaging and is targeted specifically. More typically, non-specific but impactful flow reduction to the lesion is pursued by targeting the largest, readily accessible feeders. Adequate flow control of the embolysate is required to prevent premature penetration into the vein. A variety of strategies may achieve this, including temporary balloon occlusion, coil-assisted liquid-embolic embolization, or in very small children where instrument access is limited, adenosine-induced cardiac pause. Treatment is concluded when the emergency is temporized, and clinical improvement can be expected.
Following temporization, we proceed with transarterial flow reduction, targeting all navigable feeders until we achieve the lowest flow state possible. N-butyl cyanoacrylate (NBCA) is the workhorse for this phase, rapidly producing short, occlusive plugs. As flow is reduced, the complexity of the lesion decreases. During this phase, we scrutinize the imaging for the potential of a navigable feeder supplying the fistulous draining vein at the most proximal point of the vein. In that setting, a curative embolization may be achievable by TAE. In our series, however, these specific feeders were not navigable. The transarterial phase is concluded when the lowest flow state is achieved. The two transarterial phases, temporization, and flow reduction, can occur in the same setting, as demonstrated in case 3, but they are more commonly staged. In our experience, due to the high flow of these lesions, the interval change in vessel calibers between treatments of the untreated feeders can be marked, certainly more so than AVM, for example. The caliber changes can be significant enough to alter subsequent treatment plans. Therefore, when achievable, our current practice is to keep the interval between stages relatively short, ideally 2-4 weeks.
The transvenous phase requires the most meticulous planning. The entirety of the fistulous region of the vein must be completely occluded from proximal to distal to achieve a good outcome. The sine qua non of this approach is twofold; first, the most proximal portion of the fistulous vein must be unequivocally identified and secondly, access to this point must be achievable by navigation with instruments suitable for embolization. Indeed, the final embolization strategy may well be most predicated on which instrument is able to achieve the navigation. If the fistulous vein has multiple outlets that are navigable, it may prove advantageous to divide the draining vein into separate compartments and achieve microcatheter access to the most proximal portion of each, as pursued in case 2. If these prerequisites are met, preparation of the vein can proceed.
The venous cast will extend from proximal to distal, encompassing the entire fistulous length. Preparation of the vein optimizes the likelihood of achieving this goal. The anatomy is scrutinized to identify any peculiarities that may prohibit complete occlusion. Eccentric varices at risk of incomplete embolization are preemptively targeted for focused transvenous treatment, as seen in case 2. Coil-assisted liquid embolic embolization is typically employed to minimize the likelihood of undue embolysate penetration into the main venous outlet. The anatomy of the main vein is also scrutinized for stenoses and compartmentalization that may impact creation of the final occlusive cast. In case 2, for example, a stricture compartmentalized the proximal and distal portions of the fistulous vein into two halves. We responded with a strategy to treat the compartments in turn, from proximal to distal. The preparation phase is concluded when the final venous configuration is simplified as much as possible. In our experience, preparation of the vein has been successfully performed at the same setting as definitive embolization. However, should the circumstance arise that staging this portion of the treatment proves advantageous, we would not hesitate to do so, provided we had not induced an unstable limitation to the main venous outflow.
The transvenous preparation and cure can stretch multiple hours. We perform all cases under general anesthesia with neurophysiologic monitoring. Meticulous attention to fluid management and contrast volume is imperative, particularly in very small children. We employed systemic heparin in all cases. The transvenous navigation, which may traverse a substantial course, is aided by judicious, distal intracranial guide catheter placement and advanced, image guided techniques such as 3D roadmap.
The curative phase begins with the creation of an occlusive cast encompassing the entirety of the proximal venous portion of the vein. We have a low threshold to deploy several coils to act as a scaffold for the liquid embolic while securing the proximal pouch. We have been successful with both NBCA and Onyx (Medtronic, Minneapolis, MN, USA) with the former being preferred if the target vein volume is relatively small and focal. Onyx is the preferred agent for more substantial configurations.
Once the proximal portion of the vein is secured, the embolic material is allowed to migrate more distally in the vein achieving a slowly enlarging, occlusive cast. During this process, venous-to-arterial penetration is expected and considered favorable. However, the degree of penetration into the arterial tree must be controlled to prevent ischemic complication. The compliance of the vein can be remarkable, with the embolic material slowly expanding the vein as the cast expands. This is most notable with Onyx but we have also experienced this with dilute NBCA. We continue the embolization until the entire fistulous segment is well occluded. This is confirmed by follow-up angiography which must include views from each of the involved vessel distributions. This point is exemplified in case 4, where occlusion was thought to be achieved based on carotid imaging, only to find ongoing flow on vertebral artery imaging after the venous portion of the case was closed.
The use of a detachable tip embolization microcatheter can be of great value during instrument removal. When the fistulous portion of the vein is long, the use of multiple instruments, strategically positioned along its course, may be required. In case 1, we used one set of instruments to achieve an occlusive proximal cast with coils and liquid embolic. This instrument, without detachable tip, was subsequently removed. Thereafter, we proceeded with curative embolization through a microcatheter with long detachable tip, already in place. We have not experienced a retained microcatheter, but if we felt we could not safely remove the instrument, we would prefer to cut the catheter at the access site rather than risk intracranial insult.
The post-procedural management takes place in the pediatric intensive care unit (ICU) with special attention to blood pressure management, avoiding hypertension. Unexplained neurologic compromise would raise concern for propagation of venous thrombosis to involve normal venous drainage. Anti-coagulation with heparin would be the preferred management. In case 4, we identified this complication after post-procedural hemorrhage, and therefore heparin was not initiated.
Follow-up is clinical and radiographic. In all cases in which we achieved complete occlusion at TVE, the clinical outcomes were excellent. We have not identified recurrence at long-term imaging follow-up. We intend to follow the children until they reach adulthood.
We identified four patients with multi-hole PAVF treated by a combined TAE/TVE (Table 1). Baseline cerebral angiography and follow-up after transvenous intervention for each case is presented in Fig. 3. In our cohort, the median age was 5.2 (0-14.7) years. Median follow-up of 8 (1-15) months by catheter angiography and 38 (23-53) months by MRI/MRA was obtained. Three presented with hemorrhage and the fourth presented with failure to thrive, developmental delay, and AV shunt induced cerebral atrophy. TVE achieved complete occlusion in three patients that proved durable on radiographic follow-up. We experienced 1 minor ischemic complication during the transarterial phase in case 1, hypoacusis secondary to flow alteration at the level of the labyrinthine artery. Case 2 suffered mild left-sided weakness from pre-existent, severe vasospasm, which resolved on long-term follow-up. Collectively, cases 1-3 demonstrated excellent clinical outcomes with a modified Rankin Score (mRS) of 0 or 1.
