Abstract
Purpose
Neoadjuvant chemotherapy is strongly recommended for advanced gastric cancer due to good local control and a high rate of R0 dissection with this strategy. Minimally invasive techniques such as laparoscopy-assisted or total laparoscopic approaches is becoming more and more acceptable in the treatment for gastric cancer. However, the safety and efficiency of total laparoscopic D2 gastrectomy (TLG) for advanced gastric cancer after neoadjuvant chemotherapy have not been well evaluated.
Methods
A retrospective study in a single center from 2014 to 2016 was conducted. A total of 65 locally advanced gastric cancers were treated by laparoscopy-assisted gastrectomy (LAG) or TLG. Parameters which include operation time, blood loss, complications, hospital stay, 3-year overall survival, and 3-year disease-free survival were used for comparison.
Results
The time of operation in the TLG group was shorter than in the LAG group (P = 0.013), blood loss was less (P = 0.002) and time to first flatus was shorter (P = 0.039) in the TLG group than that in the LLG group. Intraoperative and postoperative complications were comparable in both groups. No significant difference was found in 3-year overall and disease-free survival.
Conclusion
For patients with locally advanced gastric cancer after neoadjuvant chemotherapy, laparoscopic D2 gastrectomy can be considered as a safe and efficient alternative. A further multicenter prospective randomized controlled study is needed to elucidate the applicability of this technique for advanced gastric cancer.
Advanced gastric cancer is most common in patients suffering from gastric cancer in China. According to worldwide guidelines, neoadjuvant chemotherapy resulting in lower recurrence and higher R0 dissection rate is strongly recommended for advanced gastric cancer, although 5-year disease-free survival (DFS) and overall survival (OS) are still under debate compared with that after direct surgery [1]. Till now, open radical resection remains the gold standard of treatment for gastric cancer. However, more and more studies indicate that minimally invasive surgery such as laparoscopy is feasible and safe for gastric cancer, and the long-term outcomes after minimally invasive surgery are comparable with those after an open approach [23]. However, those studies were mainly focused on laparoscopy-assisted surgery in early-stage patients. Total laparoscopic surgery as a new alternative to minimally invasive surgery is convincing as an equivalent to the laparoscopic approach [4]. Nevertheless, there is no study to elucidate the feasibility and safety of total laparoscopic surgery in gastric cancer patients after neoadjuvant chemotherapy. Therefore, this study was conducted to assess short- and long-term outcomes of total laparoscopic surgery for gastric cancer after neoadjuvant chemotherapy.
This study conformed to the ethical standards of the World Medical Association Declaration of Helsinki. It was approved by the Institutional Review Board of the General Hospital of Western Theater Command (No. 2015027) and all patients had signed informed consent forms.
This retrospective study was performed between October 2014 and March 2016. The study included 65 advanced gastric cancer patients; 34 in laparoscopy-assisted D2 gastrectomy (LAG) and 31 in total laparoscopic D2 gastrectomy (TLG). All patients underwent esophagogastroduodenoscopy, endoscopic ultrasound, and enhanced computed tomography. Patients diagnosed with cT3-4N+M0 were included according to the American Joint Committee on Cancer staging manual, 7th edition. All patients received neoadjuvant chemotherapy by DCF (docetaxel, cisplatin, 5-fluorouracil), or ECF (epirubicin, cisplatin, and fluorouracil) for 3 cycles after multidisciplinary treatment discussion. Evaluation of postchemotherapy was performed according to RECIST 1.1 criteria [5].
The clinical characteristics include toxic effects, pathological stages, number of lymph node dissections, procedure types, postoperative complications, tumor restrict grade (TRG), and time to first flatus. Short-term outcomes include wound infection, pneumonia, intraabdominal bleeding, anastomotic leakage and bleeding, bow obstruction, deep venous thrombosis (DVT), and death. Long-term outcomes include anastomotic stenosis, bowel obstruction, 3-year recurrence rate, 3-year DFS, and 3-year OS. Postoperative complications classified by the Clavien-Dindo system were defined as any event that required conservative or surgical treatment after surgery. Early and late complications were defined as events occurring within and after 30 days after surgery, respectively.
