Abstract
Glucocorticoids provide a potent therapeutic response and are widely used to treat a variety of diseases, including coronavirus disease 2019 (COVID-19) infection. However, the issue of glucocorticoid-induced hyperglycemia (GIH), which is observed in over one-third of patients treated with glucocorticoids, is often neglected. To improve the clinical course and prognosis of diseases that necessitate glucocorticoid therapy, proper management of GIH is essential. The key pathophysiology of GIH includes systemic insulin resistance, which exacerbates hepatic steatosis and visceral obesity, as well as proteolysis and lipolysis of muscle and adipose tissue, coupled with β-cell dysfunction. For patients on glucocorticoid therapy, risk stratification should be conducted through a detailed baseline evaluation, and frequent glucose monitoring is recommended to detect the onset of GIH, particularly in high-risk individuals. Patients with confirmed GIH who require treatment should follow an insulin-centered regimen that varies depending on whether they are inpatients or outpatients, as well as the type and dosage of glucocorticoid used. The ideal strategy to maintain normoglycemia while preventing hypoglycemia is to combine basal-bolus insulin and correction doses with a continuous glucose monitoring system. This review focuses on the current understanding and latest evidence concerning GIH, incorporating insights gained from the COVID-19 pandemic.
Glucocorticoids provide a potent therapeutic response with few alternatives, and they are utilized in treating a broad spectrum of conditions, including inflammatory diseases, immunosuppression following transplantation, anti-allergic reactions, and improving the prognosis of shock in critically ill patients. In the UK, over 10% of hospitalized patients are prescribed glucocorticoids, with more than half of them using these drugs for longer than 10 days [1]. Despite their relatively low cost, the global market for glucocorticoids has reached nearly 5 billion dollars, a figure that is expected to grow in the aftermath of the coronavirus disease 2019 (COVID-19) pandemic [2]. The increased use of glucocorticoids has raised concerns about metabolic side effects and associated health issues, particularly glucocorticoid-induced hyperglycemia (GIH). The hyperglycemic effects of glucocorticoids are dose-dependent and can manifest rapidly, often within hours of administration [3]. Prolonged, high-dose glucocorticoid therapy heightens the risk of hyperglycemia, regardless of whether a patient has diabetes, and can lead to severely uncontrolled hyperglycemia in those with pre-existing diabetes [4]. In individuals without diabetes, glucocorticoid use is associated with a twofold higher risk of hyperglycemia [5].
Despite the increasing burden of GIH, it is often underestimated or neglected by many healthcare professionals. Despite the importance of determining baseline hyperglycemia, fewer than one-third of patients undergo glycemic evaluation prior to glucocorticoid administration [6]. In studies on GIH in inpatients, 25% to 80% of patients did not receive glucose monitoring even though they were hospitalized [1,7]. The COVID-19 pandemic has necessitated the use of a high-potency glucocorticoid (dexamethasone) to treat patients with severe COVID-19 cases [8]. Therefore, this review aims to highlight the current status of GIH and the latest evidence on its pathophysiology, clinical course, risk factor assessment, diagnosis, and treatment.
Currently, 1% of the general population and 2.5% of older adults receive oral glucocorticoid therapy [9]. In a prospective study of a primary care population diagnosed with diabetes from 2003 to 2004, the incidence of GIH resulting from oral glucocorticoid therapy was estimated to be 2% [10]. The risk of GIH is more pronounced in inpatients. According to a study by Donihi et al. [7], half of the inpatients who had not previously been diagnosed with diabetes experienced more than one episode of hyperglycemia, with blood glucose levels of 200 mg/dL or higher. Similar results were observed in a study that detected new cases of GIH using the same glucose threshold (≥200 mg/dL) with a continuous glucose monitoring system (CGMS) in steroid-naive dermatological patients undergoing systemic glucocorticoid treatment [11]. Another study involving patients without diabetes and using a lower threshold for GIH (random blood glucose between 145 and 180 mg/dL) found that a larger proportion of patients (70 to 86%) experienced hyperglycemia at least once, with nearly half of all patients having a mean blood glucose level exceeding 140 mg/dL [12].
A recent retrospective study that included 2,424 inpatients over a 4-year period reported an overall incidence of GIH of 34% [13]. After standard immunosuppressive treatment, which included intravenous methylprednisolone and oral prednisone after kidney transplantation, most patients (87%) without pretransplant diabetes exhibited hyperglycemia, and two-thirds required insulin at discharge [14]. Inpatients with community-acquired pneumonia who were treated with 50 mg of prednisone daily for 1 week had an in-hospital hyperglycemia incidence of approximately 20%, with the odds ratio (OR) for hyperglycemia compared to placebo being 1.96 [15]. A retrospective analysis of elderly individuals with an average age of 75 years showed a GIH incidence rate of 12% [16]. A subsequent review of several case-control and retrospective studies, including this one, indicated that the relative risk of developing new GIH in patients without a previous history of hyperglycemia ranged from 1.3 to 2.3, with the total dose and duration of treatment being strong predictors of GIH development [5]. A meta-analysis of approximately 35,000 participants from 13 studies in non-diabetic patients who underwent glucocorticoid treatment for at least 1 month found a GIH incidence of 32% [17], consistent with the results of other previous studies [16,18]. Another recent meta-analysis that pooled data from 118 randomized controlled trials reported a GIH prevalence of 10% (OR, 2.1), with severe hyperglycemia—defined as requiring treatment, newly diagnosed diabetes, or withdrawal from the study due to being reported as a serious adverse event—at 5% [19]. In summary, the incidence of GIH varies depending on the study design and the threshold set for hyperglycemia, but it is often observed to be in the range of 20% to 30%, with a relative risk of approximately double.
Key aspects of the pathophysiology of GIH are summarized in Fig. 1.
Insulin resistance is the core pathophysiology of diabetes [20]. This cascade could be accelerated following glucocorticoid administration. Glucocorticoid play an important role in adipogenesis and adipose tissue metabolism, increasing visceral rather than peripheral fat storage [21]. They promote the differentiation of preadipocytes into mature adipocytes, increase visceral adiposity by selectively enhancing the affinity of lipoprotein lipase for visceral adipose tissue, and stimulate lipolysis to produce non-esterified fatty acids, which are not suppressed by insulin [22]. The actions of various adipokines, including the upregulation of leptin and resistin due to an increase in white adipose tissue, contribute to insulin resistance. Interestingly, the appetite-suppressing effect of leptin is counteracted by glucocorticoids [23,24]. In contrast to the increase in white adipose tissue, a suppression of energy expenditure due to the inhibition of the browning of adipose tissue and a decline in its function have been observed in rats [25].
In addition to adipose tissue, muscle contributes to the insulin resistance caused by glucocorticoids. The increase in non-esterified fatty acids from lipolysis leads to their ectopic accumulation in skeletal muscle, which exacerbates insulin resistance [26]. Glucocorticoids further promote insulin resistance by decreasing the rate of protein synthesis and activating multiple protein degradation pathways, resulting in a reduction of skeletal muscle mass [27]. Furthermore, glucocorticoids may contribute to insulin resistance in skeletal muscle by directly inhibiting the translocation of glucose transporter 4 to the cell membrane, thereby interfering with insulin signaling and reducing glycogen synthesis kinase 3 phosphorylation [28].
Pronounced glucose intolerance due to insulin resistance leads to subsequent β-cell dysfunction. The suppressive effect on β-cells from acute glucocorticoid treatment within a few hours is counterbalanced by an increase in insulin secretion from healthy β-cells, which leads to subtle changes in blood glucose levels [29]. GIH is also associated with an early increase in β-cell mass in a dose-dependent manner [30]. However, prolonged exposure to glucocorticoids disrupts this compensatory mechanism that maintains normoglycemia. The ability of glucocorticoids to inhibit insulin synthesis by inducing apoptosis through endoplasmic reticulum stress in β-cells has been demonstrated in a rat model of insulinoma [31]. Additionally, circulating non-esterified fatty acids accumulate in the pancreas and contribute to β-cell dysfunction [27]. When glucocorticoids are administered to healthy subjects for 1 to 7 days, impaired glucose tolerance accompanied by insulin resistance is observed [32]. Populations at higher risk for diabetes due to β-cell overload include those with a family history of diabetes and those using immunosuppressants for underlying autoimmune diseases [33,34].
The liver plays a crucial role in maintaining glucose homeostasis, which can be disrupted by glucocorticoids. Schneiter and Tappy [3] demonstrated that glucocorticoids are linked to a decreased ability of plasma glucose to suppress gluconeogenesis in the liver. Glucocorticoids directly increase hepatic glucose production by altering the molecular regulation of gluconeogenic enzyme expression, including phosphoenolpyruvate carboxykinase and glucose-6-phosphatase [35]. Additionally, glucocorticoids enhance the supply of substrates for hepatic gluconeogenesis and facilitate the transport of metabolites across the mitochondrial membrane [36]. Glucocorticoids counteract the metabolic effects of insulin, promoting gluconeogenesis through the loss of insulin-mediated inhibition [35]. Furthermore, a study on nuclear peroxisome proliferator-activated receptor α knockout mice revealed that glucocorticoids induce hepatic gluconeogenesis in a manner dependent on nuclear peroxisome proliferator-activated receptor α expression [37]. The hepatic secretion of very low-density lipoprotein and the uptake of circulating non-esterified fatty acids are increased in the presence of glucocorticoids [38]. This leads to a synergistic stimulation of de novo lipogenesis and the subsequent accumulation of intrahepatic lipids, creating a vicious cycle that exacerbates hepatic insulin resistance and hyperglycemia [9].
