Abstract
Purpose
Recent advances in the treatment of breast cancer have led to the improvement of breast cancer patient’s survival. With the prolonged survival of these patients, pregnancy became an important issue, especially in young cancer patient aged 35 years or under. Increased hormone levels during pregnancy, however, raise concerns about elevating the risk of cancer recurrence. The aim of this study was to validate the notion of increased risk associated with pregnancy after breast cancer treatment in young patients.
Methods
From January 2009 to December 2020, newly diagnosed breast cancer patients 35 years old or under who underwent optimal surgery in Korea University Guro Hospital were enrolled in this study. Patients were categorized into 3 groups: nulliparous, pregnancy prior to treatment of breast cancer, and patients with pregnancy after breast cancer treatment. Their overall survival and disease-free survival were evaluated.
Results
A total of 107 patients were enrolled in this study. Thirteen patients (12.1%) conceived and successfully delivered. The mean follow-up period after surgery was 58.9 (± 33.5) months. There was no significant difference in overall survival (P = 0.608) and disease-free survival (P = 0.591) among different groups.
Conclusion
In young patients, pregnancy after treatment for breast cancer did not affect their overall survival or diseasefree survival as compared to nullipara or previously delivered groups. Therefore, pregnancy counseling should not be prevented in young breast cancer patients 35 years old or under.
Recent advances in the treatment of breast cancer have led to improved patient survival. With the prolonged survival of these patients, pregnancy has become an important issue, especially in patients aged 35 years or younger. This is emphasized in Asian countries, where the average age at first delivery is increasing, with more women being nulliparous at the time of breast cancer diagnosis [1].
Despite the desire for pregnancy, elevated hormone levels during pregnancy raise concerns regarding the increased risk of cancer recurrence, particularly in patients with hormone receptor-positive tumors. In addition, worse outcomes in young premenopausal than older premenopausal or postmenopausal patients have been widely reported. Numerous studies have reported significantly lower survival rates and higher local and distant recurrence rates in younger patients than in older patients [2]. The difference was more pronounced in patients aged ≤35 years [3], as their tumor characteristics were associated with poorer prognostic features. Accordingly, physicians often confront challenges in discussing pregnancy safety and optimal timing of conception in young breast cancer patients, especially in those aged 35 years or younger.
Moreover, this group of patients often require adjuvant chemotherapy that is reported to cause amenorrhea and premature ovarian failure [4]. Endocrine therapy using tamoxifen further impedes subsequent pregnancy as its teratogenic feature delays attempts to childbearing [4]. Treatment-related amenorrhea following the use of tamoxifen has also been reported in several studies [5]. Likewise, numerous factors including safety and fertility preservation in premenopausal patients, especially in very young cancer patients aged 35 years or younger, need to be addressed when considering pregnancy in this subgroup.
The aim of this study was to describe the nature of pregnancy in young breast cancer patients, validate the notion of increased risk associated with pregnancy after breast cancer treatment, and review fertility preservation with assisted reproduction technology in patients aged 35 years or younger with successful pregnancy.
The study was approved by the Ethics Committee of Korea University Guro Hospital (No, 2023GR0013). It was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Newly diagnosed breast cancer patients aged 35 years or younger were enrolled in this study. Data were retrospectively collected from patients who underwent curative surgery at Korea University Guro Hospital from January 2009 to December 2020. Their medical data and pathological reports were reviewed.
Patients with ductal carcinoma in situ (DCIS) were included for the purpose of observing possible local recurrence. Meanwhile, stage IV breast cancer patients and those with missing data on stage status were excluded from the analysis. Those who were diagnosed with cancer other than breast cancer, or had an atypical histology including sarcoma or melanoma, were excluded from the analysis. Patients who were pregnant at the time of breast cancer diagnosis were excluded from this study, as it aimed to observe the outcomes of pregnancy after breast cancer treatment. Patients with ectopic pregnancies were also excluded from the analysis.
Demographic data including age, body mass index, family history of breast cancer, and cancer characteristics including subtypes, grade, histology, and staging were obtained. Although different definitions exist for classifying luminal A vs. B subtypes, in this study, luminal A was defined as a tumor that is HER2(–) and Ki-67<14%. Luminal B was defined as a tumor with HER2(–) and Ki-67 ≥14%.
The follow-up data of breast cancer patients were collected from the day of surgical treatment rather than breast cancer diagnosis, regardless of the neoadjuvant chemotherapy status. This was due to the notion that patients are more likely to consider childbearing after surgery rather than from the day of diagnosis. Data on delivery were retrospectively collected by reviewing individual medical charts. The date of conception was roughly calculated by subtracting 9 months from the date of delivery.
