1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016; 387:1513–30.
2. Choi HH, Choi G, Yoon H, Ha KH, Kim DJ. Rising incidence of diabetes in young adults in South Korea: a national cohort study. Diabetes Metab J. 2022; 46:803–7.

3. Zhang X, Wu H, Fan B, Shi M, Lau ES, Yang A, et al. Lifetime risk of developing diabetes in Chinese people with normoglycemia or prediabetes: a modeling study. PLoS Med. 2022; 19:e1004045.

4. Kim SM, Lee G, Choi S, Kim K, Jeong SM, Son JS, et al. Association of early-onset diabetes, prediabetes and early glycaemic recovery with the risk of all-cause and cardiovascular mortality. Diabetologia. 2020; 63:2305–14.

5. Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu XO, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA Netw Open. 2019; 2:e192696.
6. Chan JC, So W, Ma RC, Tong PC, Wong R, Yang X. The complexity of vascular and non-vascular complications of diabetes: the Hong Kong diabetes registry. Curr Cardiovasc Risk Rep. 2011; 5:230–9.

7. Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, et al. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med. 2014; 127:616–24.

8. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011; 364:829–41.

9. Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021; 396:2019–82.

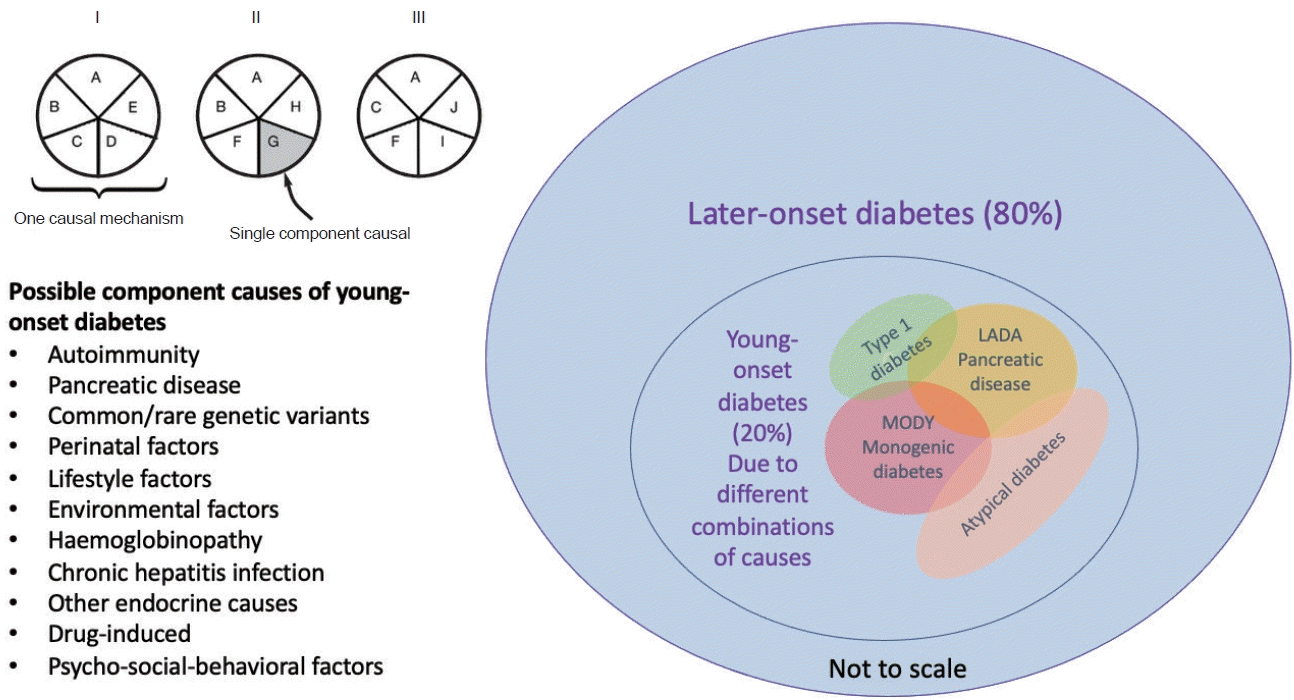
10. Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, et al. Current insights and emerging trends in earlyonset type 2 diabetes. Lancet Diabetes Endocrinol. 2023; 11:768–82.

11. Fan Y, Lau ES, Wu H, Yang A, Chow E, So WY, et al. Incidence of long-term diabetes complications and mortality in youth-onset type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2022; 191:110030.

12. Nanayakkara N, Curtis AJ, Heritier S, Gadowski AM, Pavkov ME, Kenealy T, et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia. 2021; 64:275–87.

13. Looker HC, Chang DC, Baier LJ, Hanson RL, Nelson RG. Diagnostic criteria and etiopathogenesis of type 2 diabetes and its complications: lessons from the Pima Indians. Presse Med. 2023; 52:104176.