In case 4, the youngest of the series, complete occlusion of the draining vein was not achieved at TVE. This lesion was predominantly supplied by middle cerebral artery feeders with additional small supply from the posterior cerebral artery. Embolization was therefore tracked by angiography of the carotid circulation (Fig. 7F). When complete occlusion was achieved on carotid views, embolization was thought to be concluded. Subsequent vertebral artery imaging demonstrated faint but ongoing AV shunting (Fig. 8). Further embolization was indicated but was no longer achievable due to polymerization of Onyx, and the case was closed. 12 hours later, the child suffered large intraventricular hemorrhage (IVH), subsequently followed by progressive venous thrombosis extending beyond the fistulous vein to include the deep venous system with resultant deep venous infarction. The child also developed shunt-dependent hydrocephalus and seizures. He is graded as pediatric mRS=5 three years post-procedure. MRI at three years demonstrates no persistent serpiginous or dilated veins and arrest of cerebral atrophy. Catheter angiography has not been performed and therefore definitive occlusion status remains unknown.
PAVFs are rare, direct arterial-to-venous shunts located in the subpial space of the brain [1]. PAVFs are classified as single-hole or multi-hole depending on the number of feeding arteries making fistulous connection with a single draining vein. They are distinct from vein of Galen malformations (VGAM) and Galenic PAVF by the absence of involvement of the embryonic median prosencephalic vein of Markowski [1,3]. They are distinct from dural AVF by the absence of primary involvement with the dural venous system. Approximately 200 articles in the literature have described non-Galenic PAVF, with most describing the single-hole variant or report a combined analysis of single and multi-hole variants [5,9]. As such, the true prevalence of multi-hole PAVFs is not well defined. In our review of the literature, the reported prevalence of multi-hole PAVFs vary from 0-64% [5,7,8,9,19] with most reports suggesting that the multi-hole variant is indeed less common, typically making up 20% or less of the cases [19].
We describe the largest series of the combined TAE/TVE approach for the treatment of PAVF. Complete and durable occlusion of the fistula was achieved in 3 of 4 cases leading to excellent clinical outcomes (mRS £1). We experienced 1 minor ischemic complication during the TAE stage in one patient (hypoacusis) and no complication during the transvenous phases of the three cases in which complete occlusion was achieved. In the youngest child in the series, we did not achieve complete occlusion. The patient suffered catastrophic hemorrhage followed by thrombosis of the deep venous system, hydrocephalus and seizures. MRI follow-up several years later appears consistent with progression to complete occlusion, however catheter angiography has not been performed. The patient is graded mRS=5.
Surgery for PAVF can be curative in selective cases, especially the single-hole variant and in non-eloquent locations [1,3,5,9,19,24]. However, in contemporary practice, endovascular therapy by TAE is considered first line. The success of this approach with single-hole fistulae is well documented. Cure rates between 85-92% have been achieved in case series where the single-hole variant is the predominant lesion [8,14]. Isolated case reports and individual cases as part of larger series have demonstrated success using isolated TAE in multi-hole variants [12,17].
The literature has described the limitations of TAE for multi-hole fistulae. Mehdi et al. [14] reported catastrophic hemorrhage suffered by a patient with multihole PAVF that they attributed to incomplete occlusion. Maejima et al. [11] treated a neonate with greater than 10 feeders utilizing a staged TAE approach with NBCA. They reduced flow to the lesion, but the patient died on day of life 43. Madsen et al. [10] utilized TAE in 2 patients with multi-hole PAVF. A girl under 10 was cured with TAE using coil embolization. The second case, a neonate presenting at 1 week of life with congestive heart failure (CHF), was treated by TAE with NBCA and coils. Incomplete obliteration was achieved. Post-treatment ultrasound demonstrated thalamic infarcts and the patient died at 3 weeks of life.
In one of the largest case series, Hetts et al. [7] present 16 patients with multi-hole PAVFs compared to 9 with single-hole lesions. All cases were treated with TAE. Surgery was used as salvage therapy for 10 patients, seven of which were multi-hole. The single-hole cases demonstrated a cure rate of 78%. Compared to single-hole, the multi-hole cases were more likely to present at younger ages, require more procedures, suffer more peri-procedural complications, have lower rates of complete occlusion, and have worse clinical outcomes as graded by mRS.
To our knowledge, there are 5 detailed cases of PAVF treated with TVE [2,4,13,20,22]. Of these, three used TAE as first line intervention but were not able to completely cure the fistula. One case utilized a single-stage combined TAE/TVE approach while another utilized TVE as first-line. Three cases occurred during the first year of life, while two presented in late adolescence or early adulthood. Two patients presented with CHF, one patient with pulmonary arterial hypertension, one patient with SAH, and one patient with radiological development of a new venous varix and progression of an existing varix. Four cases demonstrated immediate angiographic occlusion, while one case had small residual flow. Long-term follow-up demonstrated good neurological outcomes and durable cure of the multi-hole PAVFs.
In the analogous disease process of VGAM, TVE has been successfully applied after planned, staged TAE, or when safe TAE has been exhausted but the lesion persists [6]. TVE has also been shown to warrant consideration for VGAM with restricted arterial access [16]. The overarching strategy is to pursue TAE to achieve the lowest flow state possible and follow shortly thereafter with TVE to achieve complete occlusion. Our cohort and the reported cases in the literature highlight the high acuity and life-threatening nature of multi-hole PAVF and demonstrate that curative treatment appears to be required for sustained clinical improvement. They also demonstrate the viability of employing a combined TAE/TVE strategy in treating multi-hole PAVF. Our institutional experience suggests that young patient age, small patient size, arterial supply from separate vascular territories, and close relationship of the fistulous vein to the deep venous system, elevate the risk for this approach.
The higher flow and increased complexity of multi-hole fistulae underly the limitations of TAE. Even with advances in catheter design, some multi-hole fistulae will be beyond cure by TAE alone. Without complete occlusion, the persistent lesion is likely to develop increased flow over time, escalating the risks associated with chronic persistent AV shunting [7,14,21,25]. To improve clinical and radiographic outcomes, targeting the final outlet of lesion, the draining vein, may be the path of least resistance. With thorough technical considerations, our series indicates that TVE of multi-hole PAVF that are refractory to TAE is feasible and effective in arresting the consequences of chronic, high-flow AV shunting produced by this pathology.
This study is limited by the small number of cases presented. It is difficult to draw definitive conclusions about the potential role this approach in the management of this rare disease. Further, our study lacks follow-up angiography for the case suffering severe complication. While long term follow-up MRI in this case strongly suggests complete occlusion, it is not a replacement for catheter angiography. Lastly, our report is subject to the limitations of a retrospective design.
Congenital multi-hole PAVFs are rare and dangerous lesions. Curative treatment is preferred if achievable. Our series, while small, represents the largest series in the literature in which TVE was utilized. Our experience suggests that with meticulous planning, curative treatment may be achievable.
REFERENCES
1. Cerebral arteriovenous fistulas. Lasjaunias P, ter Brugge KG, Berenstein A, editors. Surgical Neuroangiography: Clinical and Interventional Aspects in Children. Berlin, Heidelberg: Springer;2006. p. 227–89.
2. Cooke D, Tatum J, Farid H, Dowd C, Higashida R, Halbach V. Transvenous embolization of a pediatric pial arteriovenous fistula. J Neurointerv Surg. 2012; Jul. 4(4):e14.