All operations were performed by 2 experienced surgeons. In each group, one 10-mm trocar was inserted below the umbilicus for camera. One 5-mm trocar was put at the right upper quadrants 2 cm below the right lower rib margins. The other 5-mm trocar was placed in the left flank area. One 10-mm port was placed in the right upper quadrants 2 cm below the right lower rib margins. Another 12-mm trocar was put at the left upper quadrants 2 cm below the left lower rib margins. The pneumoperitoneum was made by carbon dioxide with a pressure maintained at 10–12 mmHg. Total omentectomy with D2 lymphadenectomy in both groups was performed under laparoscopy using an ultrasonic scalpel according to the guidelines published by the Japanese Gastric Cancer Association. Total or distal gastrectomy was performed depending on tumor location.
Digestive tract reconstruction was performed under assisted incision in the LAG group and laparoscopy in the TLG group. Roux-en-Y as the only method was chosen for reconstruction in both groups. In the LAG group, a small incision (<10 cm) in the upper midline was made after lymph node dissection and division of the stomach. For distal gastrectomy, the duodenum was amputated by linear stapler, and anastomosis of the gastric-jejunum and jejunojejunostomy were performed by circular stapler. For total gastrectomy, a circular stapler was used for esophagojejunostomy and jejunojejunostomy, jejunal stump was closed by a linear stapler.
In the TLG group, digestive tract reconstruction was performed intracorporeally by linear staplers for both distal and total gastrectomy (Fig. 1). The incision below the umbilicus for 10-mm trocar was then extended (less than 5 cm), and then the specimen was pulled out through this incision.
Methods of follow-up included outpatient visits, telephone, and home visits. Assessments of follow-up included physical examinations, laboratory tests, and image review. Chest and abdominal computed tomography were performed every 6 months after surgery for 3 consecutive years, then annually. Endoscopy was performed every year or when the postgastrectomy symptoms occurred. The patients were followed up for at least 36 months.
Continuous variables were expressed as mean ± standard deviation and categorical variables were presented as frequencies and percentages. The differences were analyzed by the t-test, chi-square test, or Fisher exact test when appropriate. Survival rates were analyzed using the Kaplan-Meier method and differences between the 2 groups were analyzed with the log-rank test. All P-values were 2-sided, and the threshold value of statistical significance was set at less than 0.05. All statistical analyses were conducted with SPSS ver. 13.0 (SPSS Inc.).
There were 65 advanced gastric cancer patients enrolled in this study; 34 in the LAG group and 31 in the TLG group. There was no significant difference between the 2 groups in age, sex, body mass index, clinical TNM stage, and pathological type. DCF was used in most of the cases in both groups, and ECF was also used in a few patients. Adverse reactions in both groups were comparative. There was no statistically significant adverse reaction between the 2 groups. No severe adverse event was found in the 2 groups (Table 1). There were 2 cases of complete response in the LAG and TLG groups each. Partial response and stable disease cases were comparable in the LAG and TLG groups (P = 0.967), no progressive disease was found in either group.