14. Laakso M, Pyorala K. Age of onset and type of diabetes. Diabetes Care. 1985; 8:114–7.

15. Chan JC, Cheung CK, Swaminathan R, Nicholls MG, Cockram CS. Obesity, albuminuria and hypertension among Hong Kong Chinese with non-insulin-dependent diabetes mellitus (NIDDM). Postgrad Med J. 1993; 69:204–10.

16. Ng MC, Lee SC, Ko GT, Li JK, So WY, Hashim Y, et al. Familial early-onset type 2 diabetes in Chinese patients: obesity and genetics have more significant roles than autoimmunity. Diabetes Care. 2001; 24:663–71.
17. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–40.
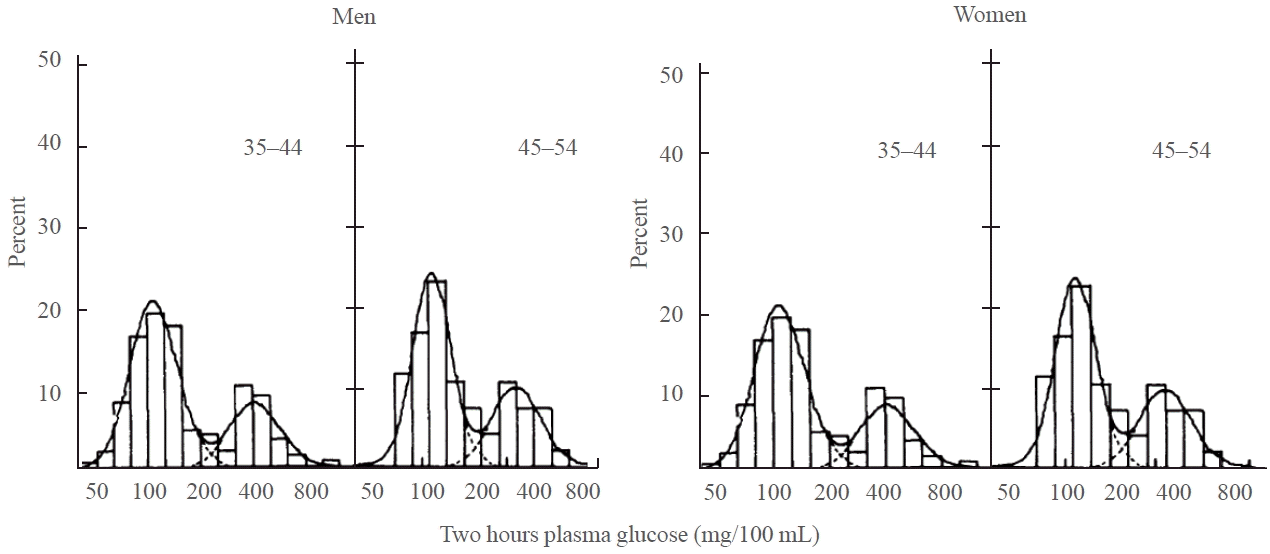
18. Rushforth NB, Bennett PH, Steinberg AG, Burch TA, Miller M. Diabetes in the Pima Indians: evidence of bimodality in glucose tolerance distributions. Diabetes. 1971; 20:756–65.

19. Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y, et al. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia. 2000; 43:1470–5.

20. Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: systematic review and meta-analysis. PLoS One. 2017; 12:e0171315.

21. Bergman M, Abdul-Ghani M, Neves JS, Monteiro MP, Medina JL, Dorcely B, et al. Pitfalls of HbA1c in the diagnosis of diabetes. J Clin Endocrinol Metab. 2020; 105:2803–11.

22. Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, et al. Global prevalence of prediabetes. Diabetes Care. 2023; 46:1388–94.

23. Ko GT, Chan JC, Chan AW, Wong PT, Hui SS, Tong SD, et al. Low levels of awareness of suboptimal health conditions in a high-risk working population: the “better health for better Hong Kong” health promotion campaign. Int J Behav Med. 2007; 14:63–9.

24. Li JK, Ng MC, So WY, Chiu CK, Ozaki R, Tong PC, et al. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2006; 22:46–52.