Due to practical use for future advice, the interval from breast cancer to subsequent pregnancy was calculated from the time of last treatment with adjuvant radiotherapy or chemotherapy. The duration of hormone therapy was not included.
The patients were categorized into 3 groups: nulliparous, previous pregnancy, and new pregnancy after breast cancer treatment. Patients without pregnancy after breast cancer treatment were divided into nulliparous and prior pregnancy groups because of controversial results being reported regarding parity and breast cancer prognosis [678910]. The nulliparous groups included patients who were not pregnant at any time before the diagnosis of breast cancer or after the curative surgery. Pregnancy prior to treatment of breast cancer included patients who became pregnant before being diagnosed with breast cancer but did not conceive after surgery. Finally, the new pregnancies after breast cancer treatment group included patients who became pregnant after curative surgery for breast cancer, regardless of their pregnancy status prior to diagnosis.
All patients received adequate consultation by the medical team before planning pregnancy. Proper preparations including fertility counseling prior to chemotherapy and careful decisions to discontinue endocrine therapy after discussing its associated risk were made once pregnancy was considered. The primary outcomes were overall survival and disease-free survival at the end of the study follow-up. Overall survival was defined as the time from curative surgery to death, while disease-free survival was defined as the time from curative surgery to the development of locoregional or distant recurrence.
The statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Corp.). The clinical characteristics were compared using the chi-square test or linear-to-linear analysis for categorical variables and analysis of variance for continuous variables. Overall and disease-free survival were estimated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. For all statistical analyses, a P-value of <0.05 was considered significant.
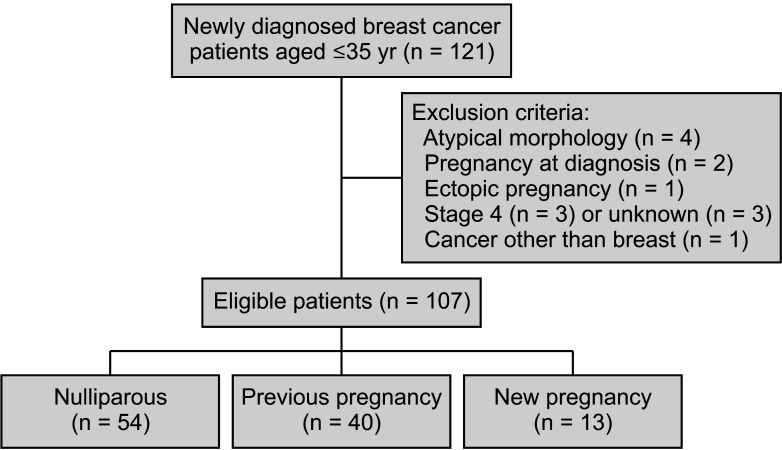
From January 2009 to December 2020, a total of 3,005 patients with either carcinoma in situ or invasive breast cancer underwent curative surgery. Among them, 121 patients were 35 years old or under. Four patients were excluded for having atypical morphology including malignant Phyllodes tumor and angiosarcoma, and 2 patients were excluded due to pregnancy at the time of breast cancer diagnosis. Another patient was excluded because of an ectopic pregnancy. Three patients with stage IV disease at the time of diagnosis and 3 patients with missing data concerning stage were also excluded from the analysis. One patient was excluded due to multiple primary malignancies. Ultimately, 107 patients were enrolled and their follow-up data were collected for analysis. The patient selection process is illustrated in Fig. 1.
Thirteen patients (12.1%) conceived and delivered successfully. Fifty-four patients (50.5%) were nulliparous, and the remaining 40 (37.4%) had a history of previous pregnancy. The mean patient age was 32.1 (±3.0) years, and the mean follow-up period was 58.9 (±33.5) months. For those who got pregnant after surgery, the mean follow-up period post-pregnancy was 43.2 (± 24.1) months.