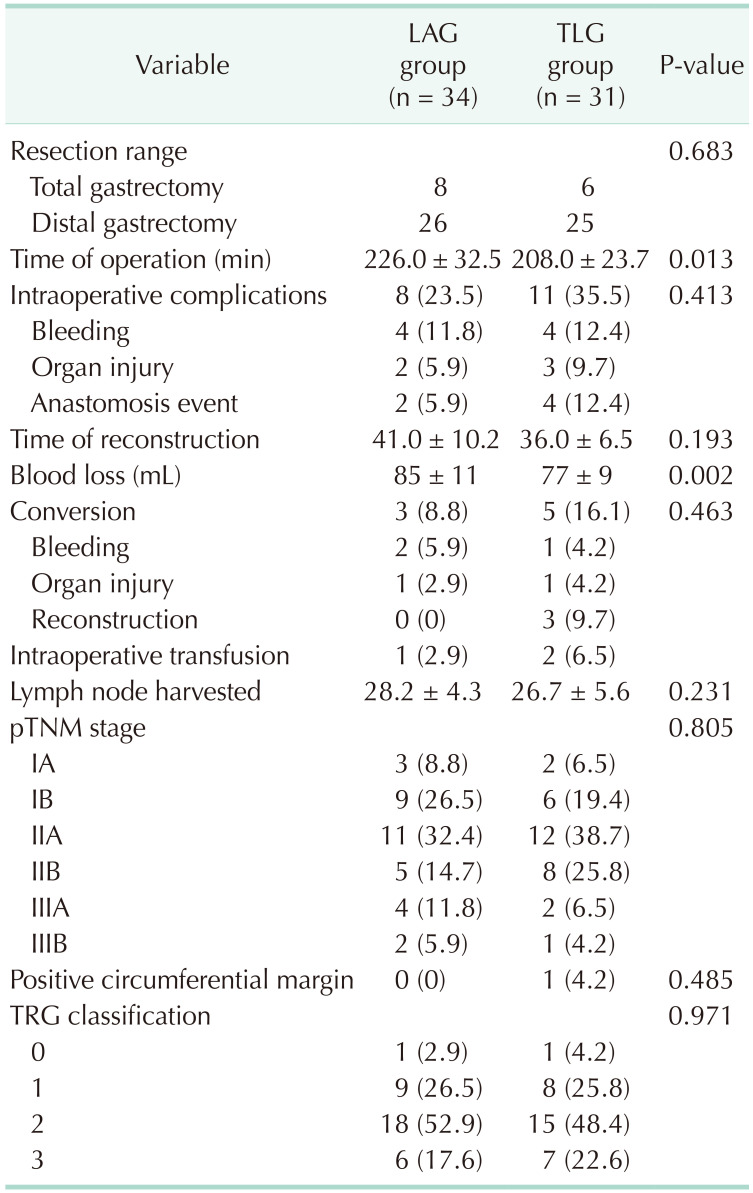
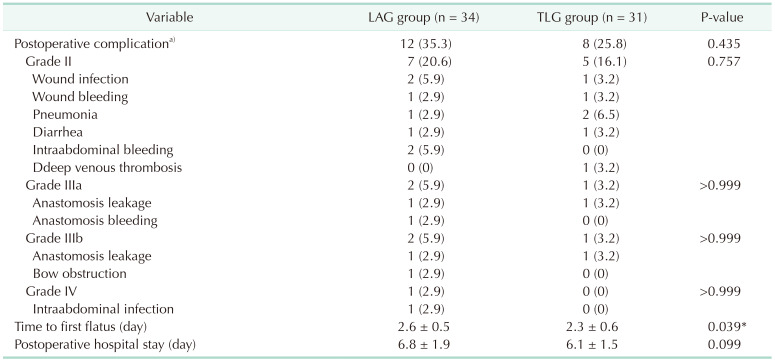
All patients were operated successfully, and no death was found in the 2 groups. Eight patients received total gastrectomy in the LAG group and 6 patients received total gastrectomy in the TLG group (P = 0.683). Operation time was 208 ± 23.7 minutes in the TLG group and 226 ± 32.5 minutes in the LAG group (P = 0.0127). Intraoperative complications and conversion rates were comparable in both groups (P = 0.413 and P = 0.463, respectively). Blood loss in the TLG group was significantly less than in the LAG group (P = 0.0018). Lymph nodes harvested were 26.7 ± 5.6 and 28.2 ± 4.3 in TLG and LAG groups, respectively (P = 0.231). No significant difference was found in terms of TRG classification (P = 0.971). One Positive circumferential margin was found in the TLG group and no such case was found in the LAG group (Table 2). Time to first flatus in the TLG and LAG groups was 2.3 ± 0.6 and 2.6 ± 0.5, respectively (P = 0.039). Postoperative hospital stay was 6.1 ± 1.5 in the TLG group and 6.8 ± 1.9 in the LAG group (P = 0.099). Postoperative complications such as anastomotic leakage and bleeding, wound infection, pneumonia, DVT, and bowel obstruction were comparable in the LAG and TLG groups (Table 3). There was no mortality in both groups after surgery.