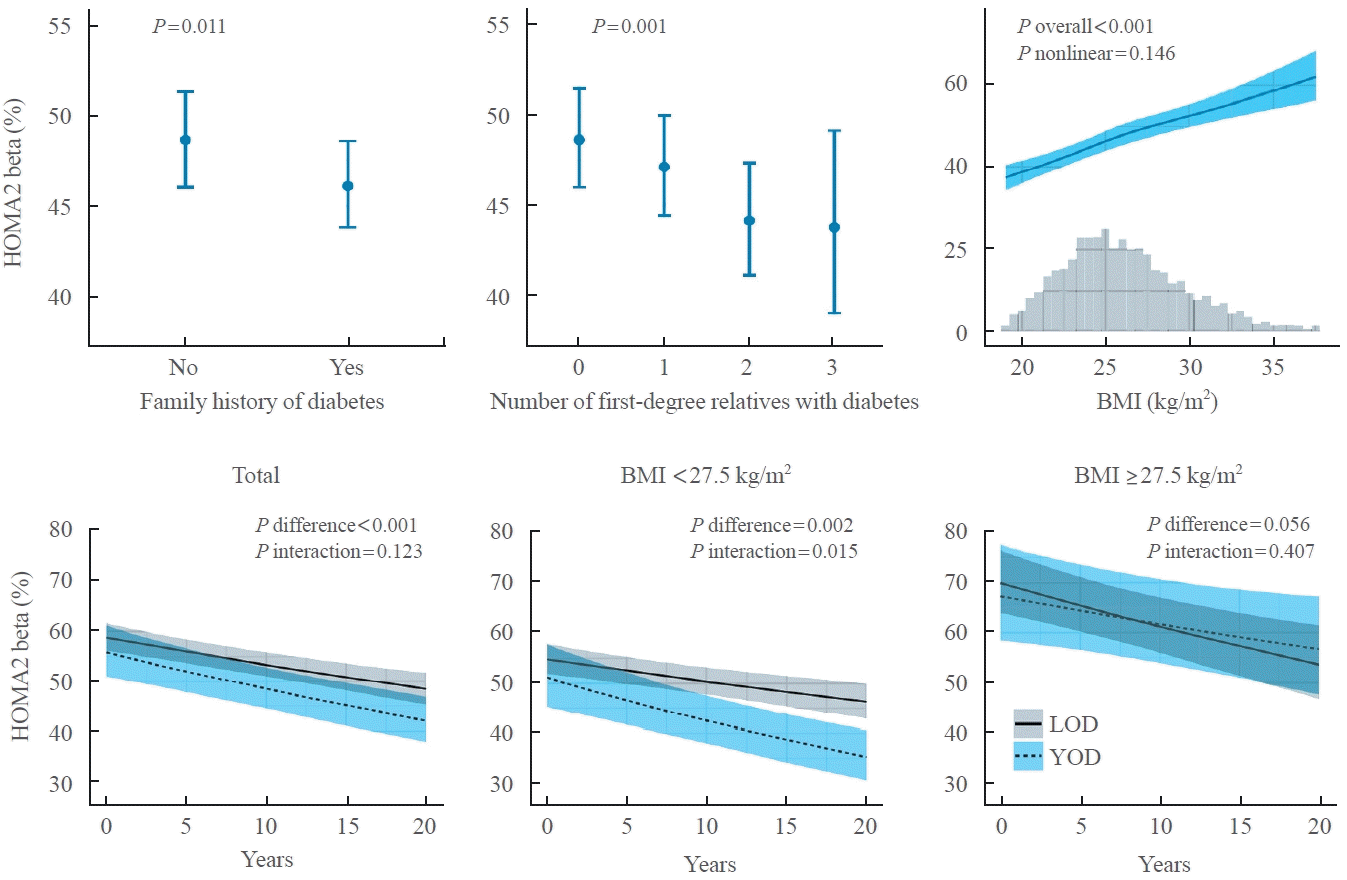
25. Zhang Y, Luk AO, Chow E, Ko GT, Chan MH, Ng M, et al. High risk of conversion to diabetes in first-degree relatives of individuals with young-onset type 2 diabetes: a 12-year follow-up analysis. Diabet Med. 2017; 34:1701–9.

26. Sathish T, Khunti K, Narayan KM, Mohan V, Davies MJ, Yates T, et al. Effect of conventional lifestyle interventions on type 2 diabetes incidence by glucose-defined prediabetes phenotype: an individual participant data meta-analysis of randomized controlled trials. Diabetes Care. 2023; 46:1903–7.

27. Bergman M, Buysschaert M, Ceriello A, Hussain A, Mohan V, Sesti G, et al. Current diagnostic criteria identify risk for type 2 diabetes too late. Lancet Diabetes Endocrinol. 2023; 11:224–6.

28. Herman WH, Ye W. Precision prevention of diabetes. Diabetes Care. 2023; 46:1894–6.

29. Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, et al. Impaired glucose tolerance as a disorder of insulin action: longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988; 318:1217–25.

30. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and metaanalysis. Diabetes Care. 2013; 36:1789–96.
31. Yabe D, Seino Y. Type 2 diabetes via β-cell dysfunction in east Asian people. Lancet Diabetes Endocrinol. 2016; 4:2–3.

