This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
The need for interoperability at the national level was highlighted in Korea, leading to a consensus on the importance of establishing national standards that align with international technological standards and reflect contemporary needs. This article aims to share insights into the background of the recent national health data standardization policy, the activities of the Health Data Standardization Taskforce, and the future direction of health data standardization in Korea.
Methods
To ensure health data interoperability, the Health Data Standardization Taskforce was jointly organized by the public and private sectors in December 2022. The taskforce operated three working groups. It reviewed international trends in interoperability standardization, assessed the current status of health data standardization, discussed its vision, mission, and strategies, engaged in short-term standardization activities, and established a governance system for standardization.
Results
On September 15, 2023, the notice of “Health Data Terminology and Transmission Standards” in Korea was thoroughly revised to improve the exchange of health information between information systems and ensure interoperability. This notice includes the Korea Core Data for Interoperability (KR CDI) and the Korea Core Data Transmission Standard (HL7 FHIR KR Core), which are outcomes of the taskforce’s efforts. Additionally, to reinforce the standardized governance system, the Health-Data Standardization Promotion Committee was established.
Conclusions
Active interest and support from medical informatics experts are needed for the development and widespread adoption of health data standards in Korea.
Go to :

Keywords: Standards, Health Information Exchange, Health Information Interoperability, Digital Health, Health Policy
I. Introduction
Digital transformation in healthcare has driven innovation worldwide. Changes in medical services have accelerated due to the widespread adoption of electronic health records (EHRs), personal health records (PHRs), and mobile health applications. In 2020, the World Health Organization established a digital health department and urged member countries to develop digital health strategies and promote policies. Standards and interoperability were emphasized in global, national, and regional digital health strategies [
1]. Health data standards are fundamental in healthcare information systems, facilitating seamless information exchange within the national health information infrastructure [
2]. These standards are crucial for sharing and utilizing data effectively. Establishing these standards can be challenging without national projects. Consequently, many countries are introducing national-level interoperability standards to support EHR and PHR projects.
Recently, there has been a growing recognition in Korea of the need for national standards to ensure interoperability and improve patient-centered health information exchange (HIE). There is also a consensus on the importance of adopting national standards that incorporate international standard technology and reflect current trends. On September 15, 2023, the Ministry of Health and Welfare (MOHW) fully revised the notice on “Health Data Terminology and Transmission Standards” in Korea. This revision aims to improve the exchange of health information between information systems by ensuring interoperability. The notice now includes the Korea Core Data for Interoperability (KR CDI) and the Korea Core Data Transmission Standard (KR Core).
The revision of the notice is a result of the efforts by the MOHW’s Health Data Standardization Taskforce, which was established in December 2022 to ensure interoperability through collaboration between the public and private sectors. In June 2023, the taskforce team updated the notice and conducted a verification process that included gathering feedback from the medical, industrial, and academic sectors.
This article aims to share recent activities of the Health Data Standardization Taskforce, outline the direction of health data standardization policy in Korea, and introduce the KR CDI and KR Core. Additionally, we will discuss overseas interoperability standardization trends, with a focus on the United States, which was readily accessible and frequently served as a benchmark for our standardization efforts, as well as the background of the recent national health data standardization policy.
Go to :