The median follow-up time for the TLG and LAG groups was 34 and 34.5 months, respectively. During the follow-up, there were 11 and 12 deaths in the TLG and LAG groups, respectively. The overall 3-year survival in the TLG and LAG groups was 64.5% and 64.7%, respectively (P = 0.957) (Fig. 2). There were 14 and 15 cases of recurrence or metastasis in the TLG and LAG groups, respectively. The 3-year DFS of the TLG and LAG groups was 54.8% and 58.5%, respectively, with no significant difference (P = 0.907) (Fig. 3).
Minimally invasive surgery is one of the most remarkable achievements in the development of surgery in the past 20 years, and now it is widely used in many surgical fields. Undoubtedly, it will be the key direction in the future development of surgery, which is also suitable for the surgical treatment of gastric cancer. There have been many studies indicating that LAG may be favorable for early or selected advanced gastric cancer, the short- and long-term outcomes are both comparable to open technique [6]. These results show that the laparoscopy-assisted technique is becoming more and more mature, and more minimally invasive methods such as TLG may be put on the agenda for gastric cancer.
It is reported that compared with LAG, TLG had less after-surgery pain, shorter hospital stays, and faster recovery of bowel function in early gastric cancer cases [4]. However, the effect of neoadjuvant chemotherapy on laparoscopic surgery, especially on total laparoscopic surgery for advanced gastric cancer is rarely reported in the literature. Our study showed that grade III–IV 30-day complications were 14.7% and 6.5% in the LAG and TLG groups, respectively, which was similar to the literature [7]. These results indicate that neoadjuvant chemotherapy does not affect the safety of total laparoscopic gastrectomy.
Compared with LAG, TLG had similar intraoperative complications, lymph nodes harvested, time of reconstruction, postoperative complications, and conversion, but less blood loss, shorter operation time, and faster flatus. On one hand, it is believed that laparoscopic surgery is less invasive and can amplify operation fields, which are very important during operation for bleeding control and careful dissection [38]. On the other hand, a skilled laparoscopic surgeon can complete the reconstruction of the digestive tract by linear stapler and barbed suture [910]. All these advantages may explain the better short-term outcomes of TLG for gastric cancer.
One obvious difference between TLG and LAG is the procedure of digestive tract reconstruction. It is reported that the average time of R-Y reconstruction by laparoscopy is about 44 minutes [11]. Our results showed that the time of reconstruction in the TLG group was 36 ± 6.5 minutes, which was compared with the LAG group (41 ± 10.2 minutes, P = 0.193). With our experience, anastomosis and suture are not so difficult after the learning curve. Therefore, reconstruction time will shorten accordingly. However, there were 3 cases of conversion in the TLG group and none in the LAG group, due to reconstruction. Among these 3 cases, 2 cases were unable to complete anastomosis due to short lower esophagus; the other one was because of gastrojejunal anastomotic bleeding. Furthermore, one upper-located tumor was found to have a positive circumferential margin in TLG. It seems as though tumors of the upper location are more likely to occur in conversion and have a positive circumferential margin. We noticed that there are studies showing similar results [1213]. These results remind us that the upper location of tumor may be a risk factor for conversion by total laparoscopic technique and should be carefully considered in D2 gastrectomy.
It was reported that anastomotic complications after surgery were approximately 1.4%–10% [1415]. Most research showed that neoadjuvant chemotherapy did not affect anastomotic complications after surgery, open or laparoscopic [1617]. Our results showed that the rate of anastomotic complications was 8.8% and 6.5% in the LAG and TLG groups, respectively. Unlike radiotherapy, chemotherapy does not increase the difficulty of surgery and anastomosis [18]. Furthermore, neoadjuvant chemotherapy does not affect tissue healing ability after surgery [19]. These reasons may explain why the incidence of anastomotic complications did not increase significantly after neoadjuvant chemotherapy. Our results also showed that there was no significant difference in anastomotic complications between the LAG and TLG groups. This indicates that TLG does not increase the rate of anastomotic complications.