3. da Silva Martins WC, de Albuquerque LA, de Souza Filho CB, Dellaretti M, de Sousa AA. Surgical treatment of the intracranial pial arteriovenous fistula. Surg Neurol Int. 2015; Jun. 6:102.

4. Eldesouky I. Transvenous endovascular embolisation of nongalenic arteriovenous fistula. EC Neurology. 2015; 1:16–21.
5. Goel A, Jain S, Shah A, Rai S, Gore S, Dharurkar P. Pial arteriovenous fistula: A brief review and report of 14 surgically treated cases. World Neurosurg. 2018; Feb. 110:e873–81.

6. Guerrero WR, Dandapat S, Ortega-Gutierrez S. Hemorrhagic cerebrovascular pathology in the pediatric population. Front Neurol. 2020; Sep. 11:1055.

7. Hetts SW, Keenan K, Fullerton HJ, Young WL, English JD, Gupta N, et al. Pediatric intracranial nongalenic pial arteriovenous fistulas: Clinical features, angioarchitecture, and outcomes. AJNR Am J Neuroradiol. 2012; Oct. 33(9):1710–9.

8. Jin H, Meng X, Quan J, Lu Y, Li Y. Role of endovascular embolisation for curative treatment of intracranial non-Galenic pial arteriovenous fistula. Stroke Vasc Neurol. 2021; Jun. 6(2):260–6.

9. Li J, Gao Z, Zhi X, Du J, Zhang H, Ling F. Clipping of a pediatric pial arteriovenous fistula located at basilar artery tip using a hybrid trapping-evacuation technique. World Neurosurg. 2018; Sep. 117:292–7.

10. Madsen PJ, Lang SS, Pisapia JM, Storm PB, Hurst RW, Heuer GG. An institutional series and literature review of pial arteriovenous fistulas in the pediatric population: Clinical article. J Neurosurg Pediatr. 2013; Oct. 12(4):344–50.
11. Maejima R, Ohshima T, Miyachi S, Matsuo N, Kawaguchi R, Takayasu M. Neonatal intracranial pial arteriovenous fistula treated with endovascular embolization: A case report. World Neurosurg. 2018; Oct. 118:261–4.

12. Mahmoud M, Abdalla RN, Mohamed AH, Farid M. Pial fistula in infancy: Report of two cases and literature review with special emphasis on the ruptured group. Interv Neuroradiol. 2018; Aug. 24(4):444–9.

13. Mao GH, Wang QH. Transvenous embolization with Onyx for a cerebral arteriovenous fistula originating from the distal lenticulostriate artery. Br J Neurosurg. 2013; Aug. 27(4):532–4.

14. Medhi G, Gupta AK, Saini J, Ramalingaiah AH, Pendharkar H, Parida S. Pial arteriovenous fistula: A clinical and neuro-interventional experience of outcomes in a rare entity. Indian J Radiol Imaging. 2020; Jul-Sep. 30(3):286–93.

15. Okazaki T, Sakamoto S, Ishii D, Oshita J, Matsushige T, Shinagawa K, et al. A pial arteriovenous fistula in infancy as the presenting manifestation of hereditary hemorrhagic telangiectasia. World Neurosurg. 2019; Feb. 122:322–5.