32. Chow EY, Chan JC. Insulin resistance versus β-cell dysfunction in type 2 diabetes: where public and personalised health meet. Lancet Diabetes Endocrinol. 2020; 8:92–3.

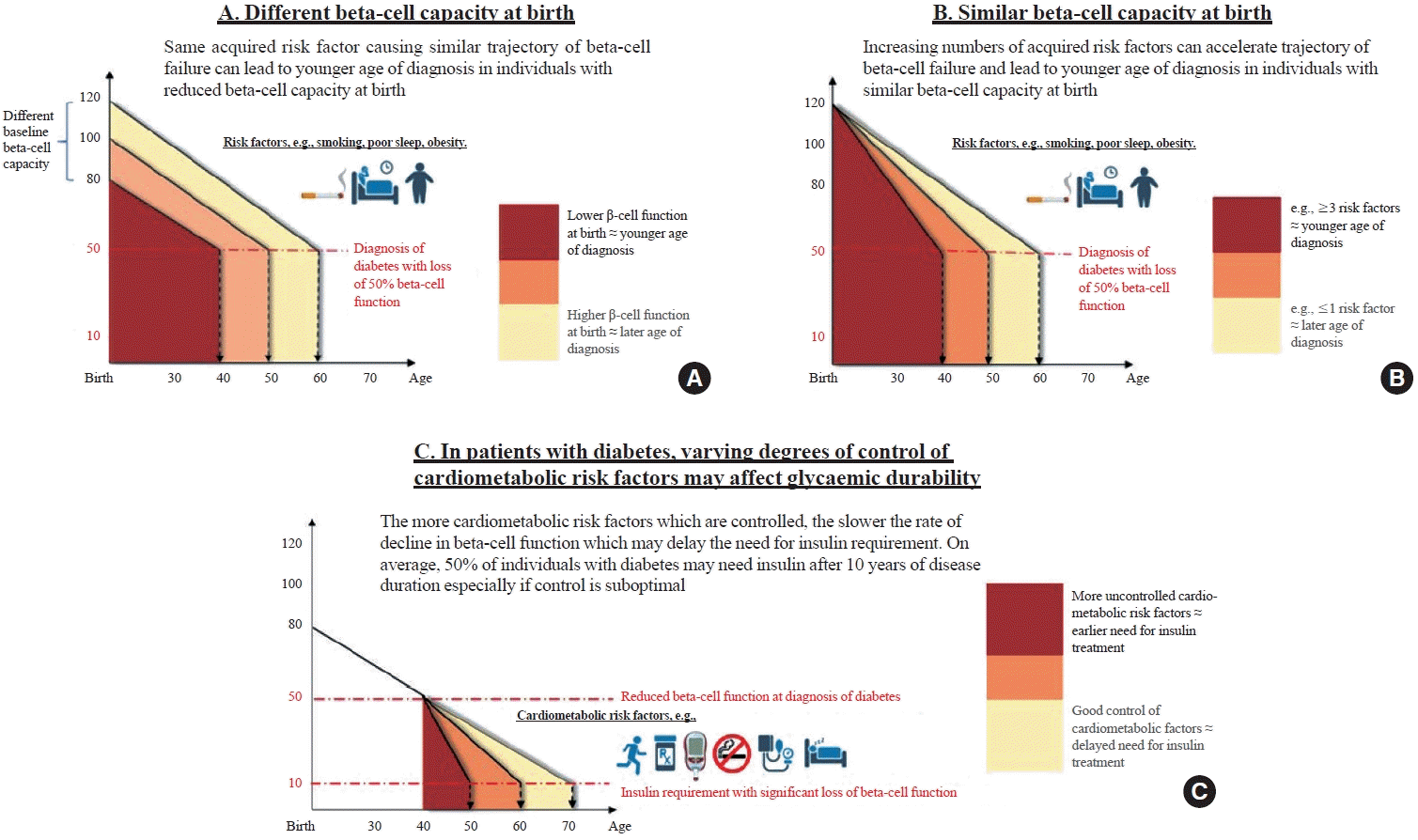
33. Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, et al. 10-Year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016; 4:27–34.

34. Fan Y, Fan B, Lau ES, Lim CK, Wu H, Ma RC, et al. Comparison of beta-cell function between Hong Kong Chinese with young-onset type 2 diabetes and late-onset type 2 diabetes. Diabetes Res Clin Pract. 2023; 205:110954.

35. Chan JC, Lim LL, Luk AO, Ozaki R, Kong AP, Ma RC, et al. From Hong Kong diabetes register to JADE program to RAMP-DM for data-driven actions. Diabetes Care. 2019; 42:2022–31.

36. Ke C, Stukel TA, Shah BR, Lau E, Ma RC, So WY, et al. Age at diagnosis, glycemic trajectories, and responses to oral glucose-lowering drugs in type 2 diabetes in Hong Kong: a population-based observational study. PLoS Med. 2020; 17:e1003316.