The research on TLG for advanced gastric cancer after neoadjuvant chemotherapy is rare, and even fewer on long-term outcomes. Our results showed that the 3-year OS and DFS were 64.5% and 54.8% in the TLG group, respectively. These results were comparable with open techniques [2021]. However, we still need time to receive 5-year OS and DFS. Our results also showed no significant difference with the LAG group. To the best of our knowledge, this study is the first report that total laparoscopic treatment for advanced gastric cancer after neoadjuvant chemotherapy had similar long-term survival results as laparoscopic adjuvant treatment for advanced gastric cancer.
This study has several limitations. Firstly, this is retrospective research from a single center and the sample size is small; although the baseline of patients is comparable in both groups, selective bias may exist. Secondly, this study does not compare the results with open techniques. At last, cancer recurrence and 5-year-long outcomes have not been analyzed. Therefore, these limitations should be considered when referring to our study. In the future, a multicenter prospective randomized controlled study is needed to evaluate the safety and efficacy of TLG for advanced gastric cancer after neoadjuvant chemotherapy.
In summary, TLG does not increase complications intraoperatively or postoperatively for patients with locally advanced gastric cancer after neoadjuvant chemotherapy compared with laparoscopy-assisted procedures, and the long-term outcomes were comparable with laparoscopy-assisted procedures. A further multicenter prospective randomized controlled study is needed to elucidate the applicability of this technique for advanced gastric cancer.
Notes
References
1. Long B, Yu ZY, Li Q, Du HR, Wang ZJ, Zhan H, et al. A systematic review and network meta-analysis protocol of neoadjuvant treatments for patients with gastric cancer. Medicine (Baltimore). 2018; 97:e0392. PMID: 29642200.
2. Kinoshita T, Uyama I, Terashima M, Noshiro H, Nagai E, Obama K, et al. Long-term outcomes of laparoscopic versus open surgery for clinical stage ii/iii gastric cancer: a multicenter cohort study in Japan (LOC-A Study). Ann Surg. 2019; 269:887–894. PMID: 29697447.
3. Kim JH, Jun KH, Chin HM. Short-term surgical outcomes of laparoscopy-assisted versus totally laparoscopic Billroth-II gastrectomy for gastric cancer: a matched-cohort study. BMC Surg. 2017; 17:45. PMID: 28431531.
4. Lee S, Lee H, Lee J. Feasibility and safety of totally laparoscopic radical gastrectomy for advanced gastric cancer: comparison with early gastric cancer. J Gastric Cancer. 2018; 18:152–160. PMID: 29984065.
5. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.
6. Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, et al. Long-term oncologic outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surgery. 2019; 165:1211–1216. PMID: 30772006.
7. Xing J, Wang Y, Shan F, Li S, Jia Y, Ying X, et al. Comparison of totally laparoscopic and laparoscopic assisted gastrectomy after neoadjuvant chemotherapy in locally advanced gastric cancer. Eur J Surg Oncol. 2021; 47:2023–2030. PMID: 33663942.
8. Brenkman HJ, van der Wielen NI, Ruurda JP, van Leeuwen MS, Scheepers JJ, van der Peet DL, et al. Surgical anatomy of the omental bursa and the stomach based on a minimally invasive approach: different approaches and technical steps to resection and lymphadenectomy. J Thorac Dis. 2017; 9:S809–S816. PMID: 28815078.
9. Lee HH, Son SY, Lee JH, Kim MG, Hur H, Park DJ. Surgeon's experience overrides the effect of hospital volume for postoperative outcomes of laparoscopic surgery in gastric cancer: multi-institutional study. Ann Surg Oncol. 2017; 24:1010–1017. PMID: 27834031.