II. Health Data Standard Governance and Strategy to Ensure Interoperability
1. International Trends in Interoperability Standardization
The number of countries utilizing Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) has increased approximately fivefold over 16 years, from 9 to 48 in 2023 [
3]. Seventeen Organization for Economic Co-operation and Development (OECD) member countries have reported developing plans to enhance the interoperability of electronic medical records (EMRs). Additionally, 15 OECD member countries indicated plans to adopt the HL7 Fast Healthcare Interoperability Resource (FHIR) Standard, according to a 2021 report [
4]. The United States, Canada, the United Kingdom, Australia, the Netherlands, Finland, and Switzerland have incorporated HL7 FHIR release 4 (R4)-based transmission standards into their national projects [
4]. More than 20 European countries operate national PHR systems, and 22 countries implement an interoperability framework based on international standards such as SNOMED-CT, HL7 FHIR, and HL7 CDA R2 [
5]. According to a survey conducted by HL7 International across 32 countries, 13 countries have established a national FHIR data model, and another 13 are in the process of developing one [
6].
In 2004, the Institute of Medicine in the United States emphasized the need for a national health information infrastructure, encompassing systems, technologies, applications, standards, and policies, to improve patient safety. They also recommended that the federal government provide various support policies, including financial assistance, to establish this infrastructure [
2]. Additionally, they advised healthcare providers to invest in EHRs. These recommendations continue to guide U.S. health information policy. The Office of the National Coordinator for Health Information Technology (ONC), created by the Health Information Technology for Economic and Clinical Health Act in 2009, has since implemented a health data standardization management system. Starting in 2010, the Meaningful Use (MU) incentive program for EHRs was launched to encourage the adoption and exchange of health information [
7]. The 21st Century Cures Act, passed in 2016, further promotes the interoperability of electronic health information and includes regulations on information blocking, as well as provisions to standardize data usage. This act came into effect in 2021. In 2018, the MU incentive program for EHRs transitioned to the Promoting Interoperability program and was integrated into the payment system [
8]. Following the enactment of the 21st Century Cures Act, separate standardization committees were consolidated and are now operational. Additionally, the Health Information Technology Advisory Committee, a public-private partnership advisory committee, was established to provide the ONC with recommendations based on the Health IT strategic plan, aiming to establish an ONC-centered standardization promotion system. The transmission and exchange of electronic health information utilize a standard known as the US Core Data for Interoperability (USCDI) via an API using the HL7 FHIR. The USCDI, defined by the ONC, is a standardized set of health data classes and constituent data elements for nationwide interoperable health information exchange [
9]. It was officially adopted as a standard in the ONC Cures Act Final Rule in May 2020 and is updated annually. According to a 2021 survey, seven out of 10 hospitals enabled patients to access their health information using applications or software based on API technology. Furthermore, patients were able to review their clinical records through hospital portals at a rate of over 80% [
10].
2. Background and Previous Health Data Standardization Policy in Korea
Given the global trend toward digital transformation in healthcare, Korea has invested significantly in digital health technologies. Moreover, the rapid aging of the population and the COVID-19 pandemic have accelerated the development of an ecosystem that supports effective health data sharing and utilization. This includes the formulation of policies aimed at enhancing public health and stimulating growth in related industries. Consequently, laws and regulations concerning health data have been amended to facilitate their utilization.
In 2010, the Center for Interoperable Electronic Healthcare Record introduced a national terminology standard known as the Korea Standard Terminology of Medicine (KOSTOM). By 2014, it was recognized as a national health data standard and underwent irregular revisions until the end of 2022. The use of KOSTOM was optional, and only a few related national projects were in place, resulting in very low adoption rates [
11]. According to a national health informatization survey conducted by the MOHW in 2020, 78% of tertiary hospitals participated in the Standard-based HIE project. However, only 32% of hospital-level medical institutions were involved [
12]. Among tertiary hospitals, the adoption rate for Clinical Document Architecture (CDA) was 21%, while the adoption rate for FHIR stood at just 10% (
Figure 1).
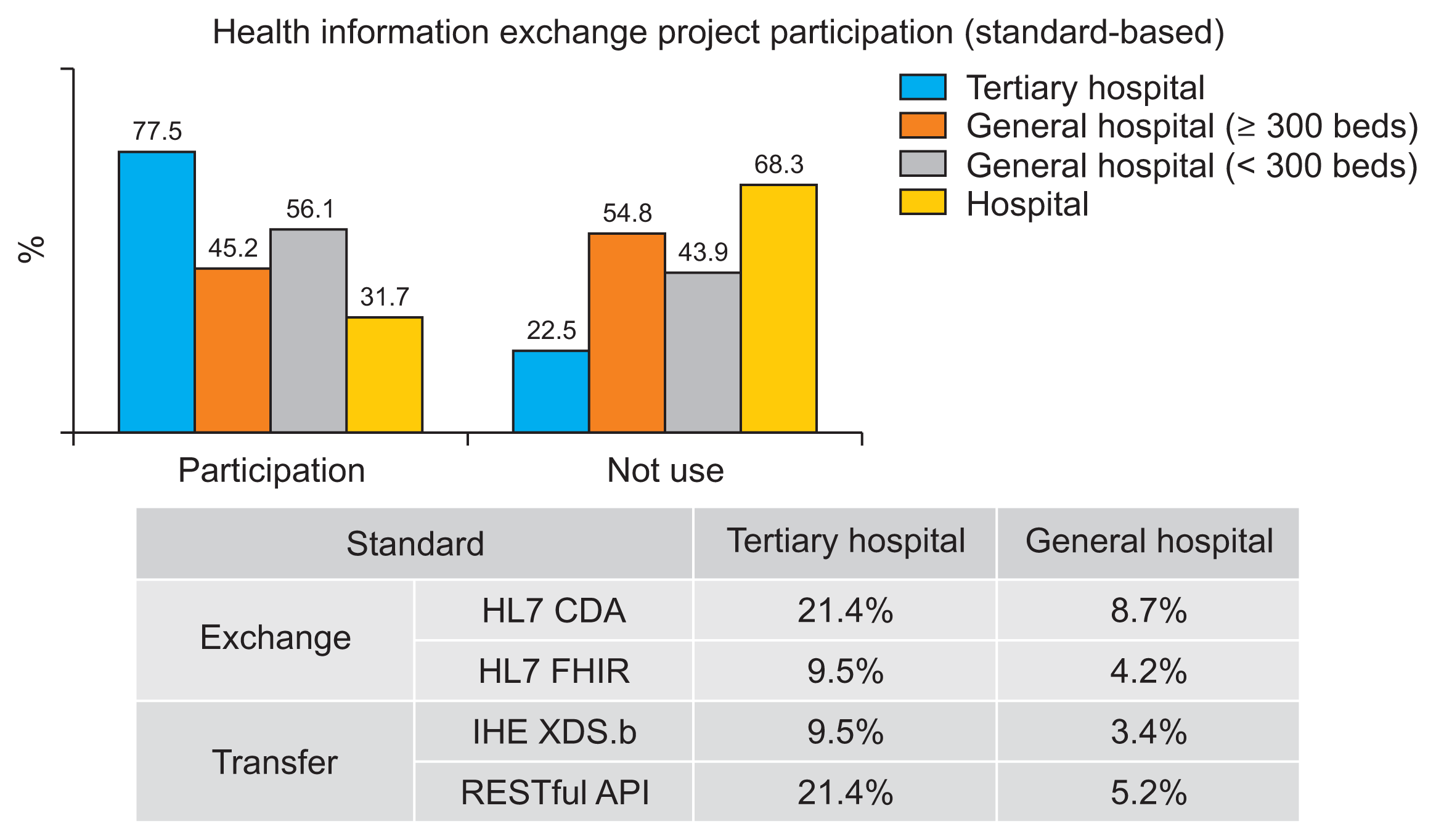 | Figure 1Status of health information exchange based on National Health Informatization Survey. Adapted from the Korea Health Information Service [ 12]. 
|
The MOHW oversees three principal projects related to health data standards: the EMR certification system, the HIE project, and a national PHR project known as MyHealthWay. Previously, no national transmission standards were in place, leading to the creation of individual standards that complicated the integration of national projects. Hospitals involved in these projects are required to adhere to various standards. Consequently, there is an increasing demand for a national health standard policy and advanced transmission standard technology to facilitate the use and sharing of health data.
To address the limitations of existing standardization policies, promote interoperability-based standardization, and establish a governance system for standardization, the Health Data Standardization Taskforce was formed. This taskforce aimed to propose a new health data standardization policy.
3. Health Data Standardization Taskforce Activities
The Health Data Standardization Taskforce, established in December 2022, is dedicated to setting standards for terminology and transmission technology. It also focuses on developing a standardization roadmap and an implementation strategy for use and dissemination in healthcare. The taskforce, co-chaired by representatives from both private and government agencies, includes 23 members. These members represent a diverse group of stakeholders from the medical and industrial sectors, including chief medical information officers, leaders in medical informatics and health information technology, and representatives from medical academia and public health agencies. Within the taskforce, three working groups (WGs) were formed: the CDI WG, the Next-Generation Technology WG, and the Governance WG, as depicted in
Figure 2. The specific roles of these WGs are detailed in
Figure 2.
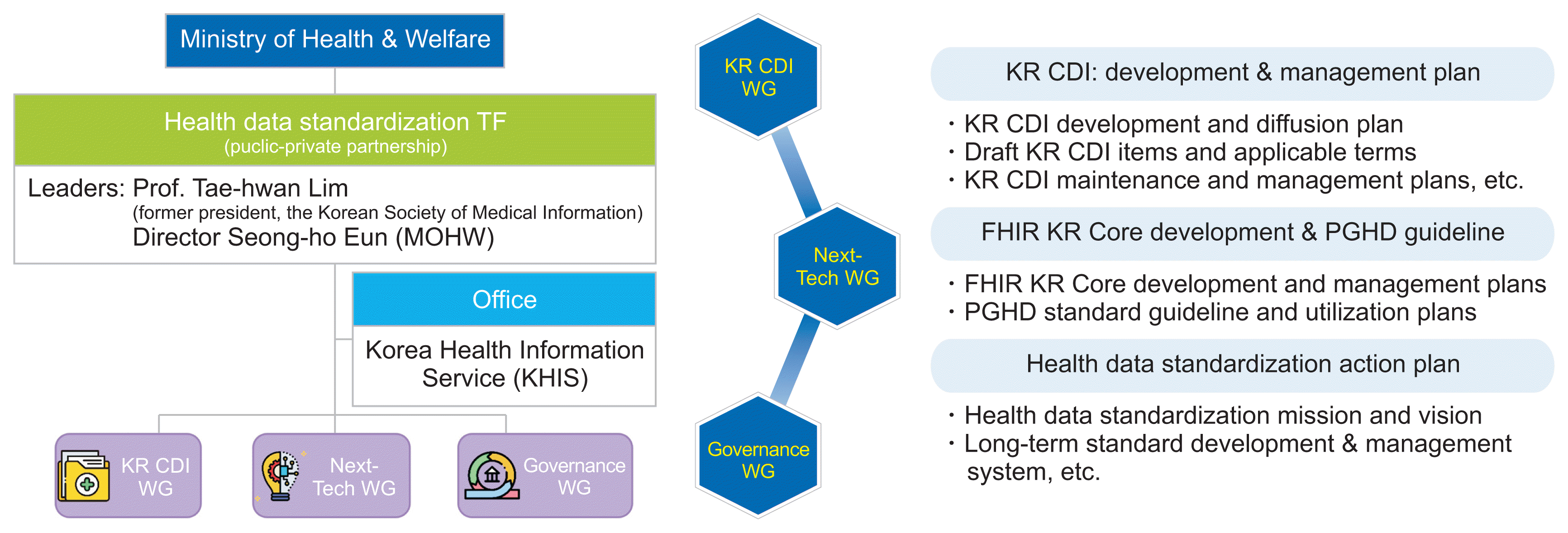 | Figure 2Organizational chart and roles of the Health Data Standardization Taskforce. KR CDI: Korea Core Data for Interoperability, MOHW: Ministry of Health and Welfare, WG: working group, PGHD: patient generated health data. 
|
The taskforce team evaluated the current status of health data standardization and discussed its vision, mission, and strategies. Over the course of 6 months, 37 meetings were held with a total attendance of 1,078 participants. During these meetings, a field-demand-based standardization promotion strategy was developed, along with health data standards (KR CDI, KR Core), and a plan to support the widespread adoption of these standards was formulated. In line with the global trend towards adopting international standards, the decision was made to discontinue updates to KOSTOM. By benchmarking advanced standardization models and gathering feedback from the medical community, key exchange items (KR CDI) and detailed specifications for their implementation (KR Core) were developed and subsequently announced. These standards are mandatory for projects based on standardization. The specific activities are depicted in
Figures 2 and
3.
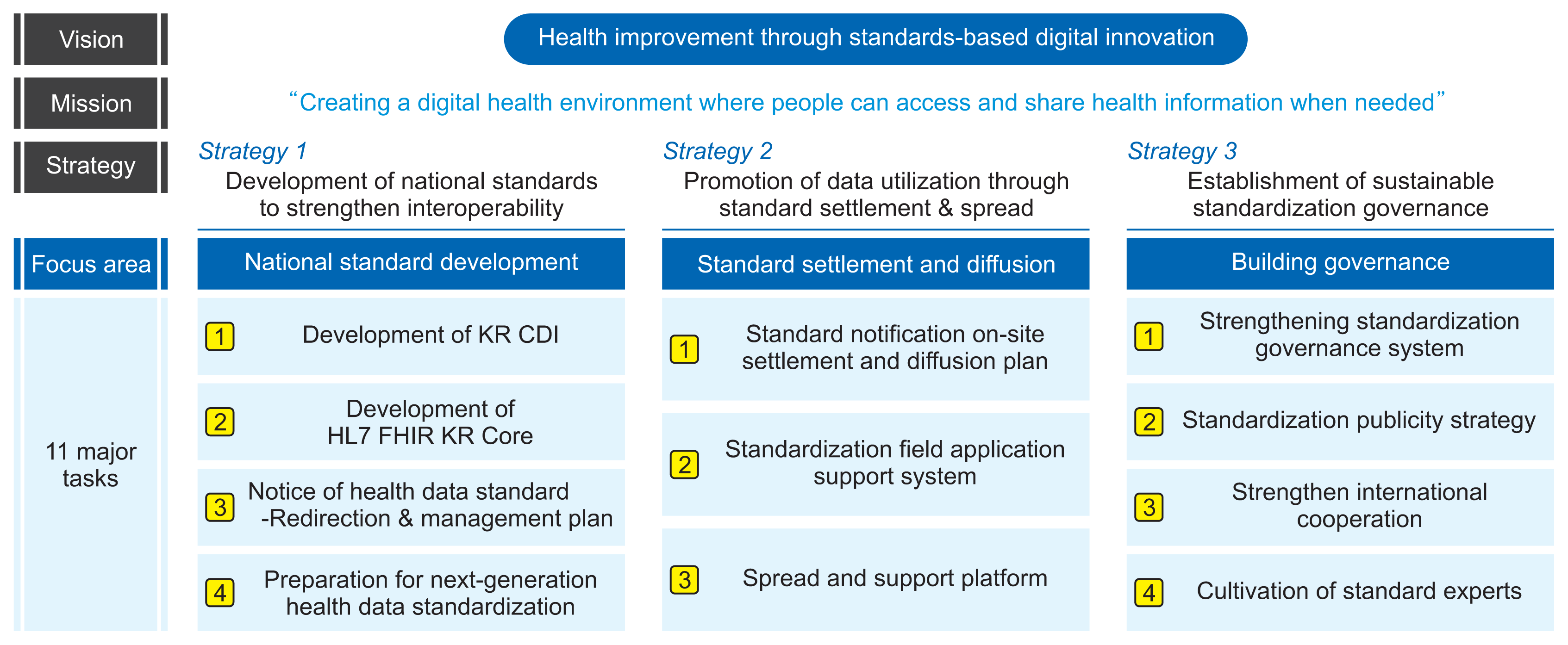 | Figure 3Vision, mission, and strategies of health data standardization. KR CDI: Korea Core Data for Interoperability, KR Core: Korea Core Data Transmission Standard. 
|
4. Strategy for Health Data Standardization
The taskforce proposed a 5-year mid- to long-term strategy along with detailed tasks to promote patient-centered HIE. Additionally, it was suggested that these tasks be undertaken by a permanent standardization organization. Following the discussions, the taskforce established the vision for health data standardization as “promoting public health improvement through standards-based digital innovation” and defined the mission as “creating a digital health environment where people can access and share health information when needed.”
Three strategies and 11 major tasks were selected (
Figure 3). Strategy 1 focuses on the “development of national standards to strengthen interoperability.” This includes the creation of the KR CDI, HL7 FHIR KR Core, health data standard notice, and addressing the needs of next-generation health data standardization. Strategy 2 aims to enhance data utilization through the establishment and diffusion of standards. This strategy includes tasks such as developing a plan for the establishment and dissemination of standard notices in the field, creating a support system for the field application of standardization, and setting up a standard diffusion and support platform. Strategy 3 centers on the “establishment of sustainable standardization governance.” It involves strengthening the standardization governance system, formulating a standardization promotion strategy, enhancing international cooperation, and developing expertise in standards.
5. Health Data Standardization Promotion Committee
To strengthen the standardized governance system, a Health Data Standardization Promotion Committee has been proposed as a permanent body to deliberate and decide on health data standardization policies. This committee includes experts from the medical field, industry, academia, and government agencies. It will develop a strategy for standard development and diffusion, oriented toward international standards to enhance the interoperability of health data. Additionally, the committee will prepare a standardization diffusion plan through the gradual application of standards in standardization-based projects and establish a corresponding legal framework. The committee oversees the Governance WG, KR CDI WG, and Standard Technology WG, which facilitate standard development and policy promotion (
Figure 4). The Industry Council and Standard Terminology Council will gather input from practitioners regarding the KR CDI, KR Core, and related topics.
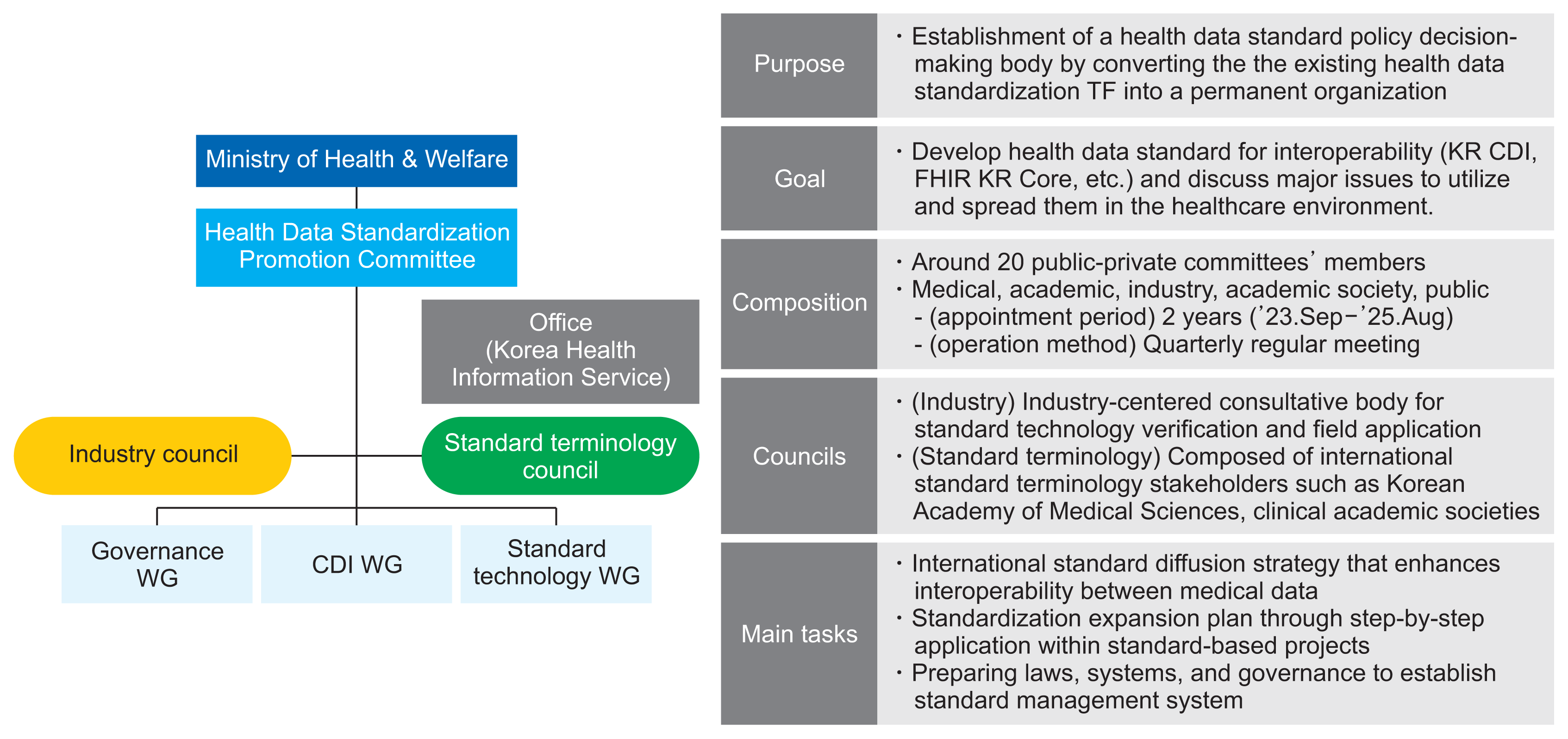 | Figure 4Organizational chart and roles of the Health Data Standardization Promotion Committee. KR CDI: Korea Core Data for Interoperability, KR Core: Korea Core Data Transmission Standard, WG: working group. 
|
Go to :