16. Orlov K, Gorbatykh A, Berestov V, Shayakhmetov T, Kislitsin D, Seleznev P, et al. Superselective transvenous embolization with Onyx and n-BCA for vein of Galen aneurysmal malformations with restricted transarterial access: Safety, efficacy, and technical aspects. Childs Nerv Syst. 2017; Nov. 33(11):2003–10.

17. Requejo F, Jaimovich R, Marelli J, Zuccaro G. Intracranial pial fistulas in pediatric population. Clinical features and treatment modalities. Childs Nerv Syst. 2015; Sep. 31(9):1509–14.

18. Terada A, Komiyama M, Ishiguro T, Niimi Y, Oishi H. Nationwide survey of pediatric intracranial arteriovenous shunts in Japan: Japanese Pediatric Arteriovenous Shunts Study (JPAS). J Neurosurg Pediatr. 2018; Nov. 22(5):550–8.

19. Tomycz L, Maris AS, Ghiassi M, Singer RJ. Open surgical disconnection for congenital, multi-hole, pial arteriovenous fistulae in non-eloquent cortex. Neurol India. 2012; Jul-Aug. 60(4):415–8.

20. Vasudevan K, Spader HS, Grossberg JA, Murphy T, Jayaraman MV. Successful endovascular treatment of a holo-hemispheric cerebral arteriovenous fistula in an infant. J Neurointerv Surg. 2012; Sep. 4(5):e26.

21. Viñuela F, Drake CG, Fox AJ, Pelz DM. Giant intracranial varices secondary to high-flow arteriovenous fistulae. J Neurosurg. 1987; Feb. 66(2):198–203.

22. Yamada H, Akiyama T, Kamamoto D, Yoshida K, Fukumura M, Toda M. Combined transarterial and transvenous embolization of multi-hole pial arteriovenous fistula with large varix. Neuroradiol J. 2022; Oct. 35(5):640–6.