10. Kim SY, Nam SH, Min JS, Kim MC. Laparoscopic reinforcement suture on staple-line of duodenal stump using barbed suture during laparoscopic gastrectomy for gastric cancer. Ann Surg Treat Res. 2017; 93:305–309. PMID: 29250509.
11. Kim MS, Kwon Y, Park EP, An L, Park H, Park S. Revisiting laparoscopic reconstruction for Billroth 1 versus Billroth 2 versus Roux-en-Y after distal gastrectomy: a systematic review and meta-analysis in the modern era. World J Surg. 2019; 43:1581–1593. PMID: 30756163.
12. Małczak P, Torbicz G, Rubinkiewicz M, Gajewska N, Sajuk N, Rozmus K, et al. Comparison of totally laparoscopic and open approach in total gastrectomy with D2 lymphadenectomy: systematic review and meta-analysis. Cancer Manag Res. 2018; 10:6705–6714. PMID: 30584365.
13. Shi Y, Li L, Xiao H, Guo S, Wang G, Tao K, et al. Feasibility of laparoscopic gastrectomy for patients with Siewert-type II/III adenocarcinoma of the esophagogastric junction: a propensity score matching analysis. PLoS One. 2018; 13:e0203125. PMID: 30256806.
14. Parisi A, Reim D, Borghi F, Nguyen NT, Qi F, Coratti A, et al. Minimally invasive surgery for gastric cancer: a comparison between robotic, laparoscopic and open surgery. World J Gastroenterol. 2017; 23:2376–2384. PMID: 28428717.
15. Oshi M, Kunisaki C, Miyamoto H, Kosaka T, Akiyama H, Endo I. Risk factors for anastomotic leakage of esophagojejunostomy after laparoscopy-assisted total gastrectomy for gastric cancer. Dig Surg. 2018; 35:28–34. PMID: 28441658.
16. Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019; 22:1044–1052. PMID: 30827001.
17. Luo H, Wu L, Huang M, Jin Q, Qin Y, Chen J. Postoperative morbidity and mortality in patients receiving neoadjuvant chemotherapy for locally advanced gastric cancers: a systematic review and meta-analysis. Medicine (Baltimore). 2018; 97:e12932. PMID: 30412102.
18. Klevebro F, Friesland S, Hedman M, Tsai JA, Lindblad M, Rouvelas I, et al. Neoadjuvant chemoradiotherapy may increase the risk of severe anastomotic complications after esophagectomy with cervical anastomosis. Langenbecks Arch Surg. 2016; 401:323–331. PMID: 27020672.
19. Berger AK, Jäger D. [Multimodal oncological therapy concepts, chemotherapy and immunosuppressive drugs: effects on surgical morbidity and mortality]. Chirurg. 2013; 84:930–936. German. PMID: 24218092.
20. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011; 29:1715–1721. PMID: 21444866.
21. Das M. Neoadjuvant chemotherapy: survival benefit in gastric cancer. Lancet Oncol. 2017; 18:e307. PMID: 28483410.
Fig. 1
Reconstruction of total laparoscopic D2 gastrectomy. (A) The distal stomach was cut off using an endoscopic linear stapler at least 5 cm from the proximal edge of the tumor. (B) Mesentery and small intestine were cut off by an endoscopic linear stapler 20 cm away from Treitz ligament. (C) Two holes were made at the proximal jejunum and 40 cm from the distal end of intestine, then an endoscopic linear stapler was inserted from the holes, and side-to-side small intestinal anastomosis was performed. (D) The common entry of intestinal anastomosis was closed by continuous barbed suture. (E) A side-to-side gastrojejunostomy was performed with the afferent loop to lesser curvature. (F) The common entry of gastrojejunostomy was closed by continuous barbed suture.