III. Development and Notification of Health Data Terminology and Transmission Standards
1. KR CDI
The KR CDI comprises a set of standard classifications, items, and terminology required for the exchange of information, tailored for the interoperability of domestic health data. The designated exchange standard for the KR CDI is HL7 FHIR R4, which is implemented through the development and distribution of FHIR KR Core. HL7 FHIR facilitates a flexible architecture that supports existing paradigms such as messaging and document exchange. Moreover, it allows for expansion into various computing environments, including mobile and cloud, through the adoption of web-based technology. While HL7 FHIR is globally recognized for health data exchange, it provides only the foundational standards. To effectively utilize these standards, each country must develop its own detailed FHIR standards at a national level that are universally applicable and tailored to specific needs. Consequently, conducting case studies is essential. Several countries have already developed detailed national FHIR specifications that are reflective of their unique medical environments and have successfully applied them to various use cases (
Table 1). As the exchange and utilization of health data become more prevalent in Korea, there is a pressing need to develop detailed FHIR specifications that are well-suited to the domestic medical environment. This necessitates the creation of a common core detailed standard (KR Core) that builds upon the KR CDI, the cornerstone of domestic interoperability (
Figure 5).
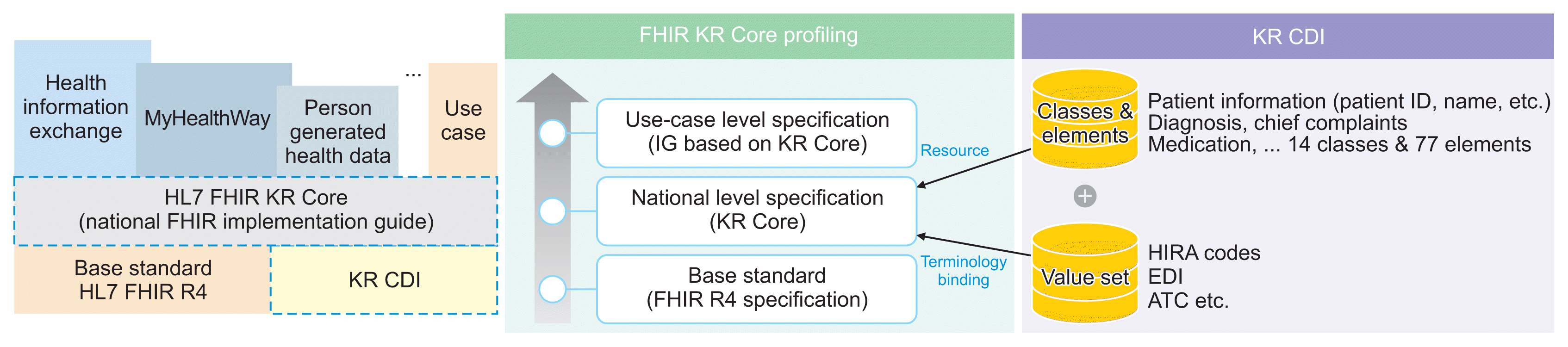 | Figure 5Linkage and utilization of FHIR-based KR CDI and KR Core-based profiling. KR CDI: Korea Core Data for Interoperability, KR Core: Korea Core Data Transmission Standard, HIRA: Health Insurance Review & Assessment Service, EDI: electronic data interchange, ATC: anatomic therapeutic chemical classification. 
|
Table 1
Selected FHIR National Implementation Guides (IGs) and use cases
|
Country |
National IG |
Use cases |
|
Unites States |
US Core |
SDOH Clinical Care |
|
Canada |
Canadian Baseline |
PS-CA |
|
United Kingdom |
UK Core |
NHS Genomic Service |
|
Australia |
AU Kore |
My Health Record (PHR) |
|
Netherland |
Netherland Base |
MedMij (PHR) |
|
Finland |
Finnish Base |
Kanta (PHR) |
|
Swiss |
Swiss Core |
Cancer Registry Law |