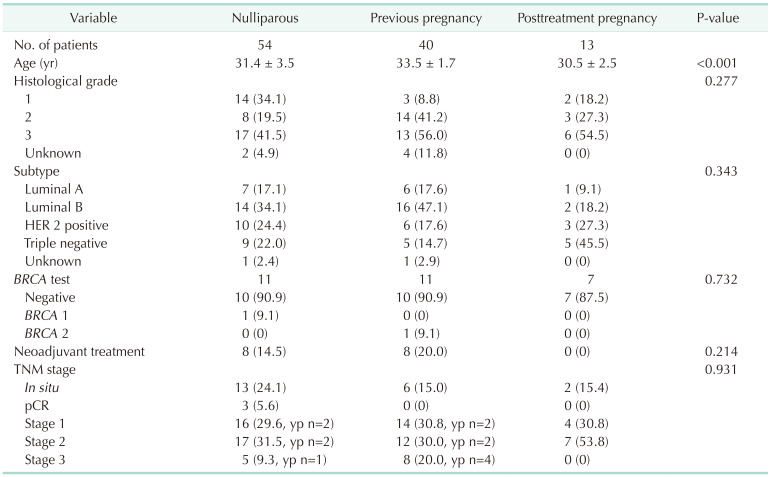
The demographic features of the patients belonging to each group are presented in Table 1. Age differed significantly among different pregnancy statuses, with the posttreatment pregnancy group being the youngest (P < 0.001). Other features including histological grade, tumor subtypes, BRCA status, neoadjuvant treatment status, and TNM staging, showed no significant differences among the groups. Twenty-one patients were diagnosed with DCIS while the other 86 patients had invasive tumors.
Adjuvant treatments patients received are shown in Table 2. Most patients, regardless of pregnancy status, received radiotherapy after surgery. There was no statistically significant difference in the adjuvant treatments patients received among different groups.
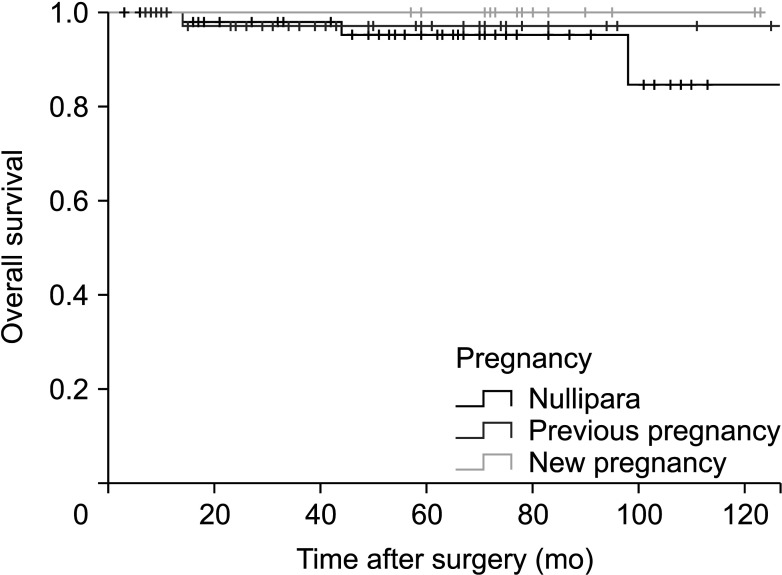
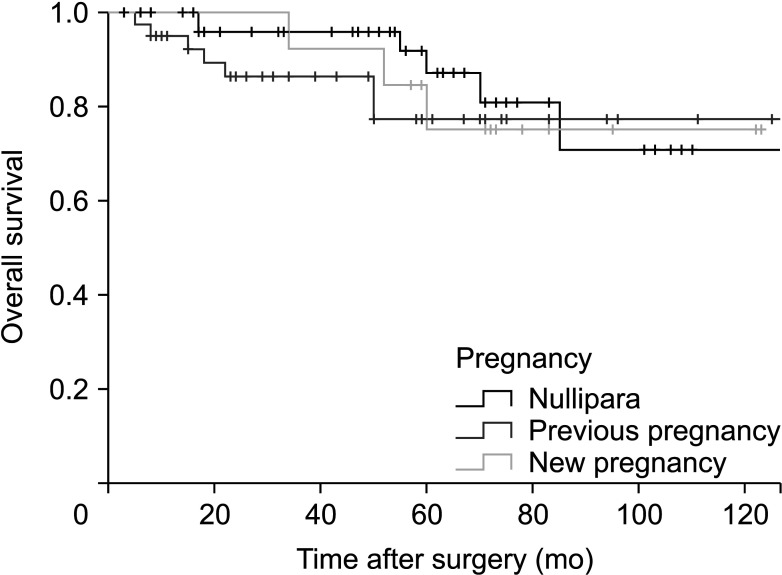
Fig. 2 shows the overall survival of the 3 groups. There was no significant difference in the overall survival (P = 0.608) among them. Fig. 3 shows disease-free survival, which also did not differ significantly among the groups (P = 0.591). Among the 16 patients who showed recurrence, 8 patients had locoregional recurrence while the rest presented with distant recurrence including bone, liver, and brain.