37. Barnett AH, Eff C, Leslie RD, Pyke DA. Diabetes in identical twins: a study of 200 pairs. Diabetologia. 1981; 20:87–93.
38. Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990; 6:1–27.

39. Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C. Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet. 1997; 60:651–6.
40. Almgren P, Lehtovirta M, Isomaa B, Sarelin L, Taskinen MR, Lyssenko V, et al. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia. 2011; 54:2811–9.

41. Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016; 536:41–7.
42. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013; 1281:64–91.

43. Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020; 582:240–5.
44. Noordam R, Lall K, Smit RA, Laisk T; Estonian Biobank Research Team; Metspalu A, et al. Stratification of type 2 diabetes by age of diagnosis in the UK biobank reveals subgroup-specific genetic associations and causal risk profiles. Diabetes. 2021; 70:1816–25.

45. Christiansen CE, Arathimos R, Pain O, Molokhia M, Bell JT, Lewis CM. Stratified genome-wide association analysis of type 2 diabetes reveals subgroups with genetic and environmental heterogeneity. Hum Mol Genet. 2023; 32:2638–45.

46. Srinivasan S, Chen L, Todd J, Divers J, Gidding S, Chernausek S, et al. The first genome-wide association study for type 2 diabetes in youth: the Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes. 2021; 70:996–1005.

47. Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017; 60:769–77.

48. Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019; 570:71–6.
49. Locke JM, Saint-Martin C, Laver TW, Patel KA, Wood AR, Sharp SA, et al. The common HNF1A variant I27L is a modifier of age at diabetes diagnosis in individuals with HNF1A-MODY. Diabetes. 2018; 67:1903–7.
50. Kettunen JL, Rantala E, Dwivedi OP, Isomaa B, Sarelin L, Kokko P, et al. A multigenerational study on phenotypic consequences of the most common causal variant of HNF1A-MODY. Diabetologia. 2022; 65:632–43.

51. Zhou K, Donnelly LA, Morris AD, Franks PW, Jennison C, Palmer CN, et al. Clinical and genetic determinants of progression of type 2 diabetes: a DIRECT study. Diabetes Care. 2014; 37:718–24.

52. Jiang G, Luk AO, Tam CH, Lau ES, Ozaki R, Chow EY, et al. Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong Diabetes Register and Hong Kong Diabetes Biobank. PLoS Med. 2020; 17:e1003209.

53. Ma RC, Lee HM, Lam VK, Tam CH, Ho JS, Zhao HL, et al. Familial young-onset diabetes, pre-diabetes and cardiovascular disease are associated with genetic variants of DACH1 in Chinese. PLoS One. 2014; 9:e84770.

54. Ma RC, Hu C, Tam CH, Zhang R, Kwan P, Leung TF, et al. Genome-wide association study in a Chinese population identifies a susceptibility locus for type 2 diabetes at 7q32 near PAX4. Diabetologia. 2013; 56:1291–305.

55. Lam VK, Ma RC, Lee HM, Hu C, Park KS, Furuta H, et al. Genetic associations of type 2 diabetes with islet amyloid polypeptide processing and degrading pathways in Asian populations. PLoS One. 2013; 8:e62378.

56. Pettitt DJ, Lawrence JM, Beyer J, Hillier TA, Liese AD, Mayer-Davis B, et al. Association between maternal diabetes in utero and age at offspring’s diagnosis of type 2 diabetes. Diabetes Care. 2008; 31:2126–30.

57. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000; 49:2208–11.

58. Tam WH, Ma RC, Ozaki R, Li AM, Chan MH, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017; 40:679–86.

59. Li LJ, Huang L, Tobias DK, Zhang C. Gestational diabetes mellitus among Asians: a systematic review from a population health perspective. Front Endocrinol (Lausanne). 2022; 13:840331.
60. Mazidi M, Banach M, Kengne AP; Lipid and Blood Pressure Meta-analysis Collaboration Group. Prevalence of childhood and adolescent overweight and obesity in Asian countries: a systematic review and meta-analysis. Arch Med Sci. 2018; 14:1185–203.

61. Ozaki R, Qiao Q, Wong GW, Chan MH, So WY, Tong PC, et al. Overweight, family history of diabetes and attending schools of lower academic grading are independent predictors for metabolic syndrome in Hong Kong Chinese adolescents. Arch Dis Child. 2007; 92:224–8.

62. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013; 310:948–59.

63. Chan JC, Chow E, Kong A, Cheung E, O T, Lim C, et al. Multifaceted nature of young-onset diabetes: can genomic medicine improve the precision of diagnosis and management? J Transl Genet Genom. 2024; 8:13–34.

64. Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014; 2:935–43.