The KR CDI was developed by reviewing the health data exchange items from existing domestic and international health data standard projects and by gathering feedback on the essential health data items needed for information sharing. For domestic projects, we examined the HIE project, the MyHealthWay project, the FHIR KR Core Draft developed in 2021 [
13], and the second cycle of EMR certification standards (draft), and we also referred to the USCDI versions 1 through 3 (V1-3). The KR CDI draft was formulated and reviewed in January 2023, with input from stakeholders in the medical field. We identified two or more common classes and elements across health data projects. Several review consultations were then held with standard experts from the medical, academic, and industry sectors to discuss the feasibility of implementing the KR CDI, its suitability as a standard, and its integration with related standard projects. After finalizing the main content, as well as the class and item names, the development of KR CDI V1 was completed.
The KR CDI V1 consists of 14 classes (data classes), 77 items (data elements), item values (value sets), and specifies whether each item and item value is mandatory. A class groups related topics or items together. Items represent the smallest units defined for data exchange, while item values are sets of terms designed to convey the same meaning across different expressions, ensuring system interoperability. For instance, in the classes concerning diagnosis and chief complaint information, the items include the diagnosis date, diagnosis name, clinical status at diagnosis, date of the chief complaint, chief complaint name, and clinical status of the chief complaint (
Table 2). The diagnosis date, name, and clinical status at diagnosis are mandatory. The KR CDI notice includes a detailed manual that provides examples of classes and items [
14].
Table 2
Examples of KR CDI version 1 (diagnosis and chief complaint information)
|
Number |
Class |
Element |
Value set |
|
|
|
Name |
Required |
Value set term |
Required |
Value set |
|
5 |
Diagnosis and chief complaint information |
Diagnosis date |
R |
- |
- |
- |
|
Diagnosis name |
R |
KCD-8 |
R |
- |
|
Clinical status at diagnosis |
R2 |
FHIR Condition-ClinicalStatus-Codes |
R2 |
(Value set) http://hl7.org/fhir/R4B/valueset-condition-clinical.html
(Value) active, recurrence, relapse, inactive, remission, resolved |
|
|
Chief complaint date |
O |
- |
- |
- |
|
Chief complaint name |
O |
- |
- |
- |
|
Clinical status at chief complaint |
O |
FHIR Condition-ClinicalStatus-Codes |
O |
(Value set) http://hl7.org/fhir/R4B/valueset-condition-clinical.html
(Value) active, recurrence, relapse, inactive, remission, resolved |