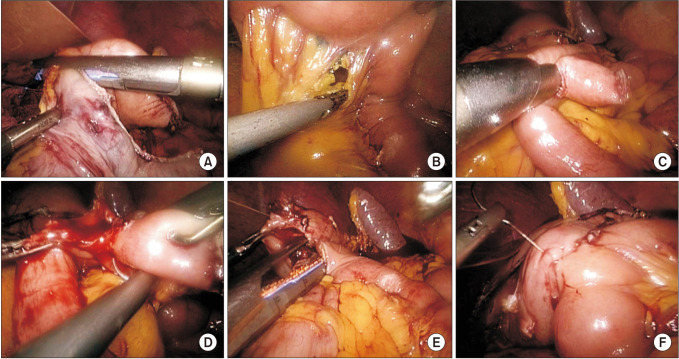
Fig. 2
Overall survival curves following total laparoscopic D2 gastrectomy (TLG) and laparoscopic-assisted D2 gastrectomy (LAG) for advanced gastric cancer after neoadjuvant chemotherapy (P = 0.951).
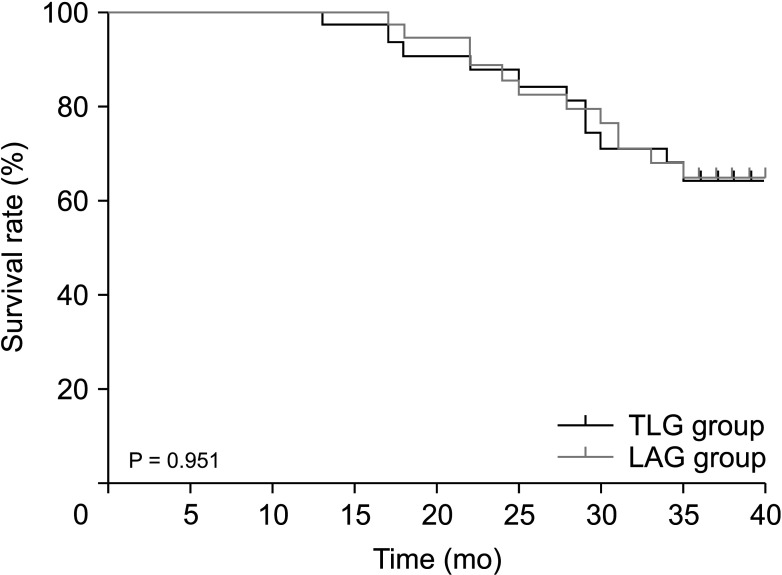
Fig. 3
Disease-free survival curves following total laparoscopic D2 gastrectomy (TLG) and laparoscopic-assisted D2 gastrectomy (LAG) for advanced gastric cancer after neoadjuvant chemotherapy (P = 0.906).
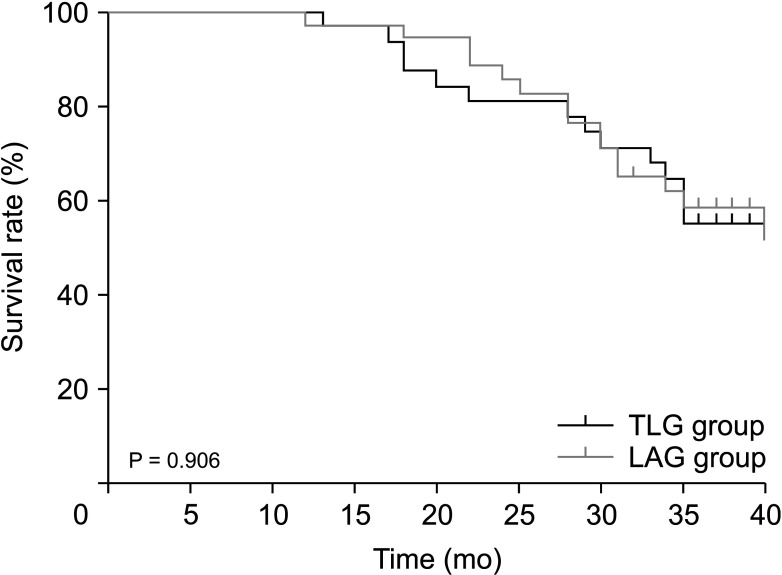