23. Yamashita K, Ohe N, Yoshimura S, Iwama T. Intracranial pial arteriovenous fistula. Neurol Med Chir (Tokyo). 2007; Dec. 47(12):550–4.
Fig. 1.
Schematic highlighting our approach to multi-hole PAVFs. ① In the acute setting, the goal is to temporize the lesion with strategically targeted embolization toward the suspected site of rupture, often with flow control techniques such as temporary balloon occlusion, to prevent unwanted premature venous occlusion. ② Concomitantly or in subsequent sessions, serial flow reduction is achieved via TAE of all navigable arterial feeders. When staging this phase, smaller, often tortuous feeders, previously masked by high-flow competitors, can become newly angiographically apparent. Decreasing the time interval between stages minimizes the impact of these previously occult feeders on the treatment plan. This stage is completed when the lesion is rendered to the lowest flow state achievable by trans-arterial approach. ③ The sine qua non of curative venous embolization is the identification of the proximal venous point, and confirmation that the point is accessible to microcatheter navigation. On occasion, internal strictures may compartmentalize the anatomy, and may be best addressed by targeting each segment separately, functionally defining more than one proximal venous point (as in Fig. 4). The vein is then prepared by simplifying the final venous structure by targeting morphologic features that might impede the creation of the final occlusive cast. Eccentric varices are embolized, taking care to avoid premature compromise to the final venous outflow. Final exit