2. FHIR KR Core V1 Publishing and Management System
FHIR KR Core is the Korean version of FHIR’s Implementation Guide (IG). It is a comprehensive standard that specifies the minimum constraints on FHIR resources and APIs for exchanging domestic health data in line with the requirements of the KR CDI. In December 2021, after analyzing the detailed FHIR specifications from leading countries such as the US Core of the United States and the AU Base of Australia, the FHIR Core Draft version was developed. This version profiles items that are frequently used in the domestic healthcare environment among FHIR resources. After discussions on the KR CDI linkage, standard notice and management plan, FHIR security, and collaboration with HL7 Korea, the Next-Generation Technology WG officially released the FHIR KR Core v1 at the end of June 2023. The announcement and distribution of the FHIR KR Core were conducted through the official HL7 standard publishing process, in collaboration with HL7 Korea. The proposal for the FHIR KR Core standard was submitted via HL7 Korea (Ballot), and feedback was gathered through a two-week initial round of member voting (balloting). The final adjustments were made based on feedback from developers’ key concerns raised during the Connectathon process and the initial ballot review. Out of 55 eligible votes, excluding 6 abstentions, 47 votes (95%) supported the standard, surpassing the 60% threshold required for approval, leading to the official publication and approval of the FHIR KR Core.
In FHIR KR Core V1, 27 profiles based on FHIR R4 resources were defined (profiled) according to the KR CDI requirements (
Table 3). The notice outlines the method for resource exchange via the FHIR API and specifies constraints to ensure compliance with the FHIR KR Core. Technical documents and manuals detailing the definitions and usage guidelines for the KR Core components are available through links and are presented on web pages that adhere to the HL7 standard template.
Table 3
27 profiles of KR Core V1 and corresponding 14 data classes in KR CDI
|
Number |
KR CDI class |
KR Core profile |
|
1 |
Patient information |
KR Core Patient Profile |
|
|
2 |
Practitioner & role information |
KR Core Practitioner Profile for Medical Doctor |
|
3 |
|
KR Core PractitionerRole Profile for Medical Doctor |
|
|
4 |
Healthcare organization information |
KR Core Healthcare Organization Profile |
|
|
5 |
Encounter information |
KR Core Encounter Profile |
|
|
6 |
Diagnosis and chief complaint information |
KR Core Condition Profile for Encounter Diagnosis |
|
7 |
|
KR Core Condition Profile for Chief Complaint |
|
|
8 |
Surgery and procedure information |
KR Core Procedure Profile |
|
|
9 |
Medication information |
KR Core Medication Profile |
|
10 |
|
KR Core MedicationRequest Profile |
|
|
11 |
Laboratory test information |
KR Core DiagnosticReport Profile for Laboratory Results |
|
12 |
|
KR Core Observation Profile for Laboratory Result |
|
|
13 |
Pathology test information |
KR Core DiagnosticReportProfile for Pathology Results |
|
|
14 |
Diagnostic imaging information |
KR Core DiagnosticReport Profile for Diagnostic Imaging |
|
15 |
|
KR Core ImagingStudy Profile |
|
|
16 |
Function test information |
KR Core DiagnosticReport Profile for Function Tests |
|
17 |
|
KR Core Observation Profile for Function Test |
|
|
18 |
Vital signs & physical measurement information |
KR Core Observation Profile for Vital Signs |
|
19 |
|
KR Core Observation Profile for Vital Signs: Blood Pressure |
|
20 |
|
KR Core Observation Profile for Vital Signs: Body Height |
|
21 |
|
KR Core Observation Profile for Vital Signs: Body Temperature |
|
22 |
|
KR Core Observation Profile for Vital Signs: Body Weight |
|
23 |
|
KR Core Observation Profile for Vital Signs: Heart Rate |
|
24 |
|
KR Core Observation Profile for Vital Signs: Pulse Oximetry |
|
25 |
|
KR Core Observation Profile for Vital Signs: Respiratory Rate |
|
|
26 |
Allergy intolerance |
KR Core AllergyIntolerance Profile |
|
|
27 |
Immunization |
KR Core Immunization Profile |