65. Luk AO, Lau ES, Lim C, Kong AP, Chow E, Ma RC, et al. Diabetes-related complications and mortality in patients with young-onset latent autoimmune diabetes: a 14-year analysis of the prospective Hong Kong diabetes register. Diabetes Care. 2019; 42:1042–50.

66. Ng MC, Li JK, So WY, Critchley JA, Cockram CS, Bell GI, et al. Nature or nurture: an insightful illustration from a Chinese family with hepatocyte nuclear factor-1 alpha diabetes (MODY3). Diabetologia. 2000; 43:816–8.
67. Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001; 24:1522–7.
68. Yang A, Wu H, Lau ES, Ma RC, Kong AP, So WY, et al. Trends in glucose-lowering drug use, glycemic control, and severe hypoglycemia in adults with diabetes in Hong Kong, 2002-2016. Diabetes Care. 2020; 43:2967–74.

69. Yang A, Wu H, Lau ES, Zhang X, Shi M, Fan B, et al. Glucose-lowering drug use, glycemic outcomes, and severe hypoglycemia: 18-year trends in 0.9 million adults with diabetes in Hong Kong (2002-2019). Lancet Reg Health West Pac. 2022; 26:100509.

70. Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005; 95 Suppl 1:S144–50.

71. Dagogo-Jack S. Diabetes mellitus in developing countries and underserved communities. Cham: Switzerland Springer International Publishing; 2016. Chapter 5, Diabetes in China and the Western Pacific Region; p. 63-84 [cited 2024 Mar 22]. Available from:
https://link.springer.com/chapter/10.1007/978-3-319-41559-8_5.
72. McCarthy M, Birney E. Personalized profiles for disease risk must capture all facets of health. Nature. 2021; 597:175–7.

73. Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020; 43:1617–35.

74. Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006; 296:421–6.

75. Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 1998; 54:1296–304.

76. Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000; 58:302–11.

77. Yokoyama H, Okudaira M, Otani T, Watanabe C, Takaike H, Miuira J, et al. High incidence of diabetic nephropathy in early-onset Japanese NIDDM patients: risk analysis. Diabetes Care. 1998; 21:1080–5.

78. Luk AO, Lau ES, So WY, Ma RC, Kong AP, Ozaki R, et al. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care. 2014; 37:149–57.

79. Lee ET, Keen H, Bennett PH, Fuller JH, Lu M. Follow-up of the WHO Multinational Study of Vascular Disease in Diabetes: general description and morbidity. Diabetologia. 2001; 44 Suppl 2:S3–13.

80. Luk AO, Ke C, Lau ES, Wu H, Goggins W, Ma RC, et al. Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: a retrospective cohort study. PLoS Med. 2020; 17:e1003052.

81. Leung CB, Cheung WL, Li PK. Renal registry in Hong Kong-the first 20 years. Kidney Int Suppl (2011). 2015; 5:33–8.

82. Lee J, Lee SH, Yoon KH, Cho JH, Han K, Yang Y. Risk of developing chronic kidney disease in young-onset type 2 diabetes in Korea. Sci Rep. 2023; 13:10100.

83. Wu H, Lau ES, Yang A, Fan B, Ma RC, Kong AP, et al. Young age at diabetes diagnosis amplifies the effect of diabetes duration on risk of chronic kidney disease: a prospective cohort study. Diabetologia. 2021; 64:1990–2000.

84. de Boer IH, Caramori ML, Chan JC, Heerspink HJ, Hurst C, Khunti K, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020; 98:839–48.

85. Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023; 148:1606–35.

86. Wu H, Lau ES, Yang A, Zhang X, Ma RC, Kong AP, et al. Data resource profile: the Hong Kong Diabetes Surveillance Database (HKDSD). Int J Epidemiol. 2022; 51:e9–17.