Among the 13 patients who were newly pregnant after treatment for breast cancer, 2 patients were diagnosed with DCIS and 11 patients were diagnosed with invasive breast cancer. Eight patients with new pregnancies had hormone receptor-positive tumors. All patients were recommended to undergo hormone therapy after surgery. Only 1 patient considered pregnancy after receiving the standard 5 years of adjuvant endocrine therapy while 1 patient refused to receive it. The remaining 6 patients initiated endocrine therapy but discontinued it in order to attempt pregnancy. The mean duration of tamoxifen administration prior to interruption in the new pregnancy group was 24.0 (± 14.1) months. Among nulliparous patients, 3 patients discontinued endocrine therapy to attempt pregnancy, while all patients with prior pregnancy maintained it.
Nine out of eleven patients with invasive breast cancer received adjuvant chemotherapy. All these patients received gonadotropin-releasing hormone (GnRH) agonists for ovarian suppression prior to or during chemotherapy. Only 1 patient with a new pregnancy after chemotherapy required assisted reproductive technology. The rest of the patients with new pregnancies after breast cancer treatment did not require other invasive fertility preservation or assisted reproductive technology. The average time from treatment termination, including radiotherapy and chemotherapy, to pregnancy was 34.6 (± 17.6) months.
This study was conducted to determine the safety of pregnancy in very young breast cancer patients aged 35 years or under. Neither overall nor disease-free survival was significantly different among patients with different pregnancy statuses.
While opinions conflict on the safety of pregnancy in patients with breast cancer, a meta-analysis in 2014 showed that pregnancy does not increase the risk of breast cancer recurrence and may even increase overall survival [11]. Another multicenter study by Lambertini et al. [12] in 2018 demonstrated no difference in disease-free and overall survival between pregnant and nonpregnant estrogen receptor-positive patients. A cohort study conducted in Korea by Lee et al. [1] concluded the same outcome in Korean women, with subsequent pregnancy after breast cancer treatment not leading to worse survival outcomes compared to those who did not become pregnant. Hence, the results of this study align with those of previous studies showing that pregnancy after breast cancer diagnosis does not impair survival.
Fertility preservation in young breast cancer patients undergoing chemotherapy is another issue frequently addressed when considering pregnancy after breast cancer treatments. Techniques to preserve ovarian function include ovarian suppression, oocyte and embryo cryopreservation, immature oocyte retrieval, in vitro maturation, and ovarian tissue cryopreservation [13]. In this study, only 1 patient gained assistance from in vitro fertilization. The remaining 8 patients who had posttreatment pregnancy following adjuvant chemotherapy solely received GnRH agonists before or during chemotherapy. This opens the possibility that further invasive fertility preservation and assisted reproductive technology may not be mandatory for subsequent pregnancy in very young patients under the age of 35 years.
A systematic review conducted in 1994 advised patients to wait at least 2 years after completion of treatment before attempting to conceive, to allow for screening of the manifestation of more aggressive diseases [14]. Subsequent studies have been conducted to determine the effect of pregnancy on survival, and many have reported at least equivalent, or survival benefits, in patients who conceived after breast cancer [15]. Another population-based study in 2006 showed that an interval of 6 months after the completion of breast cancer treatment may provide a sufficient safety window for women to conceive [16]. Recently published results for the POSITIVE trial, which evaluated the safety of interrupting adjuvant endocrine therapy in order to allow pregnancy in young breast cancer patients with early hormone receptor-positive breast cancer, showed promising future [17]. Temporary interruption of endocrine therapy to attempt pregnancy did not lead to a higher risk of a new breast cancer event. Likewise, various studies have been conducted to determine the optimal time of pregnancy after breast cancer treatment in young patients.
In this study, patients with hormone receptor-positive tumors were encouraged to receive endocrine therapy for the standard 5-year duration. When patients chose to attempt pregnancy, they were once again recommended to undergo at least 2 years of hormone therapy, and careful consultation on the risk associated with its interruption was given. Subsequently, the mean duration of tamoxifen therapy in patients who had planned pregnancy following breast cancer treatment was 24.0 (± 14.1) months. Regardless of hormone therapy duration, however, pregnancy did not affect overall or disease-free survival.