3. Revision of the Health Data Standard Notice
To ensure health data interoperability and foster an ecosystem for its utilization, the taskforce team updated the existing “Health Terminology Standard” notice to the “Health Data Terminology and Transmission Standard” notice. This revision incorporates key items related to the exchange of health data, including transmission technology and management systems. The revised notice now includes the KR CDI, FHIR KR Core, and a standard data management system (
Figure 6). The United States’ standards development and management procedures are systematic and well-established. The standard management system was developed by referencing the US model. The US ONC operates the Standards Version Advancement Process to adopt and announce both current and future standards [
15]. The latest versions of the USCDI and US Core are subject to a period for collecting public opinion, after which the approved standards are announced and implemented. Similarly, the revised notice will be updated by collecting feedback from stakeholders to develop standards. The completed standard notice revision (draft) will be presented to the committee, and the revised notice will be disseminated promptly. Detailed information about health data standards and the “Health Data Terminology and Transmission Standard” notice will be available on the Health Information Standards System website.
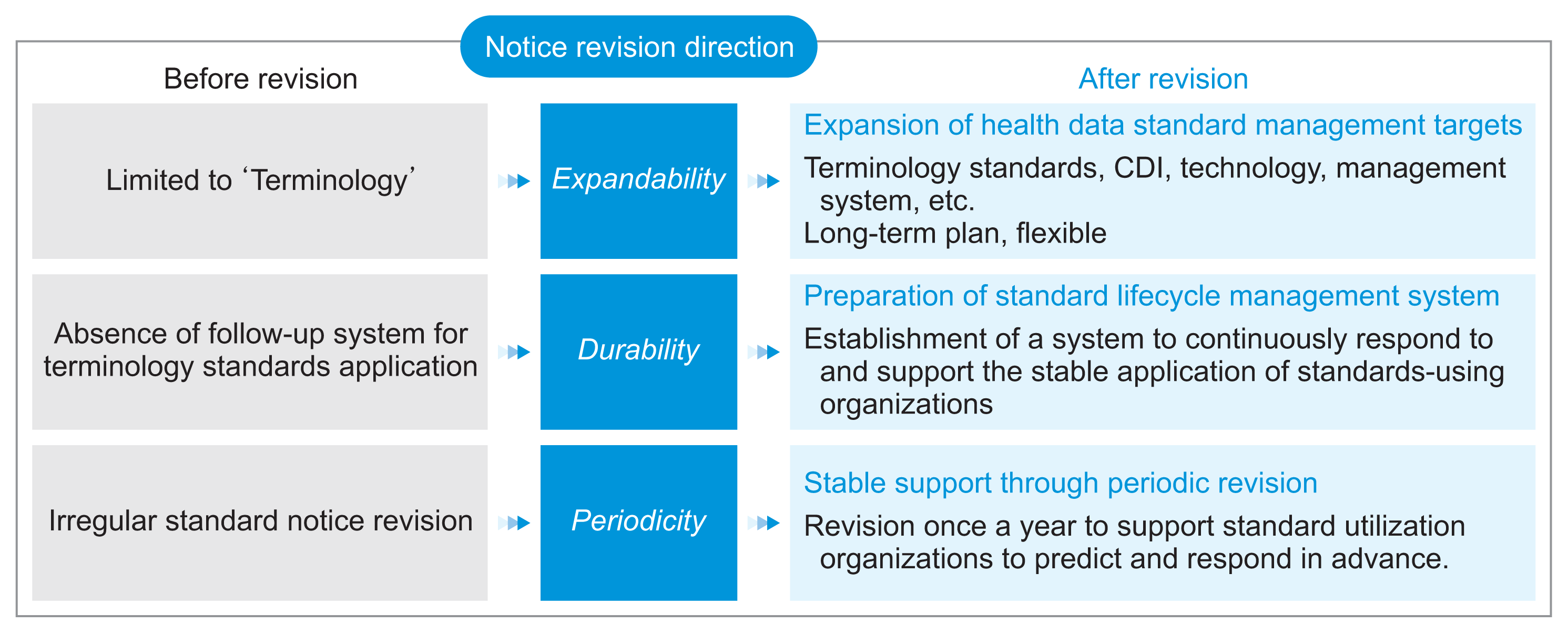 | Figure 6Directions of the Health Data Terminology and Transmission Standards Notice. CDI: Core Data for Interoperability. 
|
Go to :