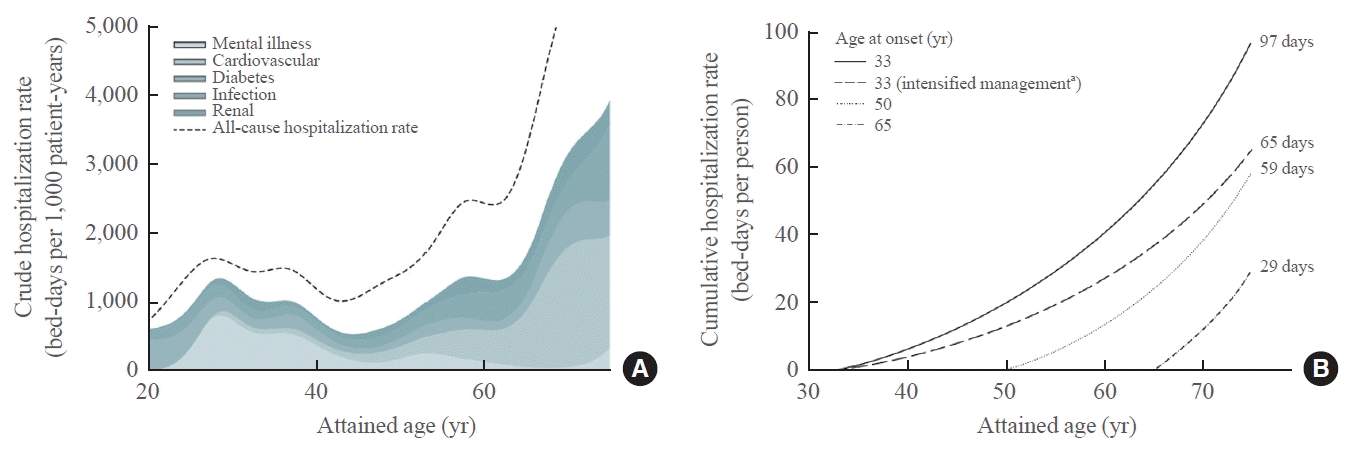
87. Ke C, Lau E, Shah BR, Stukel TA, Ma RC, So WY, et al. Excess burden of mental illness and hospitalization in young-onset type 2 diabetes: a population-based cohort study. Ann Intern Med. 2019; 170:145–54.

88. Wu H, Yang A, Lau ES, Zhang X, Fan B, Shi M, et al. Ageand sex-specific hospital bed-day rates in people with and without type 2 diabetes: a territory-wide population-based cohort study of 1.5 million people in Hong Kong. PLoS Med. 2023; 20:e1004261.
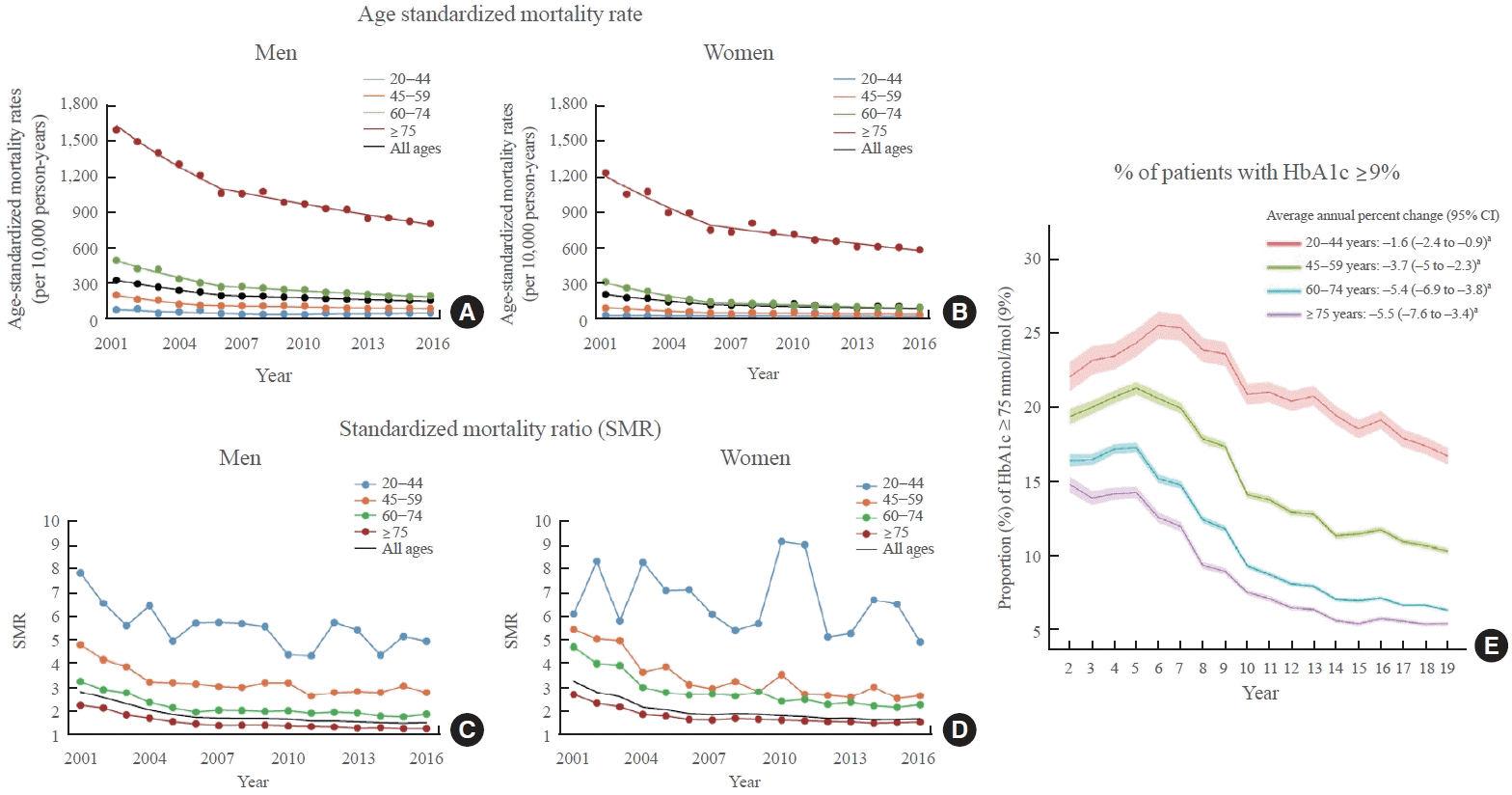
89. Wu H, Lau ES, Ma RC, Kong AP, Wild SH, Goggins W, et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001-2016: a retrospective cohort study. Diabetologia. 2020; 63:757–66.