Hyperglycemia is linked to a worsening prognosis in a variety of diseases and conditions. Acute hyperglycemia following a cardiovascular disease diagnosis has been identified as a predictor of mortality and poor prognosis, even in patients without prior diabetes [39,40]. This impact of stress-induced hyperglycemia has also been observed in critically ill inpatients with trauma and sepsis, conditions not directly related to the pathophysiology of diabetes [41,42]. Conversely, effective management of transient hyperglycemia is associated with reduced mortality and morbidity, whether or not the patient is critically ill [43,44]. GIH is a known trigger for poor prognosis in clinical practice. Ongoing glucocorticoid therapy for inflammatory diseases has been shown to increase the risk of future cardiovascular events through multiple pathways [45]. A retrospective analysis assessing the 30-day prognosis for mortality, cardiovascular events, and infection rates found that these outcomes were significantly more common in patients with GIH than in those with normoglycemia [13].
Glucocorticoids suppress cell-mediated immune responses, increasing the risk of infection [46], while acute hyperglycemia itself compromises the host’s innate immunity against infection [47]. Studies involving patients with hematologic malignancies, such as acute lymphocytic leukemia and multiple myeloma, have demonstrated an association between hyperglycemia induced by induction therapy, including high-dose glucocorticoids, and severe infections [48,49]. In the context of solid tumors, approximately 20% of non-diabetic cancer patients developed GIH after receiving the antiemetic dexamethasone during chemotherapy [50]. Diabetes in post-transplant patients treated with potent immunosuppressive therapy, including glucocorticoids, is associated with serious complications such as rejection and graft failure, infection, cardiovascular disease, and death [51]. It will be necessary to understand the clinical course of glucocorticoids across a broad spectrum of diseases and to identify strategies to control and improve patient prognosis.
Since its initial identification in China in 2019, the COVID-19 pandemic has resulted in over 700 million reported cases and 7 million deaths by 2023 [52]. The development of hyperglycemia and diabetes following COVID-19 infection is part of a bidirectional relationship involving a complex interplay of immune responses, either directly or indirectly triggered by the virus, stress-induced hyperglycemia, and the use of glucocorticoids [53]. The proinflammatory metabolic state induced by COVID-19 infection lays the groundwork for an intensified viral inflammatory response, leading to insulin resistance and stress hyperglycemia. This situation is further exacerbated by systemic shock and multiorgan failure [54]. COVID-19 has a direct toxic effect on pancreatic β-cells and hepatocytes, exacerbating hyperglycemia through β-cell dysfunction and increased gluconeogenesis [53,55]. A meta-analysis examining the link between hyperglycemia and COVID-19 infection found that the risk was especially significant in cases of severe disease [56]. Hyperglycemia, or the coexistence of diabetes, contributes to a worse clinical course and prognosis following COVID-19 infection. Pre-existing diabetes and a fasting blood glucose level of 126 mg/dL or higher upon admission were identified as independent predictors of a poor prognosis [57]. Furthermore, an increased incidence of diabetic ketoacidosis has been associated with COVID-19 infection, which is believed to result from β-cell dysfunction and systemic inflammation [53,58]. The pathogenesis of hyperglycemia in hospitalized COVID-19 patients is depicted in Fig. 2.
To improve the prognosis of hospitalized adults with COVID-19 who are receiving respiratory support, remdesivir is recommended for patients with mild symptoms requiring conventional oxygen [59]. For those with moderate to severe symptoms requiring high-flow oxygen or invasive ventilation, immunomodulators such as baricitinib are suggested [59]. It is important to note that dexamethasone administration is recommended in both scenarios, except for patients who require minimal conventional oxygen. This recommendation is based on the pivotal results of the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, which demonstrated the therapeutic effect of dexamethasone [8]. However, hyperglycemia was the most frequently reported serious adverse event in this trial. As dexamethasone has become the standard treatment, the need for management and expertise in GIH has grown [60]. The subsequent COVID STEROID 2 trial indicated that a higher dose of dexamethasone—12 mg as opposed to the 6 mg used in the RECOVERY trial—may benefit patients requiring non-invasive or mechanical ventilation [61], raising concerns about an increase in GIH.
GIH induced by dexamethasone was particularly difficult to control in patients with pre-existing diabetes [62]. Conversely, controlling hyperglycemia with appropriate insulin therapy appears to be an effective way to improve the clinical outcomes of COVID-19 patients [63]. This is supported by research showing that patients who received insulin infusions had a lower risk of developing severe disease compared to those who did not [64]. Considering the “triple insult” of impaired glucose metabolism, insulin resistance, and impaired insulin production due to COVID-19 and dexamethasone treatment, basal insulin doses may need to be increased in hospitalized COVID-19 patients [65]. The late peak and prolonged duration of action of neutral protamine Hagedorn (NPH) insulin align more closely with the pathophysiological insulin resistance and hyperglycemia seen in these patients. Therefore, the use of NPH in combination with increased doses of basal insulin is generally considered an effective treatment [66]. However, a recent retrospective study found no difference in mean blood glucose levels and other outcomes when comparing NPH, insulin glargine, and a combination of NPH and insulin glargine [62]. Further research is necessary to determine the effectiveness of oral antidiabetic agents and the most appropriate method of insulin administration to improve the clinical course of GIH following COVID-19 infection.
As highlighted in the introduction, the first and most crucial step in the effective management of GIH is to maintain clinical vigilance for the occurrence of GIH and to conduct a comprehensive patient evaluation, ensuring that the associated risks are not underestimated. Patients prescribed glucocorticoids should undergo risk stratification to anticipate potential GIH. It is also necessary to assess whether they have pre-existing hyperglycemia or undiagnosed diabetes and to monitor them to determine whether the GIH reaches a threshold that necessitates intervention.
Several studies have been conducted to identify risk factors for GIH, as shown in Table 1. Notable risk factors include the type, route, dosage, and regimen of glucocorticoid administration, previous GIH events, impaired fasting glucose or glucose tolerance, elevated glycated hemoglobin (HbA1c), a family history of diabetes, advanced age, obesity, a low estimated glomerular filtration rate, and the concurrent use of immunosuppressants [9,67]. In patients hospitalized with rheumatic or renal disease without diabetes, presence of one or more of these three risk factors—defined as older age (≥65 years), higher HbA1c (≥ 6.0%), or estimated glomerular filtration rate (<40 mL/min/1.73 m2)—more than doubled the risk of developing GIH [68]. The Joint British Diabetes Societies have indicated that a supraphysiologic dose of prednisolone equivalent to 5 mg or more can be the threshold for the development of GIH, according to their guidelines [69]. They also acknowledged the potential for GIH to occur at lower doses.
Glucocorticoids administered through various routes can cause GIH. Since the risk of hyperglycemia increases with the dose of glucocorticoids, the relative risk of GIH from intravenous administration—which is typically preferred for high doses—was more than double that of oral administration [19]. Furthermore, a positive dose-response relationship between inhaled corticosteroids and diabetes has been demonstrated in multiple large observational studies [70]. The use of topical steroids has also been linked to the development of diabetes, with the cumulative dose and duration of use being more influential than the potency of the steroid, although the evidence for this association is limited [71]. Continuous administration of glucocorticoids was associated with a higher risk of GIH compared to a cyclic regimen, with the incidence doubling [72]. The concurrent use of mycophenolate mofetil and a high-dose glucocorticoid was identified as an independent risk factor for GIH in patients with systemic lupus erythematosus [34]. A previous study confirmed the association of a family history of diabetes with GIH due to β-cell dysfunction [33]. This finding was further supported by a recent study that identified a significant association between GIH and a polygenic score for diabetes, which predicts an individual’s metabolic response to glucocorticoid treatment [73].
Metabolic deterioration associated with insulin resistance, including obesity, hyperlipidemia, and a history of hyperglycemia, should be assessed, as these factors predispose individuals to the development of GIH [9]. Furthermore, patients at high-risk should be thoroughly questioned about their diabetes history, and HbA1c levels should be tested before initiating glucocorticoid therapy to exclude undiagnosed diabetes [67]. It is important to recognize that HbA1c has diagnostic limitations due to factors that alter red blood cell lifespan [74]. These factors include advanced age, use of immunosuppressants, autoimmune diseases, chronic kidney disease, and hemoglobinopathies—conditions in which glucocorticoids are commonly prescribed. Therefore, detailed blood glucose monitoring is necessary to corroborate HbA1c results in these situations that can affect its reliability.
Initial blood glucose monitoring is necessary for detecting the onset of GIH, yet it is often neglected [1,6]. For inpatients prescribed more than 5 mg of prednisolone or its equivalent, point-of-care blood glucose monitoring is advised within the first 1 to 2 days of starting glucocorticoid therapy [43,69]. This is particularly important for those at an elevated risk of GIH, as indicated in Table 1. Patients without a history of diabetes should have their blood glucose levels checked at least once daily, ideally before lunch or dinner if the glucocorticoid is administered in the morning. If blood glucose levels exceed 216 mg/dL, it is recommended to increase monitoring frequency to four times daily [69]. Glucose monitoring can be stopped if blood glucose levels remain below 140 mg/dL for a consistent period of 24 to 48 hours in patients without diabetes, while maintaining vigilance for potential GIH [43,75]. For inpatients with pre-existing diabetes, the frequency of glucose monitoring should be increased, with at least four checks per day as per the standard hospital protocol for patients with diabetes [43,44]. Outpatients should be thoroughly educated about the symptoms of hyperglycemia and insulin deficiency. They should also understand the importance of regular blood glucose monitoring, which should include not only fasting blood glucose in the morning but also postprandial levels after meals such as lunch or dinner. It is important to note that blood glucose levels may not normalize immediately after discontinuing glucocorticoid treatment [76]. Therefore, ongoing and careful monitoring is required to assess the persistent risk of GIH.