The strength of this study is that it showed the same result in patients up to 35 years of age. Patients in this group are more likely to consider childbearing, and various studies have demonstrated a substantially unfavorable prognosis [31819]. Based on the results discussed, pregnancy is less likely to interfere with clinical outcomes, and further counseling regarding pregnancy may be more successful. In addition, the study was also able to show that despite the unfavorable conditions surrounding young breast cancer patients after adjuvant therapies, they frequently did not require further invasive fertility preservation and assisted reproductive technology when attempting pregnancy. With further studies to elaborate on this result, patients may be more accessible to diverse options when considering future pregnancy.
The study had several limitations. First, it was retrospective in nature, as pregnancy is difficult to organize prospectively. Second, a small number of patients were enrolled, especially those who were newly pregnant after breast cancer diagnosis. Larger population-based studies are needed to overcome this issue. The results of this prospective study may provide further evidence regarding the optimal timing for young breast cancer patients.
In conclusion, the overall and disease-free survival of young breast cancer patients aged 35 years or younger with new pregnancy were not inferior to those of the nulliparous or previous pregnancy groups. Counseling regarding pregnancy in these young patients requires more active investigation.
References
1. Lee MH, Kim YA, Hong JH, Jung SY, Lee S, Kong SY, et al. Outcomes of pregnancy after breast cancer in Korean women: a large cohort study. Cancer Res Treat. 2020; 52:426–437. PMID: 31476846.
2. de la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993; 341:1039–1043. PMID: 8096955.
3. Han W, Kim SW, Park IA, Kang D, Kim SW, Youn YK, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004; 4:82. PMID: 15546499.
4. Wang SS, Loong H, Chung JP, Yeo W. Preservation of fertility in premenopausal patients with breast cancer. Hong Kong Med J. 2020; 26:216–226. PMID: 32482909.
5. Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015; 107:djv202. PMID: 26307641.
6. Anderson PR, Hanlon AL, Freedman GM, Nicolaou N. Parity confers better prognosis in older women with early-stage breast cancer treated with breast-conserving therapy. Clin Breast Cancer. 2004; 5:225–231. PMID: 15335456.
7. Papatestas AE, Mulvihill M, Josi C, Ioannovich J, Lesnick G, Aufses AH. Parity and prognosis in breast cancer. Cancer. 1980; 45:191–194. PMID: 7351001.
8. Phillips KA, Milne RL, Friedlander ML, Jenkins MA, McCredie MR, Giles GG, et al. Prognosis of premenopausal breast cancer and childbirth prior to diagnosis. J Clin Oncol. 2004; 22:699–705. PMID: 14966094.
9. Rosenberg L, Thalib L, Adami HO, Hall P. Childbirth and breast cancer prognosis. Int J Cancer. 2004; 111:772–776. PMID: 15252849.
10. Sun X, Nichols HB, Tse CK, Bell MB, Robinson WR, Sherman ME, et al. Association of parity and time since last birth with breast cancer prognosis by intrinsic subtype. Cancer Epidemiol Biomarkers Prev. 2016; 25:60–67. PMID: 26545404.
11. Luo M, Zeng J, Li F, He L, Li T. Safety of pregnancy after surgical treatment for breast cancer: a meta-analysis. Int J Gynecol Cancer. 2014; 24:1366–1372. PMID: 25188887.
12. Lambertini M, Kroman N, Ameye L, Cordoba O, Pinto A, Benedetti G, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018; 110:426–429. PMID: 29087485.
13. Carneiro MM, Cota AM, Amaral MC, Pedrosa ML, Martins BO, Furtado MH, et al. Motherhood after breast cancer: can we balance fertility preservation and cancer treatment? A narrative review of the literature. JBRA Assist Reprod. 2018; 22:244–252. PMID: 29932615.
14. Petrek JA. Pregnancy safety after breast cancer. Cancer. 1994; 74:528–531. PMID: 8004628.
15. Sankila R, Heinävaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: "healthy mother effect". Am J Obstet Gynecol. 1994; 170:818–823. PMID: 8141209.
16. Ives A, Saunders C, Bulsara M, Semmens J. Pregnancy after breast cancer: population based study. BMJ. 2007; 334:194. PMID: 17158581.
17. Partridge AH, Niman SM, Ruggeri M, Peccatori FA, Azim HA Jr, Colleoni M, et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023; 388:1645–1656. PMID: 37133584.
18. Bouferraa Y, Haibe Y, Chedid A, Jabra E, Charafeddine M, Temraz S, et al. The impact of young age (< 40 years) on the outcome of a cohort of patients with primary non-metastatic breast cancer: analysis of 10-year survival of a prospective study. BMC Cancer. 2022; 22:27. PMID: 34980002.
19. Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013; 5 Suppl 1:S2–S8. PMID: 23819024.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download