IV. Discussion
The Health Data Standardization Promotion Committee has initiated strategic tasks aimed at continuing the standardization of health data and building consensus. Customized public relations strategies for stakeholders have been developed and are now being implemented. Processes for the proposal, review, and approval of CDIs have been established. The KR CDI version upgrade is underway to enhance the coverage of interoperable data. The KR CDI, FHIR KR Core, and new standards will be developed and periodically updated within the standard data management system. The MyHealthWay IG was published in December 2023, based on the FHIR KR Core [
16]. A standard guideline for patient-generated health data is set to be announced in 2024.
In medical informatics, standards are a traditional and longstanding topic; however, their adoption has posed significant challenges. In recent years, the development of national EHR systems in the US, EU, and OECD member countries, along with the introduction of patient portals and the rapid expansion of PHR services, have been closely linked to the proliferation of health information transmission standards [
4–
10]. The EHR certification system, the HIE project, the MyHealthWay project, and other current and future initiatives that utilize health data and are promoted by the government, must ensure the exchange of health information. Therefore, it is necessary to establish and manage standards at the national level. Plans are underway to broaden the scope of these standards, including increasing the involvement of relevant government agencies, expanding councils and expert groups, analyzing foreign legal and compensation systems, and enhancing legal frameworks. Although the standardization policy was initiated slightly later than similar international efforts, it is being implemented both systematically and rapidly. Active interest and support from medical informatics experts are essential for the development and widespread adoption of health data standards.
Go to :