An important consideration in the diagnosis of GIH is that it should not be assessed solely by fasting blood glucose levels in the morning, regardless of whether the patient is hospitalized or has pre-existing diabetes. Typically, diabetes can be diagnosed when a fasting blood glucose level of 126 mg/dL or higher is confirmed on more than two occasions, or when these findings are consistent with other testing methods [74]. There are additional factors to consider in the diagnosis of GIH, and understanding the characteristics of glucocorticoids is crucial for accurate evaluation and to prevent missed diagnoses. The risk of early hyperglycemia associated with glucocorticoid use is primarily due to an early impairment of oral glucose tolerance and an increase in postprandial blood glucose levels rather than fasting levels [3]. The predominant mechanism involves extrahepatic insulin resistance coupled with impaired insulin secretion, which leads to postprandial hyperglycemia [20]. In addition, disproportionate hyperglycemia often occurs during the day, yet patients frequently achieve glucose targets overnight and the following morning, regardless of treatment. This pattern can result in a missed diagnosis of GIH, particularly when the fasting blood glucose level is used as the sole indicator and when a short to intermediate-acting glucocorticoid, such as prednisolone, is administered in a single morning dose [74,77].
Identifying postprandial hyperglycemia has a higher diagnostic sensitivity in these patients [45]. In a study involving primary renal disease patients without diabetes who were administered prednisolone at a dose of 0.75 mg/kg daily, 42% of patients exhibited postprandial blood glucose levels of 200 mg/dL or higher, even though their fasting blood glucose remained within the normal range [78]. A study that utilized CGMS to determine the pattern of GIH in patients with chronic obstructive pulmonary disease found that GIH occurred 3 to 4 hours post-administration. This study revealed a pattern of postprandial hyperglycemia lasting 12 to 16 hours, suggesting that monitoring postprandial hyperglycemia after lunch or dinner is useful for diagnosing GIH [79]. In contrast, administering short- to intermediate-acting glucocorticoids in divided doses more than twice a day, or using long-acting glucocorticoids such as dexamethasone, may lead to hyperglycemic effects lasting over 24 hours [77]. Furthermore, it was unclear whether fasting or postprandial hyperglycemia was the predominant issue when low doses of intermediate-acting glucocorticoids were administered chronically for more than 6 months [80].
The oral glucose tolerance test (OGTT) can diagnose diabetes with higher specificity and is the preferred method for diagnosing patients with post-transplantation diabetes [74]. However, the OGTT may present limitations in diagnosing GIH because it is typically conducted in the morning on an empty stomach, when the diabetogenic effects of short- to medium-acting glucocorticoids are not yet evident [81]. HbA1c is more convenient and can be useful in diagnosis due to its high specificity, although it is not recommended as a screening tool for identifying hyperglycemia because of its lower sensitivity compared to other tests, such as the OGTT [74]. HbA1c is not particularly helpful for diagnosing recent-onset hyperglycemia in patients who have just begun glucocorticoid therapy, as it reflects blood glucose levels over the past 2 to 3 months. Imatoh et al. [82] proposed that monitoring changes in HbA1c (the absolute difference between baseline and maximum HbA1c levels) as a detection algorithm for GIH resulted in higher sensitivity and specificity than the pre-existing criteria of a fasting blood glucose level exceeding 125 mg/dL or an HbA1c of 8.1% or higher. However, a significant limitation of this study was its failure to account for postprandial hyperglycemia.
In summary, measuring postprandial blood glucose levels or obtaining an OGTT result of 200 mg/dL or higher currently offers the most accurate method for diagnosing GIH. Diagnosis based on fasting glucose levels should not rely solely on morning, which can underestimate its prevalence. Further research is needed to establish new diagnostic criteria that consider various factors, including the degree of insulin resistance, the type and dosage of glucocorticoids administered, differences between inpatient and outpatient settings, and the utilization of HbA1c.
Despite the absence of high-quality, evidence-based guidelines for formal treatment strategies, it is clear that patients with GIH require effective hyperglycemic management to reduce morbidity and mortality during hospitalization or treatment, as well as to prevent diabetes-related complications. We suggest initiating treatment for GIH when fasting glucose levels are 140 mg/dL or higher, including pre-prandial glucose levels at lunch or dinner, or if random glucose levels are 200 mg/dL or higher more than once during the day [4]. The target is to maintain blood glucose levels within the range of 140 to 180 mg/dL, which aligns with the guidelines recommended for other diabetic patients, whether critically or non-critically ill [44,69].
Maintaining a strict blood glucose target of 110 to 140 mg/dL in hospitalized patients may lead to better prevention of complications and improved clinical outcomes [44]. However, hypoglycemia presents a major challenge in achieving this goal. Both hypoglycemia and glycemic variability are independent predictors that significantly affect microvascular and macrovascular outcomes, as well as mortality [83,84]. Glucocorticoid use is independently associated with increased glycemic variability, regardless of the severity of the underlying disease [85]. In a prospective study of patients undergoing high-dose glucocorticoid therapy with prednisolone at doses ranging from 0.75 to 2.0 mg/kg, those who developed GIH exhibited significantly higher glycemic variability than those who did not [11]. Furthermore, inpatients with GIH were at a higher risk of frequent hypoglycemic episodes, and hypoglycemia itself was identified as a strong and significant risk factor for poor prognosis [13]. The risk of nocturnal hypoglycemia is increased due to the cumulative effect of insulin administered during the day for GIH, the inhibition of endogenous cortisol production, and the waning glycemic effect of glucocorticoids [86]. This finding is corroborated by a study showing that administering prednisolone in divided doses mitigated GIH and reduced glycemic variability without causing hypoglycemia [87]. Failure to modify the treatment plan, such as adjusting the insulin dose when rapid euglycemia occurs after tapering or discontinuing glucocorticoid therapy, heightens the risk of subsequent hypoglycemia. Patients who are using medications with a high-risk of hypoglycemia, such as insulin secretagogues or insulin, should consider adjusting their dosage or switching medications when tapering off glucocorticoids.
CGMS provide more informed decisions regarding insulin dosing due to their ability to account for glycemic variability, and they are particularly effective in detecting nocturnal hypoglycemia [44], as compared to traditional capillary glucose monitoring. Several studies have utilized CGMS to investigate the patterns of GIH and to evaluate the efficacy and safety, thereby contributing to the development of formal treatment strategies for GIH [88-90]. CGMS has proven useful in identifying unrecognized hypoglycemia and in achieving optimal glucose control in patients undergoing glucocorticoid replacement therapy for central hypoadrenalism [91]. In a recent study involving patients with severe COVID-19 and diabetes who were receiving glucocorticoids, the utilization of intermittently scanned CGM achieved superior blood glucose control without being associated with an increased risk of hypoglycemia, even though higher total daily insulin doses were administered compared to patients monitored with regular capillary glucose testing [92]. This approach also reduced the frequency of healthcare worker-patient contact. In summary, CGMS can be an effective tool for managing hypoglycemia and glycemic variability associated with glucocorticoid use. If CGMS overcomes current challenges related to simplicity and cost, and further evidence demonstrates its advantages over point-of-care glucose monitoring, it is likely to become increasingly valuable for the management of GIH in the near future.
To date, there is no international consensus on the role of oral hypoglycemic agents in managing GIH due to insufficient evidence. Given that the main pathophysiological features of GIH include central and peripheral insulin resistance, as well as β-cell dysfunction, it is reasonable to infer that insulin sensitizers and incretin-based agents could complement the use of insulin or secretagogues.
Metformin is an oral hypoglycemic agent that has been widely prescribed for decades, offering a range of therapeutic mechanisms, such as improving insulin resistance. It increases insulin sensitivity by inducing the expression of glucose transporter 4 and enhancing its translocation [93], potentially counteracting the inhibitory effects of glucocorticoids. In a double-blind, placebo-controlled trial involving patients without diabetes, metformin administration proved beneficial in maintaining stable baseline glucose levels following glucocorticoid administration and improved insulin resistance [94]. Furthermore, in a randomized controlled trial of patients with inflammatory diseases and treated with continuous glucocorticoids, metformin not only improved metabolic profiles, including glucose and lipid levels, but also lowered inflammatory markers and reduced carotid intima-media thickness [95]. Metformin may be a viable option for managing GIH in patients with metabolic issues, except for those with hemodynamic instability or renal insufficiency, who are at an increased risk of lactic acidosis.
Thiazolidinediones (TZDs) are potent insulin sensitizers that activate peroxisome proliferator-activated receptor-γ. They also have significant effects on β-cells, including enhancing insulin secretion, preserving β-cell mass and islet structure, and providing protection from oxidative stress [96]. In a study by Willi et al. [97], TZDs were shown to improve glycemic control and lipid profiles, as well as to reduce fasting leptin concentrations in patients with GIH, despite an associated weight gain. These findings were echoed in a study of patients who continued prednisone use post-transplantation, although the improvement in lipid profile was not deemed significant [98]. However, it is important to consider the increased risk of fluid retention and fractures, risks that are exacerbated by glucocorticoids, before prescribing TZDs for patients with GIH.
