Abstract
Objective
To develop a computerized visuomotor integration system for assessment and training of visual perception impairments and evaluate its safety and feasibility in patients with a stroke. Visual field defects and spatial neglect lead to substantial poststroke impairment. Most diagnostic assessments are anchored in traditional methods, and clinical effects of rehabilitation treatments are limited.
Methods
The CoTras Vision system included two evaluations and four training modules. The evaluation modules were based on the Albert’s test and Star cancellation test, and training modules were based on visual tracking, central-peripheral integration, and visuomotor perception techniques. Bland–Altman plots for agreement with the traditional paper-and-pencil test were performed, and the modified Intrinsic Motivation Inventory, Patient Satisfaction Questionnaire, and Simulator Sickness Questionnaire were conducted.
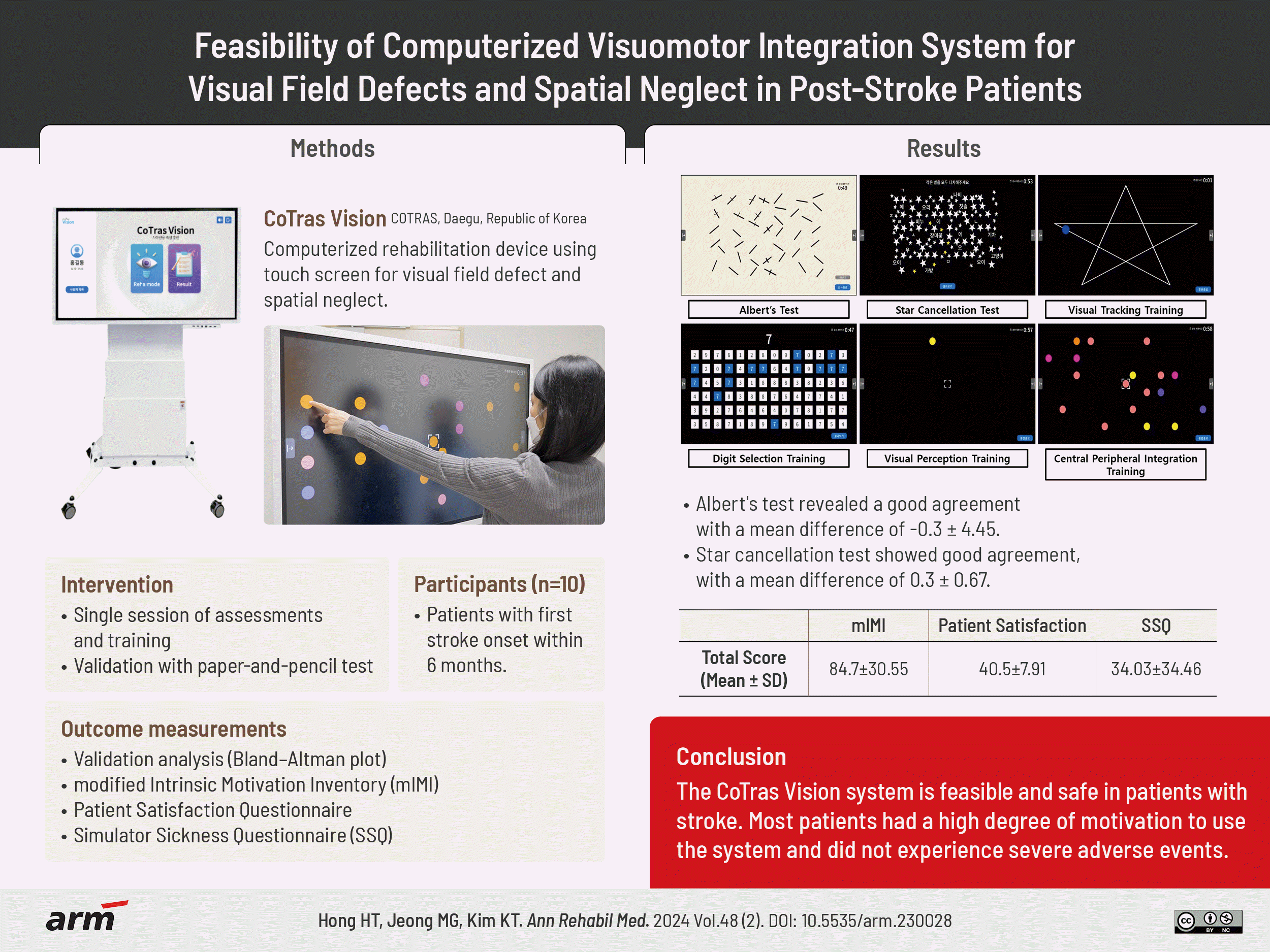
Results
Ten patients with acute stroke completed the study. Bland–Altman plots revealed good agreements for Albert’s test (mean difference, -0.3±4.5) and Star cancellation test (mean difference, 0.3±0.7). The mean±standard deviation scores of the modified Intrinsic Motivation Inventory, Patient Satisfaction Survey, and Simulator Sickness Questionnaire were 84.7±30.6, 40.5±7.9, and 34.0±34.5 respectively.