Notes
Go to :

Acknowledgments
This research is partly based on the KOSMI Issue Report (2023), supported by the Ministry of Health and Welfare, Republic of Korea.
This article was written based on materials provided by the Health Data Standardization Taskforce. We thank the Ministry of Health and Welfare and the Korea Health Information Service for their support.
Go to :

References
2. Institute of Medicine (US) Committee on Data Standards for Patient Safety. Patient safety: achieving a new standard for care. Washington (DC): National Academies Press;2004.
3. SNOMED International. Members of SNOMED International [Internet]. London, UK: SNOMED International;c2024. [cited at 2024 Apr 30]. Available from:
https://www.snomed.org/members.
4. Slawomirski L, Lindner L, de Bienassis K, Haywood P, Hashiguchi TC, Steentjes M, et al. Progress on implementing and using electronic health record systems: developments in OECD countries as of 2021 (OECD Health Working Papers 160. Paris, France: OECD Publishing;2023.
https://doi.org/10.1787/4f4ce846-en.

7. Modi S, Feldman SS. The value of electronic health records since the Health Information Technology for Economic and Clinical Health Act: systematic review. JMIR Med Inform. 2022; 10(9):e37283.
https://doi.org/10.2196/37283.

8. Gettinger A, Zayas-Caban T. HITECH to 21st century cures: clinician burden and evolving health IT policy. J Am Med Inform Assoc. 2021; 28(5):1022–5.
https://doi.org/10.1093/jamia/ocaa330.

9. Gordon WJ, Gottlieb D, Kreda D, Mandel JC, Mandl KD, Kohane IS. Patient-led data sharing for clinical bioinformatics research: USCDI and beyond. J Am Med Inform Assoc. 2021; 28(10):2298–300.
https://doi.org/10.1093/jamia/ocab133.

11. Lee JH, Lee HY, Lee SY, Kwon AK, Lee GJ, Sung YN, et al. Standardization project and direction to ensure interoperability of healthcare data [Internet]. Seoul, Korea: KOSMI Issue Report;2023. [cited at 2024 Apr 26]. Available from:
https://www.maillink.co.kr/agency/html/20231229/REPORT6.pdf.
16. Korea Health Information Service. MyHealthWay [Internet]. Seoul, Korea: Korea Health Information Service;2023. [cited at 2024 Apr 30]. Available from:
https://hins.or.kr/fhir/MyHealthWay/.
Go to :







 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download