Dipeptidyl peptidase 4 inhibitors (DPP-4is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) are therapies derived from GLP-1, an incretin hormone produced in the gut. GLP-1 is released in response to glucose and enhances insulin secretion, inhibits glucagon release, slows gastric emptying, and decreases food intake. These actions contribute to improved postprandial blood glucose levels, addressing the primary issue in GIH. Glucocorticoid-induced insulin resistance diminishes the early insulin-stimulating effects of GLP-1, making the overcoming of this resistance a potential therapeutic target [99]. DPP-4is are favored for their relatively few side effects and safety in renal failure. However, DPP-4is have not shown a definitive benefit in ameliorating GIH, despite their advantages in reducing glycemic variability and insulin requirements [90,100].
GLP-1RAs are promising agents anticipated to counteract the pathophysiology of glucocorticoids. They not only improve hyperglycemia without increasing the risk of hypoglycemia but also suppress appetite, reduce weight, and enhance metabolic profiles through systemic mechanisms [101]. In a study involving healthy subjects and a meal challenge test, exenatide was shown to prevent glucose intolerance and islet cell dysfunction caused by high-dose glucocorticoids [102]. Furthermore, a retrospective study of patients with GIH demonstrated that adding dulaglutide to insulin therapy resulted in glycemic control with a reduced insulin dose and frequency, while maintained safety with respect to gastrointestinal symptoms and hypoglycemia [103]. Despite these findings, there is still a need for more long-term research to conclusively determine the efficacy and safety of GLP-1RAs in the management of GIH.
Among insulin secretagogues, the second-generation sulfonylureas—glimepiride, glipizide, and glyburide—are commonly used in clinical practice. These medications have a sufficiently long half-life to allow for once-daily dosing and are effective in reducing fasting blood glucose levels. A case report from Japan indicated that glimepiride, due to its dual effects on β-cell function and insulin sensitivity, provided benefits that were sustained for 24 weeks in patients with GIH [104]. However, sulfonylureas are generally not recommended for most cases of GIH because of several limitations, including their β-cell-dependent action and a narrow therapeutic window [105]. An exception is made for long-term dexamethasone administration, which is associated with a relatively low risk of nocturnal hypoglycemia.
Glinides, such as repaglinide and nateglinide, address some of the limitations of sulfonylureas. They have a relatively rapid onset and shorter duration of action, which helps to lower the risk of hypoglycemia. Additionally, they stimulate a transient release of insulin from β-cells in a glucose-dependent manner. This mechanism is particularly effective for controlling postprandial hyperglycemia and appears to be ideal for improving GIH [105]. Glinides also have a relative advantage over sulfonylureas in terms of the side effect of weight gain associated with hyperinsulinemia [106]. Patients with diabetes that developed post-kidney transplantation have achieved successful glucose control with less hypoglycemia when treated with repaglinide, despite the limitations of the observational study design [107]. In a small-scale study involving individuals with thyroid ophthalmopathy, repaglinide demonstrated more favorable glycemic control for GIH induced by methylprednisolone pulse therapy, as observed through CGMS [108]. However, the requirement for frequent dosing before each meal remains a challenge for the use of glinides.
Sodium-glucose cotransporter-2 inhibitors (SGLT-2is) induce glucose-dependent glycosuria independently of insulin and offer benefits such as weight loss, blood pressure control, improved cardiovascular risk reduction, and renal protection [109]. Therefore, they are anticipated to be beneficial in mitigating metabolic deterioration following glucocorticoid administration. However, a randomized controlled study using dapagliflozin did not show superior glycemic control in terms of average blood glucose and time-in-range, as confirmed by CGMS, although the treatment was deemed safe compared to placebo [110]. The results of the ongoing EmpAgliflozin compared to NPH Insulin for sTeroId diabetes (EANITIATE) study, a randomized controlled multicenter trial comparing the safety and efficacy of empagliflozin with NPH insulin in patients with new-onset GIH, are awaited [111].
Numerous studies have investigated the appropriate insulin therapy for patients with GIH. GIH is a common issue encountered during hospital stays, and insulin is considered the optimal treatment for managing GIH, aiming to maintain blood glucose levels at or below 180 mg/dL to improve the clinical outcomes for inpatients [44]. Insulin therapy offers the flexibility to adjust dosages in response to the rapidly fluctuating patterns of GIH, and it allows for high doses to be administered via subcutaneous or intravenous infusion without a maximum dose limit, which is crucial for addressing severe hyperglycemia. In cases where GIH occurs in patients with pre-existing diabetes who are already on insulin therapy, it is recommended to increase the insulin dosage to maintain euglycemia, typically by 20% to 30% of the total daily dose [9,112]. For most hospitalized patients with diabetes, a regimen that includes multiple insulin injections—comprising basal, bolus, and corrective doses—is the preferred treatment approach [44]. Basal-bolus insulin (BBI) therapy has been shown to be more effective and safer than sliding scale therapy for diabetic inpatients receiving dexamethasone [113]. However, a factorial survey conducted by Gerard et al. [114] reported that up to 62% of patients with GIH who require insulin therapy prefer the use of sliding scale insulin.
For outpatients learning to self-administer insulin for the first time, a single insulin injection can offer convenient benefits, especially for those unaccustomed to multiple daily injections. Given that the onset and duration of GIH caused by an intermediate-acting glucocorticoid like prednisolone align with the late peak and prolonged action of NPH insulin [79], a once-daily morning regimen combining an intermediate-acting glucocorticoid with NPH is a sensible approach. A retrospective study comparing the management of prednisone-associated GIH found that NPH and insulin glargine were equally effective in controlling blood sugar and preventing hypoglycemia [115]. Notably, the NPH regimen required lower doses of basal and corrective insulin in this study. However, for persistent hyperglycemia throughout the day when using divided doses of intermediate-acting glucocorticoid or long-acting dexamethasone, selecting insulin glargine or detemir may be advisable due to their relatively longer duration of action [77]. When initiating insulin therapy, the appropriate dose is calculated based on body weight and adjusted according to the type and dose of glucocorticoid (Table 2) [5].
Stable and consistent glycemic control is crucial for improving the prognosis of hospitalized patients. Therefore, the addition of bolus insulin to basal insulin is necessary for persistent GIH, rather than relying on a sliding scale regimen [44,113]. When employing a BBI regimen in patients taking intermediate-acting glucocorticoids, insulin glargine and NPH insulin were found to be equally effective as basal insulin. However, there was a relative increase in the required dose of bolus insulin with NPH, along with a trend toward more hypoglycemic events, although this was not statistically significant [88,112]. In a cross-sectional study that followed a protocol where half of the calculated insulin dose was administered as basal and the other half as bolus, the BBI group exhibited a higher mean daily glucose concentration compared to the control group, which did not use prednisolone [116]. This necessitated subsequent increases in the doses of bolus correction insulin at lunch and dinner. BBI and 50/50 premixed insulin were found to be similarly effective in achieving treatment goals [117]. However, the premixed insulin group in this study had an 8% higher proportion of glucose values within the target range and showed a trend toward a lower risk of hypoglycemia [117].
Spanakis et al. [118] suggested that to achieve normoglycemia, a higher proportion of the total daily insulin dose should be administered as bolus insulin—approximately 65% to 70%— with the remaining 30% to 35% as basal insulin. Studies that have incorporated corrective insulin, including NPH, into BBI therapy have indicated glycemic control that is non-inferior or even superior, with a favorable risk profile for hypoglycemia and no significant difference in the total daily insulin dose [119,120]. Therefore, BBI therapy, which can flexibly address postprandial hyperglycemia, is preferred for inpatients with confirmed GIH who require insulin treatment. It is crucial to tailor bolus and correction doses to the patient. For patients with severe GIH who experience high glycemic variability, the ideal management strategy to maintain normoglycemia while preventing hypoglycemia is to combine BBI and correction doses with a CGMS.
Patients on glucocorticoid therapy require comprehensive lifestyle management to prevent hyperglycemia. This includes regular glucose monitoring for early detection, diligent management without side effects, and education to prevent hypoglycemia. Glucose monitoring should be maintained until levels return to the normoglycemic range that was present before glucocorticoid treatment commenced [76]. Additionally, an HbA1c test is recommended 3 months after starting glucocorticoids to screen for the onset of GIH [69]. Patients should be informed about the symptoms of acute hyperglycemia, such as polyuria, polydipsia, and weight loss. Education is also essential for patients to recognize potential side effects of various glucose-lowering agents. For instance, those using insulin secretagogues or insulin, who are at risk for hypoglycemia, should understand the importance of regular fasting blood glucose tests and know how to adjust their medication to prevent hypoglycemia, particularly when planning to taper or discontinue glucocorticoid therapy. Patients using TZDs need information about worsening edema and increased risk of fractures, which can be intensified by glucocorticoids. Those using SGLT-2is should be vigilant for genitourinary infections, as the risk of infection may be heightened by glucocorticoid use. In cases of thyrotoxicosis, high doses of glucocorticoids should be administered with caution to patients with periodic paralysis, as they may exacerbate hypokalemia, especially when used in conjunction with insulin [121].
Glucocorticoids offer potent therapeutic benefits and have seen increased use during the COVID-19 pandemic. However, GIH demands greater attention in clinical practice, as it remains a neglected problem. Collaborative efforts among experts in diverse fields are crucial to gather high-quality evidence regarding the pathophysiology of GIH, as well as to refine patient evaluation, diagnosis, and management strategies. Furthermore, it is essential to prevent complications associated with GIH and improve patient outcomes by identifying novel therapeutic targets or achieving consensus on optimal management practices beyond current standards.
REFERENCES
1. Narwani V, Swafe L, Stavraka C, Dhatariya K. How frequently are bedside glucose levels measured in hospital inpatients on glucocorticoid treatment? Clin Med (Lond). 2014; 14:327–8.