strategies, often utilizing detachable tip microcatheters, are devised. ④ Curative embolization is then performed, typically with liquid embolic from proximal to distal. Onyx is often the agent of choice due to its long polymerization time. The cast is considered final when follow-up angiography of all vessel distributions supplying the lesion demonstrate complete occlusion. PAVF, pial arteriovenous fistula; TAE, transarterial endovascular embolization
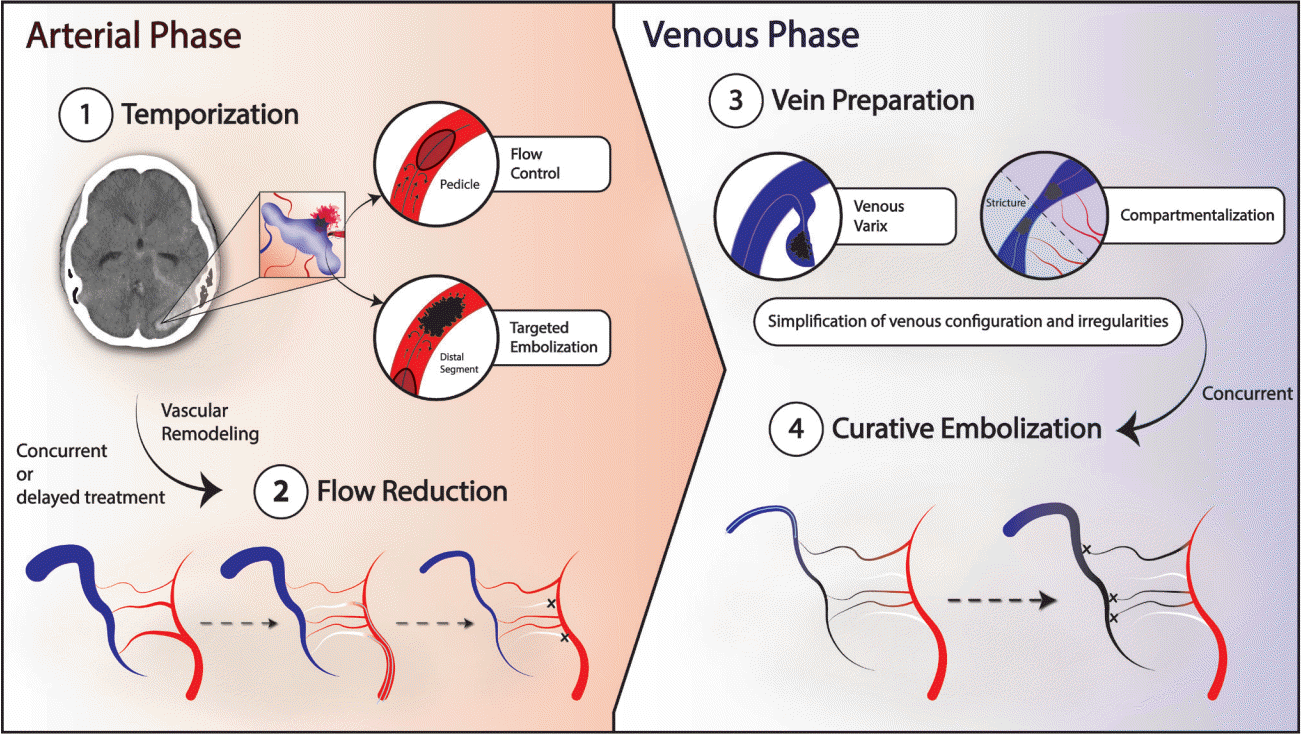
Fig. 2.
Schematic of case 2. (A) Ruptured multi-hole PAVF involving the basal right frontal lobe in a 17-month-old. (B) Emergent and subsequent staged TAE targeting high-flow navigable feeders with concentrated NBCA (glue casts in white). Innumerable non-navigable feeders not shown. (C) Trans-venous approach to prepare the vein, targeting an eccentric varix with coils and NBCA. (D) The fistulous vein was functionally separated by an internal stricture, creating a frontal compartment draining to the SSS and a sylvian compartment draining to
the transverse sinus. The compartments were treated one after the other with Onyx delivered by detachable tip microcatheters navigated to the proximal most element of each compartment at the internal stricture. (E) The final cast was created from proximal to distal in each compartment, resulting in a singular, completely occlusive cast. PAVF, pial arteriovenous fistula; TAE, transarterial endovascular embolization; NBCA, N-butyl cyanoacrylate; SSS, superior saggital sinus
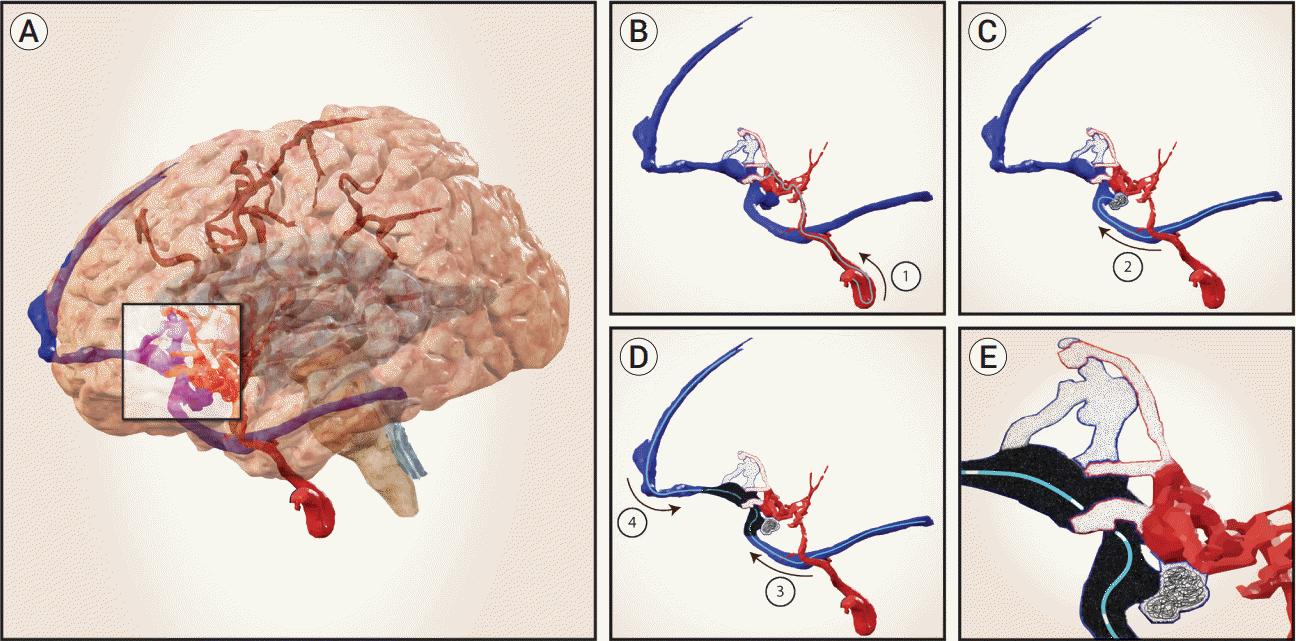
Fig. 3.