90. Ke C, Shah BR, Luk AO, Di Ruggiero E, Chan JC. Cardiovascular outcomes trials in type 2 diabetes: time to include young adults. Diabetes Obes Metab. 2020; 22:3–5.

91. Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, et al. High prevalence of depressive symptoms in patients with type 1 and type 2 diabetes in developing countries: results from the International Diabetes Management Practices Study. Diabetes Care. 2021; 44:1100–7.

92. Seng JJ, Kwan YH, Lee VS, Tan CS, Zainudin SB, Thumboo J, et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care. 2020; 43:1048–56.

93. Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019; 381:603–13.

94. Ramos EL, Dayan CM, Chatenoud L, Sumnik Z, Simmons KM, Szypowska A, et al. Teplizumab and β-cell function in newly diagnosed type 1 diabetes. N Engl J Med. 2023; 389:2151–61.

95. Zhang L, Zhang Y, Shen S, Wang X, Dong L, Li Q, et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: a multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2023; 11:567–77.

96. Herman WH. The cost-effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clin Diabetes Endocrinol. 2015; 1:9.

97. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014; 2:474–80.

98. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019; 7:344–55.

99. Wu H, Yang A, Lau ES, Zhang X, Fan B, Ma RC, et al. 1-Year weight change after diabetes diagnosis and long-term incidence and sustainability of remission of type 2 diabetes in real-world settings in Hong Kong: an observational cohort study. PLoS Med. 2024; 21:e1004327.

100. Chan JC, Paldanius PM, Mathieu C, Stumvoll M, Matthews DR, Del Prato S. Early combination therapy delayed treatment escalation in newly diagnosed young-onset type 2 diabetes: a subanalysis of the VERIFY study. Diabetes Obes Metab. 2021; 23:245–51.
101. Cheung JT, Yang A, Wu H, Lau ES, Kong AP, Ma RC, et al. Early treatment with dipeptidyl-peptidase 4 inhibitors reduces glycaemic variability and delays insulin initiation in type 2 diabetes: a propensity score-matched cohort study. Diabetes Metab Res Rev. 2024; 40:e3711.
102. Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017; 5:951–64.
103. Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008; 359:1565–76.

104. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care. 2019; 42:416–26.

105. Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018; 6:361–9.

106. Ke C, Narayan KM, Chan JC, Jha P, Shah BR. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022; 18:413–32.

107. Lim LL, Lau ES, Kong AP, Davies MJ, Levitt NS, Eliasson B, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care. 2018; 41:1312–20.

108. Chan JC, Thewjitcharoen Y, Nguyen TK, Tan A, Chia YC, Hwu CM, et al. Effect of a web-based management guide on risk factors in patients with type 2 diabetes and diabetic kidney disease: a JADE randomized clinical trial. JAMA Netw Open. 2022; 5:e223862.
109. Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019; 7:64.

110. Kong AP, Luk AO, Chan JC. Detecting people at high risk of type 2 diabetes: how do we find them and who should be treated? Best Pract Res Clin Endocrinol Metab. 2016; 30:345–55.

111. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965; 58:295–300.

112. Basu R. Precision medicine in diabetes: a multidisciplinary approach to an emerging paradigm. Cham: Springer; 2022. Chapter 5, Implementation of precision genetic approaches for type 1 and 2 diabetes; p. 111-29 [cited 2024 Mar 22]. Available from:
https://link.springer.com/chapter/10.1007/978-3-030-98927-9_5.
113. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care. 2009; 32:977–82.
114. Chan JC, Sui Y, Oldenburg B, Zhang Y, Chung HH, Goggins W, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med. 2014; 174:972–81.

115. Naoum V, Kyriopoulos D, Charonis A, Athanasakis K, Kyriopoulos J. The Pareto principle (“80− 20 Rule”) in healthcare services in Greece. Value Health. 2016; 19:A618.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download