2. 360ResearchReports. Steroid-corticosteroids market size in 2023: share, trends, opportunities analysis forecast report by 2030 [Internet]. Sunnyvale: Linkedin;2023. [cited 2024 Feb 29]. Available from: https://www.linkedin.com/pulse/steroid-corticosteroids-market-size-2023.
3. Schneiter P, Tappy L. Kinetics of dexamethasone-induced alterations of glucose metabolism in healthy humans. Am J Physiol. 1998; 275:E806–13.

4. Perez A, Jansen-Chaparro S, Saigi I, Bernal-Lopez MR, Minambres I, Gomez-Huelgas R. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014; 6:9–20.
6. Fardet L, Petersen I, Nazareth I. Monitoring of patients on long-term glucocorticoid therapy: a population-based cohort study. Medicine (Baltimore). 2015; 94:e647.
7. Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006; 12:358–62.

8. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021; 384:693–704.

9. Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022; 18:540–57.

10. Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care. 2006; 29:2728–9.

11. Kleinhans M, Albrecht LJ, Benson S, Fuhrer D, Dissemond J, Tan S. Continuous glucose monitoring of steroid-induced hyperglycemia in patients with dermatologic diseases. J Diabetes Sci Technol. 2023; Jan. 5. [Epub]. https://doi.org/10.1177/19322968221147937.

12. Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013; 99:277–80.

13. Delfs N, Struja T, Gafner S, Muri T, Baechli C, Schuetz P, et al. Outcomes of hospitalized patients with glucocorticoid-induced hyperglycemia: a retrospective analysis. J Clin Med. 2020; 9:4079.

14. Chakkera HA, Weil EJ, Castro J, Heilman RL, Reddy KS, Mazur MJ, et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol. 2009; 4:853–9.

15. Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015; 385:1511–8.

16. Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med. 2002; 17:717–20.

17. Liu XX, Zhu XM, Miao Q, Ye HY, Zhang ZY, Li YM. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014; 65:324–32.

18. Chan HW, Cheung CY, Liu YL, Chan YH, Wong HS, Chak WL, et al. Prevalence of abnormal glucose metabolism in Chinese renal transplant recipients: a single centre study. Nephrol Dial Transplant. 2008; 23:3337–42.

19. Kulkarni S, Durham H, Glover L, Ather O, Phillips V, Nemes S, et al. Metabolic adverse events associated with systemic corticosteroid therapy: a systematic review and meta-analysis. BMJ Open. 2022; 12:e061476.
20. Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007; 56:1703–11.
21. Morton NM, Seckl JR. 11Beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res. 2008; 36:146–64.
22. Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014; 1842:473–81.

23. Udden J, Bjorntorp P, Arner P, Barkeling B, Meurling L, Rossner S. Effects of glucocorticoids on leptin levels and eating behaviour in women. J Intern Med. 2003; 253:225–31.

24. Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004; 145:3754–62.

25. Kong X, Yu J, Bi J, Qi H, Di W, Wu L, et al. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes. 2015; 64:393–404.

26. Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. 2009; 23:1161–70.

27. van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009; 39:81–93.

28. Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005; 48:2119–30.

29. Rafacho A, Ortsater H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. 2014; 223:R49–62.

30. Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induce increased beta-cell proliferation in pancreatic rat islets. Am J Physiol Endocrinol Metab. 2009; 296:E681–9.
31. Linssen MM, van Raalte DH, Toonen EJ, Alkema W, van der Zon GC, Dokter WH, et al. Prednisolone-induced beta cell dysfunction is associated with impaired endoplasmic reticulum homeostasis in INS-1E cells. Cell Signal. 2011; 23:1708–15.