(A) AP vertebral artery arteriograms at baseline, (B) lowest flow state after serial TAE, and (C) 14-month follow-up after TVE demonstrating durable complete occlusion of the fistula (dashed line highlights venous cast). AP, anteroposterior; TAE, transarterial endovascular embolization; TVE, transvenous embolization
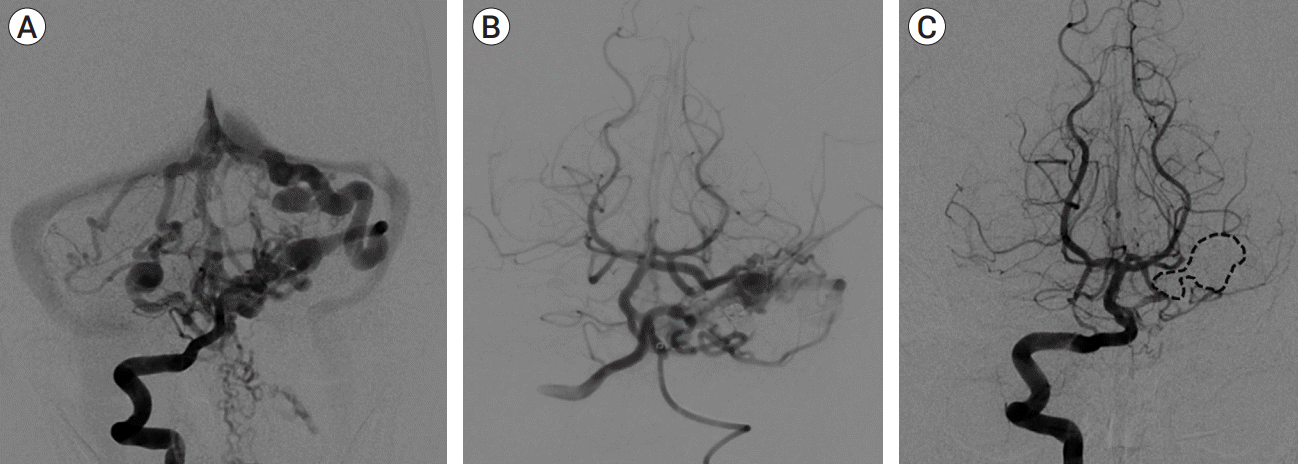
Fig. 4.
(A) AP right ICA arteriograms at baseline demonstrating severe, diffuse cerebral vasospasm and extensive fistulous drainage, (B) lowest flow state after serial TAE, (C) 18-month follow-up after TVE demonstrating durable complete occlusion of the fistula (dashed line highlights venous cast). AP, anteroposterior; ICA, internal carotid artery; TAE, transarterial endovascular embolization; TVE, transvenous embolization
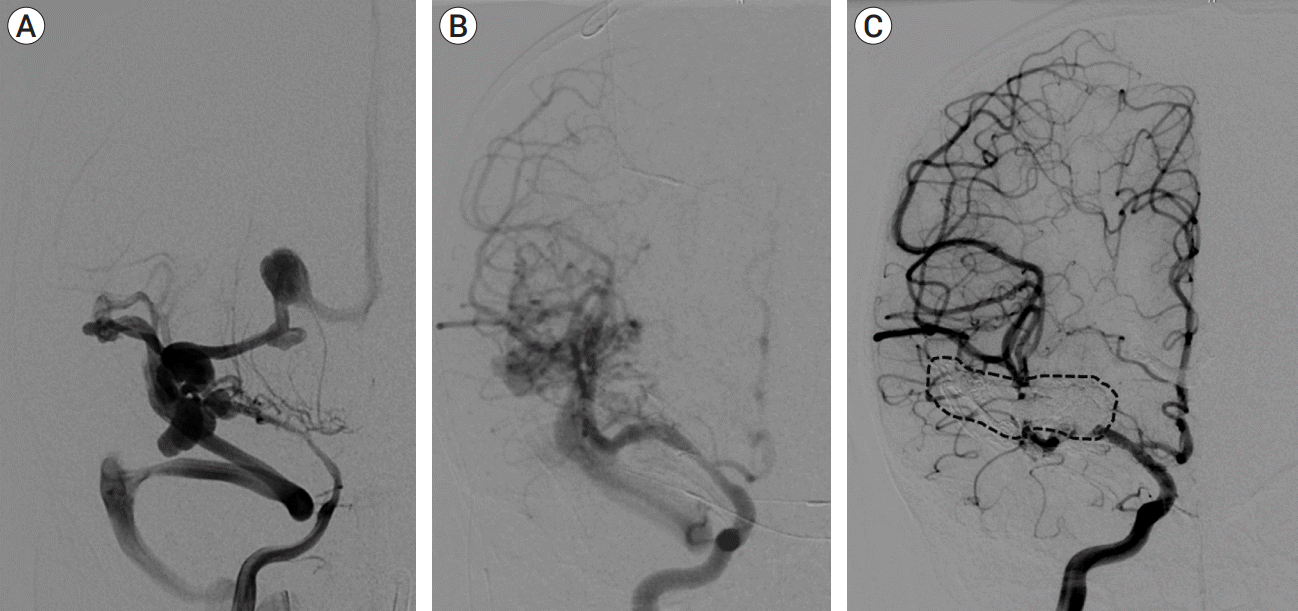
Fig. 5.
(A) Lateral vertebral artery arteriograms at baseline, (B) lowest flow state after TAE, and (C) 8-month follow-up after TVE demonstrating durable complete occlusion of the fistula (dashed line highlights venous cast). TAE, transarterial endovascular embolization; TVE, transvenous embolization
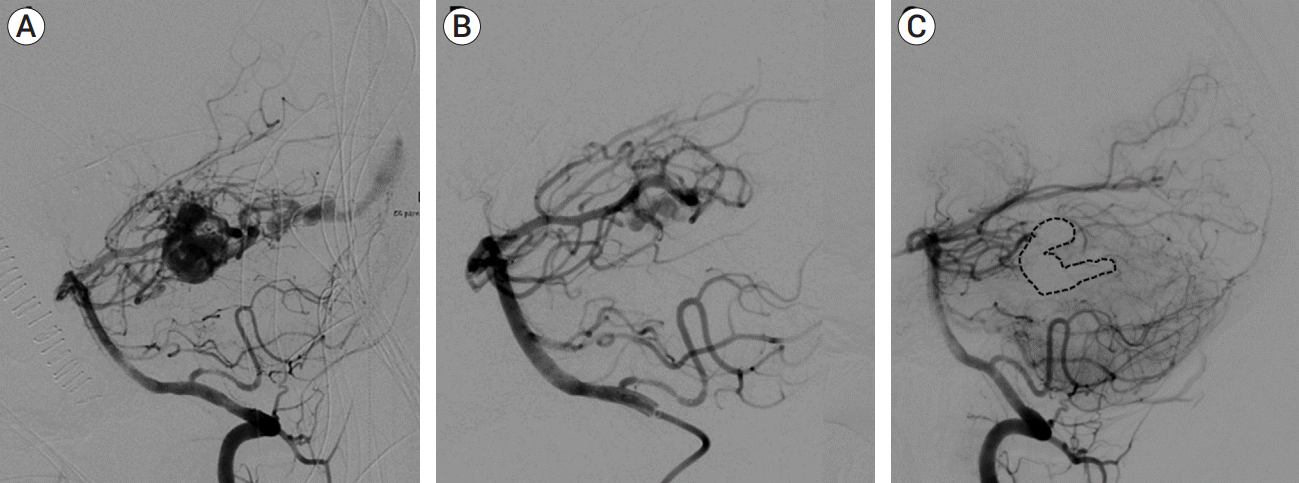
Fig. 6.
(A) Baseline axial T2 MRI demonstrating complex vascular malformation with variceal involvement of vein of Galen, hydrocephalus, and global volume loss. (B) Post-procedure axial CT demonstrating intraventricular hemorrhage and hydrocephalus. (C) Axial MRI DWI sequence demonstrating bilateral basal ganglia/internal capsule diffusion restriction secondary to thrombosis of the deep venous drainage (white arrows highlights areas of diffusion restriction). (D) 4-year follow-up axial T2 MRI demonstrating complete regression of previously dilated pathologic veins, normalization of cerebral volume, and no obvious persistent pathology. DWI, diffusion-weighted imaging