Table 1
Patient’s clinical and pathological characteristics of the 2 groups
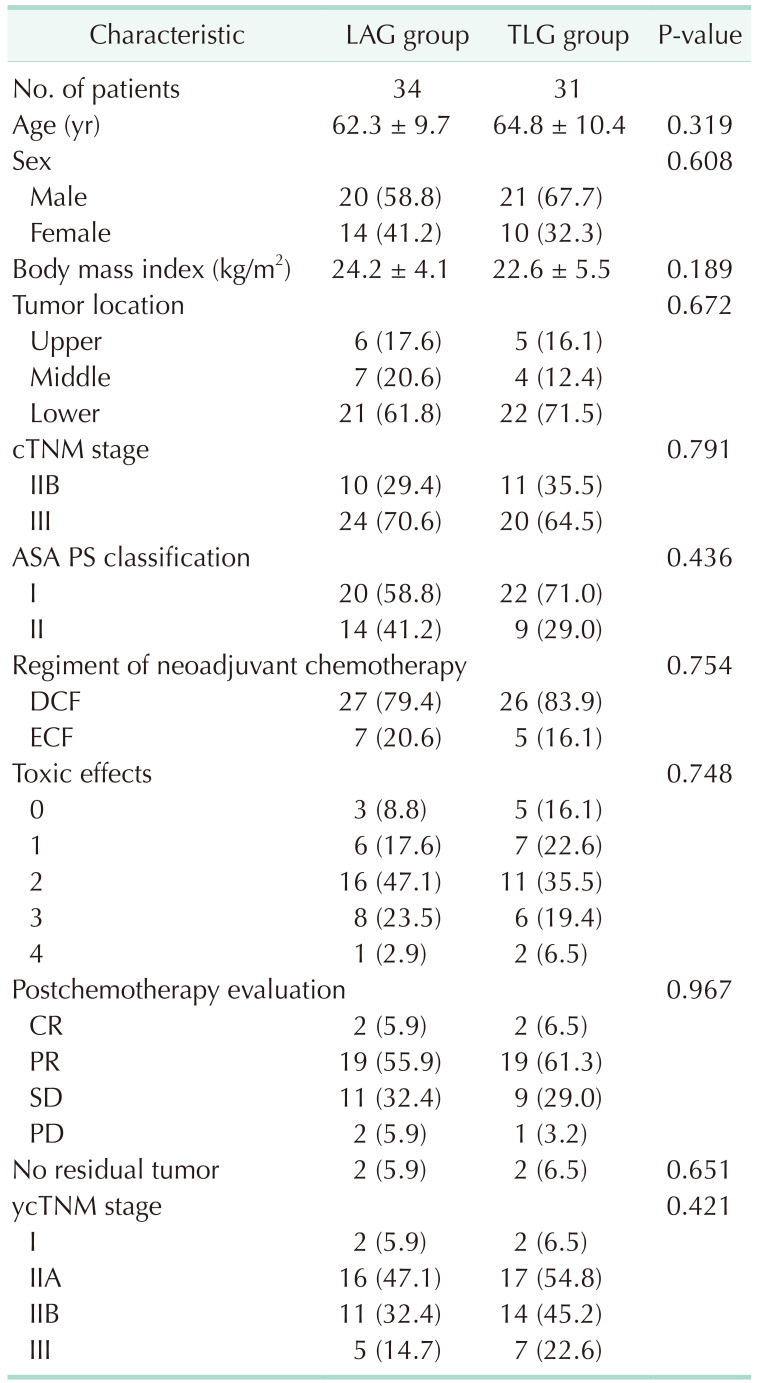
Values are presented as number only, mean ± standard deviation, or number (%).
LAG, laparoscopic-assisted D2 gastrectomy; TLG, total laparoscopic D2 gastrectomy; ASA, American Society of Anesthesiologists; PS, physical status; DCF, docetaxel, cisplatin, 5-fluorouracil; ECF, epirubicin, cisplatin, and fluorouracil; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download