32. Pagano G, Cavallo-Perin P, Cassader M, Bruno A, Ozzello A, Masciola P, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest. 1983; 72:1814–20.

33. Henriksen JE, Alford F, Ward GM, Beck-Nielsen H. Risk and mechanism of dexamethasone-induced deterioration of glucose tolerance in non-diabetic first-degree relatives of NIDDM patients. Diabetologia. 1997; 40:1439–48.

34. Ha Y, Lee KH, Jung S, Lee SW, Lee SK, Park YB. Glucocorticoid-induced diabetes mellitus in patients with systemic lupus erythematosus treated with high-dose glucocorticoid therapy. Lupus. 2011; 20:1027–34.

35. Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007; 275:43–61.

36. Kraus-Friedmann N. Hormonal regulation of hepatic gluconeogenesis. Physiol Rev. 1984; 64:170–259.

37. Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RH, Leone TC, et al. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003; 9:1069–75.

38. Rahimi L, Rajpal A, Ismail-Beigi F. Glucocorticoid-induced fatty liver disease. Diabetes Metab Syndr Obes. 2020; 13:1133–45.
39. Zhang L, Wang Z, Xu F, Han D, Li S, Yin H, et al. Effects of stress hyperglycemia on short-term prognosis of patients without diabetes mellitus in coronary care unit. Front Cardiovasc Med. 2021; 8:683932.

40. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001; 32:2426–32.

41. Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003; 55:33–8.

42. Leonidou L, Michalaki M, Leonardou A, Polyzogopoulou E, Fouka K, Gerolymos M, et al. Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am J Med Sci. 2008; 336:467–71.

43. Korytkowski MT, Muniyappa R, Antinori-Lent K, Donihi AC, Drincic AT, Hirsch IB, et al. Management of hyperglycemia in hospitalized adult patients in non-critical care settings: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2022; 107:2101–28.

44. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of care in diabetes-2024. Diabetes Care. 2024; 47(Suppl 1):S295–306.
45. Kwon S, Hermayer KL, Hermayer K. Glucocorticoid-induced hyperglycemia. Am J Med Sci. 2013; 345:274–7.

46. Klein NC, Go CH, Cunha BA. Infections associated with steroid use. Infect Dis Clin North Am. 2001; 15:423–32.

47. Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005; 33:1624–33.

48. Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004; 100:1179–85.

49. Jung SH, Jang HC, Lee SS, Ahn JS, Yang DH, Kim YK, et al. The impact of hyperglycemia on risk of severe infections during early period of induction therapy in patients with newly diagnosed multiple myeloma. Biomed Res Int. 2014; 2014:413149.

50. Jeong Y, Han HS, Lee HD, Yang J, Jeong J, Choi MK, et al. A pilot study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Treat. 2016; 48:1429–37.

51. Galindo RJ, Fried M, Breen T, Tamler R. Hyperglycemia management in patients with posttransplantation diabetes. Endocr Pract. 2016; 22:454–65.

52. World Health Organization. Number of COVID-19 cases reported to WHO [Internet]. Geneva: WHO;2024. [cited 2024 Feb 29]. Available from: https://data.who.int/dashboards/covid19/cases?n=c.
53. Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care. 2021; 44:2645–55.

54. Gianchandani R, Esfandiari NH, Ang L, Iyengar J, Knotts S, Choksi P, et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes. 2020; 69:2048–53.

55. Barreto EA, Cruz AS, Veras FP, Martins R, Bernardelli RS, Paiva IM, et al. COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis. Proc Natl Acad Sci U S A. 2023; 120:e2217119120.

56. Chen J, Wu C, Wang X, Yu J, Sun Z. The impact of COVID-19 on blood glucose: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020; 11:574541.

57. Liu SP, Zhang Q, Wang W, Zhang M, Liu C, Xiao X, et al. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract. 2020; 167:108338.

58. Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes Metab Syndr. 2020; 14:1563–9.

59. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet]. Bethesda: National Institutes of Health;2023. [cited 2024 Feb 29]. Available from: https://www.covid19treatmentguidelines.nih.gov/.
60. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JA, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020; 324:1330–41.
61. Granholm A, Munch MW, Myatra SN, Vijayaraghavan BK, Cronhjort M, Wahlin RR, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022; 48:45–55.

62. Fornwald CR, Tuttle NS, Murphy JA. NPH insulin versus insulin glargine versus NPH insulin plus insulin glargine for the treatment of dexamethasone-induced hyperglycemia in patients with COVID-19: a retrospective cohort study. J Pharm Technol. 2023; 39:68–74.

63. Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020; 31:1068–77.

64. Sardu C, D’Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, Messina V, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020; 43:1408–15.

65. Rayman G, Lumb AN, Kennon B, Cottrell C, Nagi D, Page E, et al. Dexamethasone therapy in COVID-19 patients: implications and guidance for the management of blood glucose in people with and without diabetes. Diabet Med. 2021; 38:e14378.

66. Brooks D, Schulman-Rosenbaum R, Griff M, Lester J, Low Wang CC. Glucocorticoid-induced hyperglycemia including dexamethasone-associated hyperglycemia in COVID-19 infection: a systematic review. Endocr Pract. 2022; 28:1166–77.

67. Barker HL, Morrison D, Llano A, Sainsbury CA, Jones GC. Practical guide to glucocorticoid induced hyperglycaemia and diabetes. Diabetes Ther. 2023; 14:937–45.

68. Katsuyama T, Sada KE, Namba S, Watanabe H, Katsuyama E, Yamanari T, et al. Risk factors for the development of glucocorticoid-induced diabetes mellitus. Diabetes Res Clin Pract. 2015; 108:273–9.

69. Roberts A, James J, Dhatariya K; Joint British Diabetes Societies (JBDS) for Inpatient Care. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet Med. 2018; 35:1011–7.
70. See KC. Impact of inhaled and intranasal corticosteroids on glucose metabolism and diabetes mellitus: a mini review. World J Diabetes. 2023; 14:1202–11.

71. Phan K, Smith SD. Topical corticosteroids and risk of diabetes mellitus: systematic review and meta-analysis. J Dermatolog Treat. 2021; 32:345–9.

72. Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, Gomez-Almaguer D, Lavalle-Gonzalez FJ, Tamez-Perez HE, et al. Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol Metab Syndr. 2013; 5:18.

73. Deutsch AJ, Schroeder PH, Mandla R, Kang S, Erenler F, Mercader JM, et al. Type 2 diabetes polygenic score predicts the risk of glucocorticoid-induced hyperglycemia in patients without diabetes. Diabetes Care. 2023; 46:1541–5.

74. American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2024. Diabetes Care. 2024; 47(Suppl 1):S20–42.
75. Goyal A, Gupta S, Gupta Y, Tandon N. Proposed guidelines for screening of hyperglycemia in patients hospitalized with COVID-19 in low resource settings. Diabetes Metab Syndr. 2020; 14:753–6.

76. Mills E, Devendra S. Steroid-induced hyperglycaemia in primary care. London J Prim Care (Abingdon). 2015; 7:103–6.

77. Wallace MD, Metzger NL. Optimizing the treatment of steroid-induced hyperglycemia. Ann Pharmacother. 2018; 52:86–90.

78. Uzu T, Harada T, Sakaguchi M, Kanasaki M, Isshiki K, Araki S, et al. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract. 2007; 105:c54–7.

79. Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab. 2011; 96:1789–96.

80. Burt MG, Willenberg VM, Petersons CJ, Smith MD, Ahern MJ, Stranks SN. Screening for diabetes in patients with inflammatory rheumatological disease administered long-term prednisolone: a cross-sectional study. Rheumatology (Oxford). 2012; 51:1112–9.

81. Aberer F, Hochfellner DA, Sourij H, Mader JK. A practical guide for the management of steroid induced hyperglycaemia in the hospital. J Clin Med. 2021; 10:2154.

82. Imatoh T, Sai K, Hori K, Segawa K, Kawakami J, Kimura M, et al. Development of a novel algorithm for detecting glucocorticoid-induced diabetes mellitus using a medical information database. J Clin Pharm Ther. 2017; 42:215–20.

83. Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010; 363:1410–8.

84. Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care. 2015; 38:1610–4.