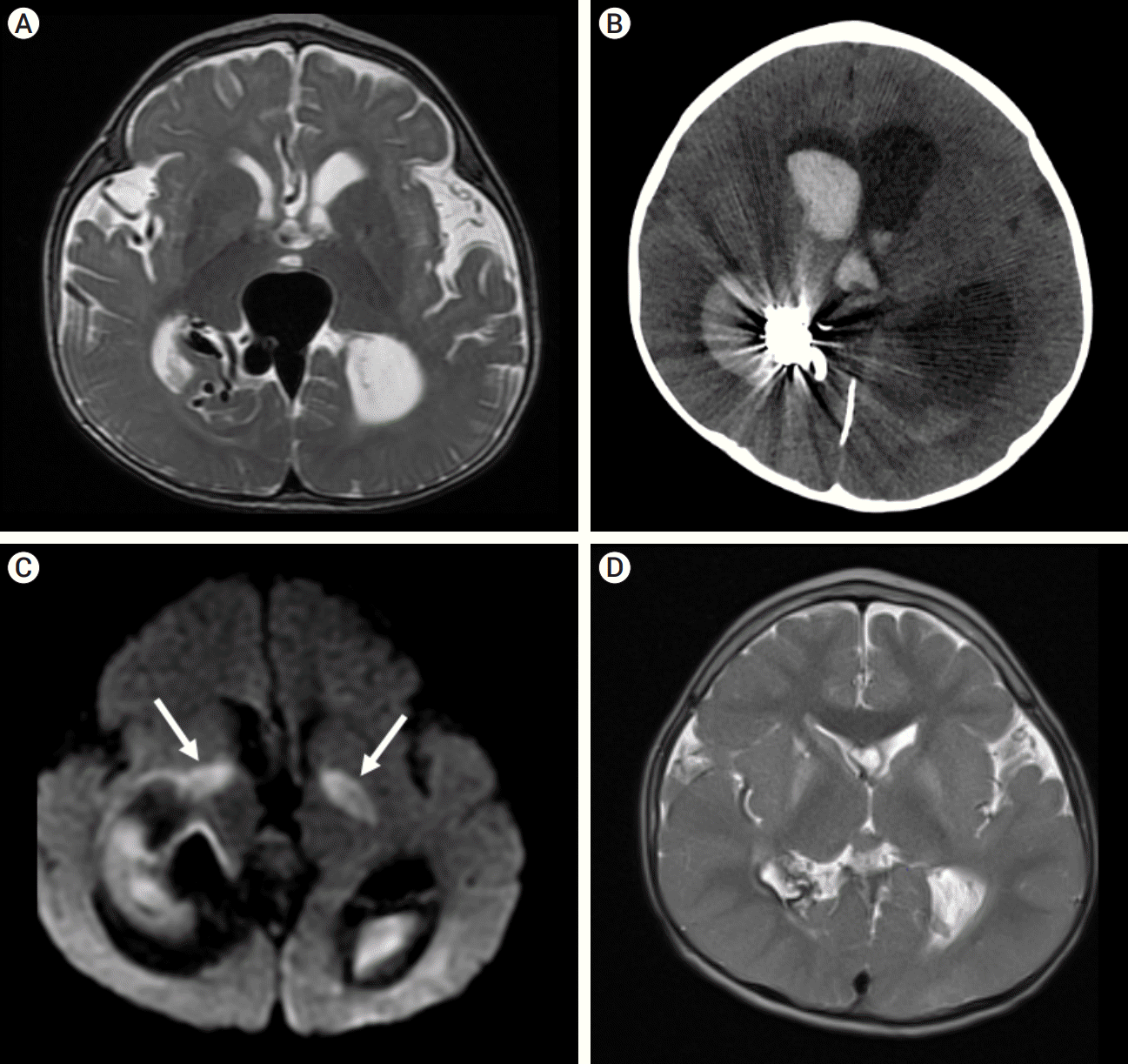
Fig. 7.
(A) AP right ICA arteriograms at baseline (B) lowest flow state after serial TAE, and (C) immediate post-treatment follow-up after TVE demonstrating apparent complete occlusion. (D) AP vertebral artery arteriograms at baseline, (E) lowest flow state after serial TAE, and (F) arterial phase immediate post-treatment follow-up after TVE. Dashed line highlights venous cast. (Continued in Fig. 8). AP, anteroposterior; ICA, internal carotid artery; TAE, transarterial endovascular embolization; TVE, transvenous embolization
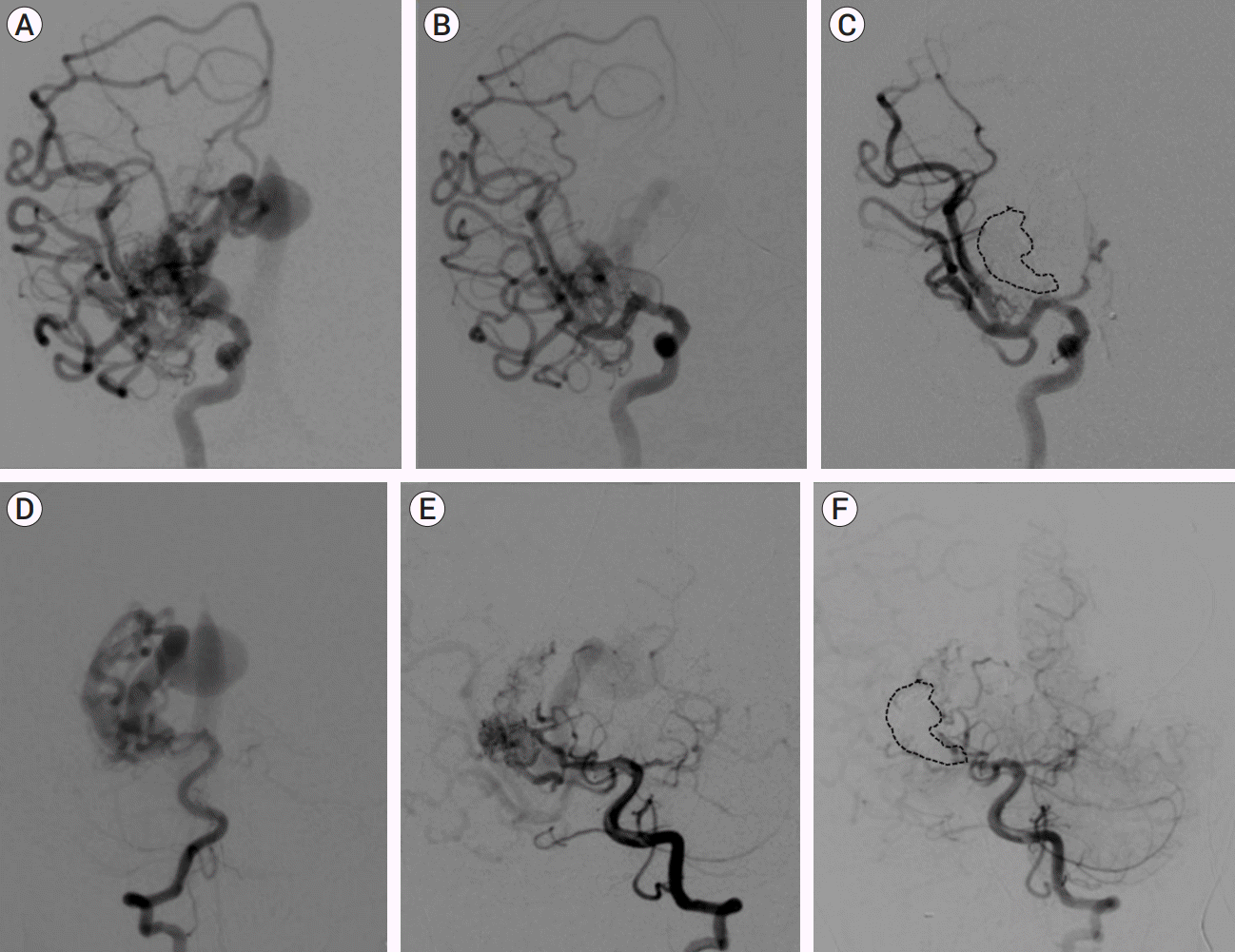
Fig. 8.
Immediate post-treatment AP vertebral artery injection, early venous phase. Dashed line indicates venous cast. Arrowheads indicate hyperemia and congestion in the choroid plexus. Arrow indicates persistent, early venous drainage signifying that the fistula was not fully occluded. AP, anteroposterior
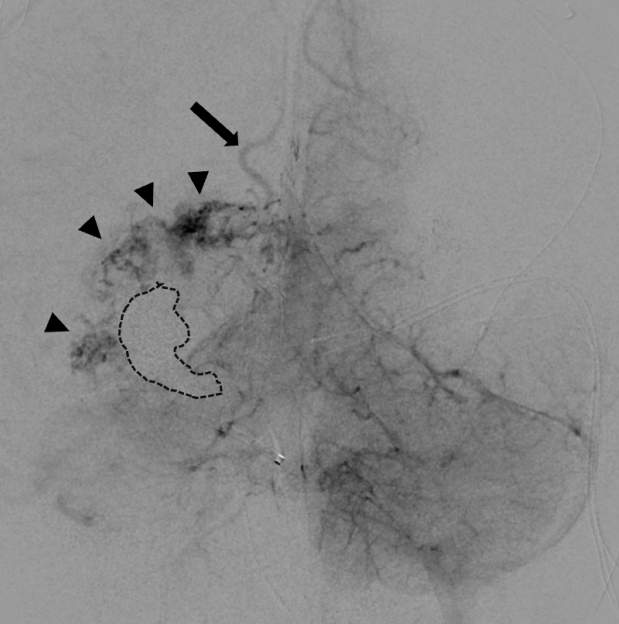
Table 1.
Demographic, clinical, and procedural characteristics
mRS, modified Rankin Scale; IPH, intraparenchymal hemorrhage; AICA, anterior inferior cerebellar artery; SCA, superior cerebellar artery; TAE, transarterial endovascular embolization; TVE, transvenous embolization; MCA, middle cerebral artery; ACA, anterior cerebral artery; SDH, subdural hemorrhage; PCA, posterior cerebral artery; IVH, intraventricular hemorrhage




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download