85. van Hooijdonk RT, Binnekade JM, Bos LD, Horn J, Juffermans NP, Abu-Hanna A, et al. Associations between bolus infusion of hydrocortisone, glycemic variability and insulin infusion rate variability in critically Ill patients under moderate glycemic control. Ann Intensive Care. 2015; 5:34.

86. Khanimov I, Boaz M, Shimonov M, Wainstein J, Leibovitz E. Systemic treatment with glucocorticoids is associated with incident hypoglycemia and mortality: a historical prospective analysis. Am J Med. 2020; 133:831–8.

87. Yates CJ, Fourlanos S, Colman PG, Cohney SJ. Divided dosing reduces prednisolone-induced hyperglycaemia and glycaemic variability: a randomized trial after kidney transplantation. Nephrol Dial Transplant. 2014; 29:698–705.

88. Ruiz de Adana MS, Colomo N, Maldonado-Araque C, Fontalba MI, Linares F, Garcia-Torres F, et al. Randomized clinical trial of the efficacy and safety of insulin glargine vs. NPH insulin as basal insulin for the treatment of glucocorticoid induced hyperglycemia using continuous glucose monitoring in hospitalized patients with type 2 diabetes and respiratory disease. Diabetes Res Clin Pract. 2015; 110:158–65.

89. Gerards MC, de Maar JS, Steenbruggen TG, Hoekstra JB, Vriesendorp TM, Gerdes VE. Add-on treatment with intermediate-acting insulin versus sliding-scale insulin for patients with type 2 diabetes or insulin resistance during cyclic glucocorticoid-containing antineoplastic chemotherapy: a randomized crossover study. Diabetes Obes Metab. 2016; 18:1041–4.

90. Yata Y, Hosojima M, Kabasawa H, Ishikawa T, Kaseda R, Iino N, et al. The assessment of the efficacy of dipeptidyl peptidase-4 inhibitors in patients with glucocorticoid-induced diabetes by continuous glucose monitoring. Intern Med. 2017; 56:2555–62.

91. Watanabe T, Ozawa A, Ishii S, Tomaru T, Shibusawa N, Saito T, et al. Usage of continuous glucose monitoring (CGM) for detecting an unrecognized hypoglycemia and management of glucocorticoid replacement therapy in adult patients with central hypoadrenalism. Endocr J. 2018; 65:547–56.

92. Uchihara M, Kodani N, Bouchi R, Saito S, Miyazato Y, Sugimoto H, et al. Glycemic control using intermittently scanned continuous glucose monitoring in patients with diabetes requiring methylprednisolone therapy for severe COVID-19. Glob Health Med. 2022; 4:336–40.

93. Herman R, Kravos NA, Jensterle M, Janez A, Dolzan V. Metformin and insulin resistance: a review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int J Mol Sci. 2022; 23:1264.

94. Seelig E, Meyer S, Timper K, Nigro N, Bally M, Pernicova I, et al. Metformin prevents metabolic side effects during systemic glucocorticoid treatment. Eur J Endocrinol. 2017; 176:349–58.

95. Pernicova I, Kelly S, Ajodha S, Sahdev A, Bestwick JP, Gabrovska P, et al. Metformin to reduce metabolic complications and inflammation in patients on systemic glucocorticoid therapy: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. Lancet Diabetes Endocrinol. 2020; 8:278–91.

96. Campbell IW, Mariz S. Beta-cell preservation with thiazolidinediones. Diabetes Res Clin Pract. 2007; 76:163–76.
97. Willi SM, Kennedy A, Brant BP, Wallace P, Rogers NL, Garvey WT. Effective use of thiazolidinediones for the treatment of glucocorticoid-induced diabetes. Diabetes Res Clin Pract. 2002; 58:87–96.

98. Luther P, Baldwin D Jr. Pioglitazone in the management of diabetes mellitus after transplantation. Am J Transplant. 2004; 4:2135–8.

99. Eriksen M, Jensen DH, Tribler S, Holst JJ, Madsbad S, Krarup T. Reduction of insulinotropic properties of GLP-1 and GIP after glucocorticoid-induced insulin resistance. Diabetologia. 2015; 58:920–8.

100. Miyawaki Y, Sada KE, Asano Y, Hayashi K, Yamamura Y, Hiramatsu S, et al. An open-label pilot study on preventing glucocorticoid-induced diabetes mellitus with linagliptin. J Med Case Rep. 2018; 12:288.

101. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018; 27:740–56.

102. van Raalte DH, van Genugten RE, Linssen MM, Ouwens DM, Diamant M. Glucagon-like peptide-1 receptor agonist treatment prevents glucocorticoid-induced glucose intolerance and islet-cell dysfunction in humans. Diabetes Care. 2011; 34:412–7.

103. Uchinuma H, Ichijo M, Harima N, Tsuchiya K. Dulaglutide improves glucocorticoid-induced hyperglycemia in inpatient care and reduces dose and injection frequency of insulin. BMC Endocr Disord. 2020; 20:58.

104. Kasayama S, Tanaka T, Hashimoto K, Koga M, Kawase I. Efficacy of glimepiride for the treatment of diabetes occurring during glucocorticoid therapy. Diabetes Care. 2002; 25:2359–60.

105. Davies MJ. Insulin secretagogues. Curr Med Res Opin. 2002; 18 Suppl 1:s22–30.
106. Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015; 36:2288–96.

107. Turk T, Pietruck F, Dolff S, Kribben A, Janssen OE, Mann K, et al. Repaglinide in the management of new-onset diabetes mellitus after renal transplantation. Am J Transplant. 2006; 6:842–6.

108. Tanaka K, Okada Y, Mori H, Torimoto K, Arao T, Tanaka Y. The effects of mitiglinide and repaglinide on postprandial hyperglycemia in patients undergoing methylprednisolone pulse therapy. Intern Med. 2018; 57:65–70.

109. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018; 61:2108–17.

110. Gerards MC, Venema GE, Patberg KW, Kross M, Potter van Loon BJ, Hageman IM, et al. Dapagliflozin for prednisone-induced hyperglycaemia in acute exacerbation of chronic obstructive pulmonary disease. Diabetes Obes Metab. 2018; 20:1306–10.

111. Klarskov CK, Holm Schultz H, Persson F, Moller Christensen T, Almdal TP, Snorgaard O, et al. Study rationale and design of the EANITIATE study (EmpAgliflozin compared to NPH Insulin for sTeroId diAbeTEs): a randomized, controlled, multicenter trial of safety and efficacy of treatment with empagliflozin compared with NPH-insulin in patients with newly onset diabetes following initiation of glucocorticoid treatment. BMC Endocr Disord. 2020; 20:86.
112. Radhakutty A, Stranks JL, Mangelsdorf BL, Drake SM, Roberts GW, Zimmermann AT, et al. Treatment of prednisolone-induced hyperglycaemia in hospitalized patients: insights from a randomized, controlled study. Diabetes Obes Metab. 2017; 19:571–8.
113. Gosmanov AR, Goorha S, Stelts S, Peng L, Umpierrez GE. Management of hyperglycemia in diabetic patients with hematologic malignancies during dexamethasone therapy. Endocr Pract. 2013; 19:231–5.

114. Gerards MC, Tervaert EC, Hoekstra JB, Vriesendorp TM, Gerdes VE. Physician’s attitudes towards diagnosing and treating glucocorticoid induced hyperglycaemia: sliding scale regimen is still widely used despite guidelines. Diabetes Res Clin Pract. 2015; 109:246–52.

115. Dhital SM, Shenker Y, Meredith M, Davis DB. A retrospective study comparing neutral protamine hagedorn insulin with glargine as basal therapy in prednisone-associated diabetes mellitus in hospitalized patients. Endocr Pract. 2012; 18:712–9.

116. Burt MG, Drake SM, Aguilar-Loza NR, Esterman A, Stranks SN, Roberts GW. Efficacy of a basal bolus insulin protocol to treat prednisolone-induced hyperglycaemia in hospitalised patients. Intern Med J. 2015; 45:261–6.

117. Merkofer F, Struja T, Delfs N, Spagnuolo CC, Hafner JF, Kupferschmid K, et al. Glucose control after glucocorticoid administration in hospitalized patients: a retrospective analysis. BMC Endocr Disord. 2022; 22:8.
118. Spanakis EK, Shah N, Malhotra K, Kemmerer T, Yeh HC, Golden SH. Insulin requirements in non-critically ill hospitalized patients with diabetes and steroid-induced hyperglycemia. Hosp Pract (1995). 2014; 42:23–30.

119. Grommesh B, Lausch MJ, Vannelli AJ, Mullen DM, Bergenstal RM, Richter SA, et al. Hospital insulin protocol aims for glucose control in glucocorticoid-induced hyperglycemia. Endocr Pract. 2016; 22:180–9.

Fig. 1.
Key aspects of the pathophysiology of glucocorticoid-induced hyperglycemia. Modified from Li et al. [9], with permission from Springer Nature.
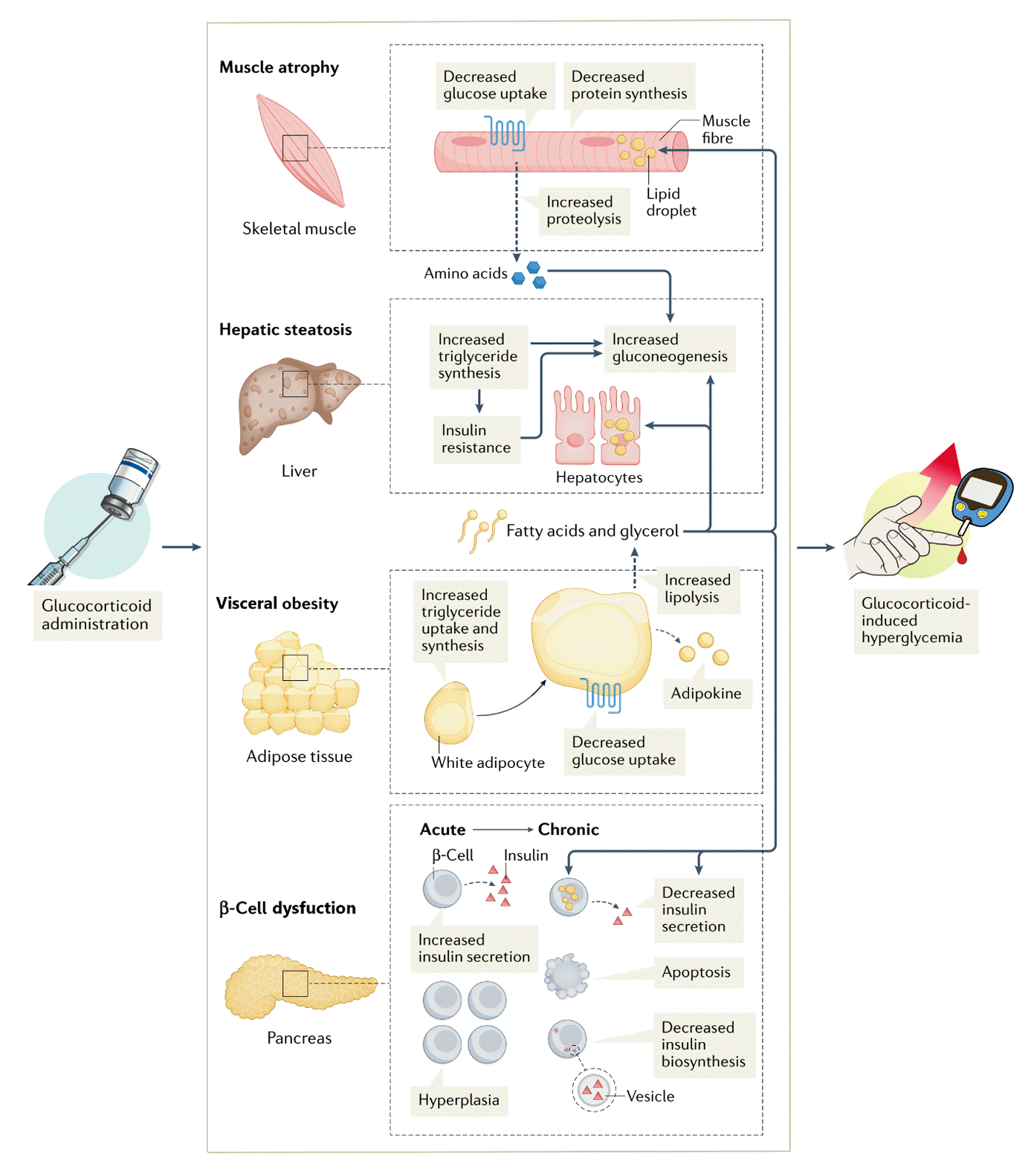
Fig. 2.
Schematic diagram of hyperglycemia in hospitalized patients with coronavirus disease 2019 (COVID-19) infection receiving respiratory support.
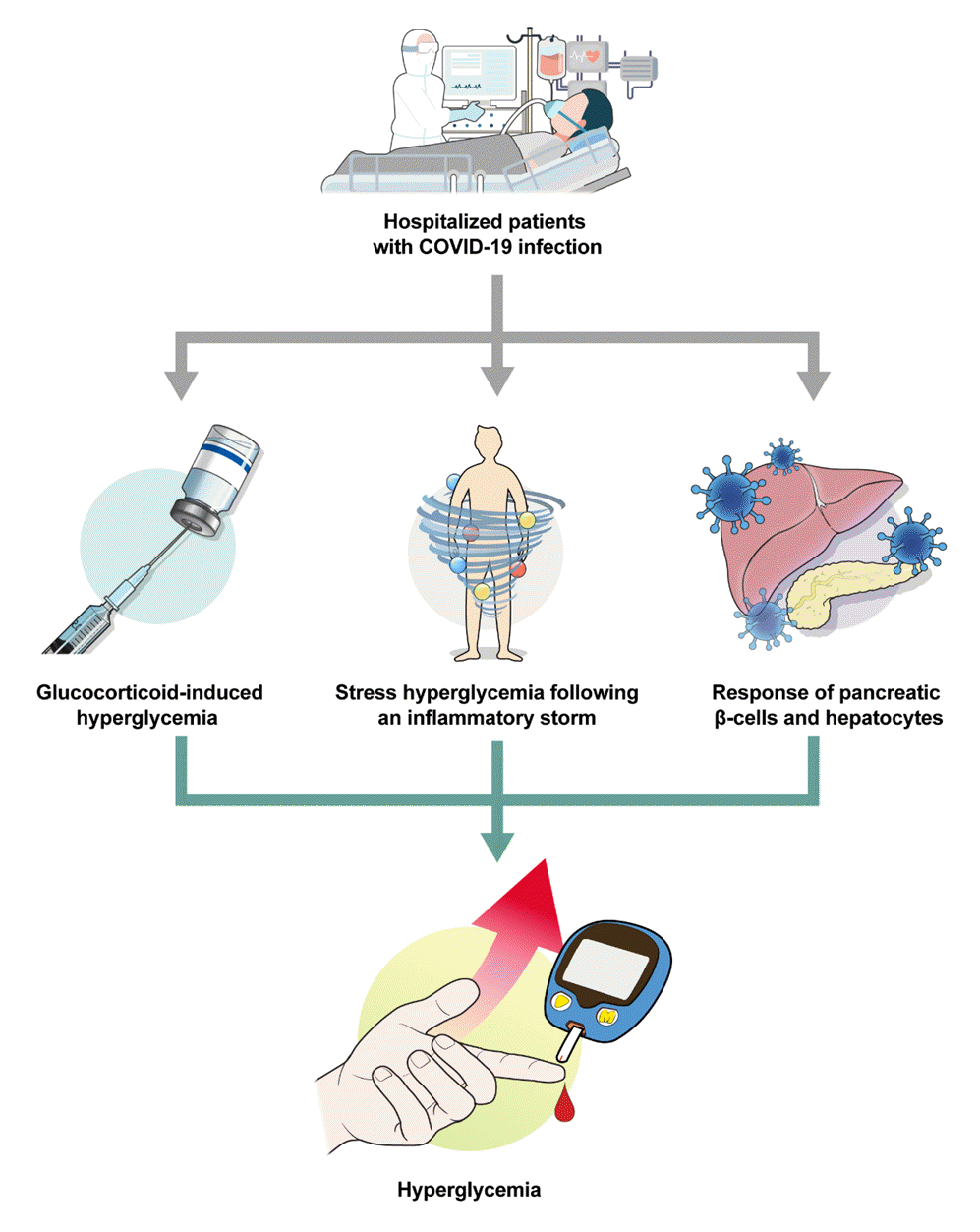
Table 1.
Risk Factors for Glucocorticoid-Induced Hyperglycemia




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download