Conclusion
The CoTras Vision system is feasible and safe in patients with stroke. Most patients had a high degree of motivation to use the system and did not experience severe adverse events. Further studies are needed to confirm its usefulness in stroke patients with visual field defects and hemineglect symptoms. Furthermore, a large, well-designed, randomized controlled trial will be needed to confirm the treatment effect of the CoTras Vision system.
The prevalence of stroke is increasing globally, and it has become the second most common cause of death [1]. A number of patients who experience a stroke develop visual perception disorders, including visual field defects (VFDs) and spatial neglect [2]. In stroke-related VFD, the optic nerve is not directly injured, but the damage to the visual center—which perceives visual information—results in a blind spot within the field of view [3,4]. In spatial neglect, the attention and spatial perception on the side contralateral to that of the brain lesion decrease, rendering it unresponsive to external stimuli [5]. Visual perception disorders in patients who have had a stroke greatly impact the safety of daily activities, such as walking or driving, increase the medical burden due to sequelae, and reduce the quality of life of the patient and their caregivers [6,7].
The current assessment tools for VFD and spatial neglect are not advanced or refined beyond the traditional paper-and-pencil test, and most rehabilitation strategies are anchored in the compensatory method [8-10]. Non-invasive brain stimulation and compensatory techniques using virtual reality, mirror therapy, prism eyeglasses, and devices such as the Dynavision (Dynavision International) have been developed to assist patients in recovering from visual perception disorders [11,12]. However, these treatments are still being under investigation and have yielded limited outcomes. Therefore, a more effective and efficient treatment method is needed [13-15].
Recently, a variety of computer-based rehabilitation systems and smart device applications have been developed for patients with VFDs and spatial neglect and are being used in clinical practice [16-18]. The recent coronavirus disease pandemic also led researchers to conduct such studies. In this study, we aimed to develop a computer-based visuomotor integration system for the functional assessment and rehabilitation training in patients with VFDs and spatial neglect following a stroke. Additionally, we assessed its usability, safety, and level of satisfaction in patients with acute stroke.
This study was conducted after obtaining approval from the Institutional Review Board (IRB) of Keimyung University Dongsan Hospital (IRB No. 2022-11-048). Patients and their caregivers were given a sufficient explanation of the objectives and procedures of the study, after which they had to provide informed consent before being enrolled.
Patients who were admitted to our hospital from March 2023 to May 2023 for a stroke were enrolled in this study. The inclusion criteria were as follows: (1) first stroke; (2) age between 18 and 85 years; and (3) stroke within the past 6 months. The exclusion criteria were as follows: (1) severe upper extremity weakness (Medical Research Council scale score<3); (2) cognitive impairment rendering a patient unable to follow directions; and (3) diagnosis of psychiatric or neurological disease(s) prior to stroke. Since the main purpose of this study was to assess the usability, safety, and applicability of the newly developed device, we included the entire cohort of stroke patients with or without hemineglect or VFDs symptoms.
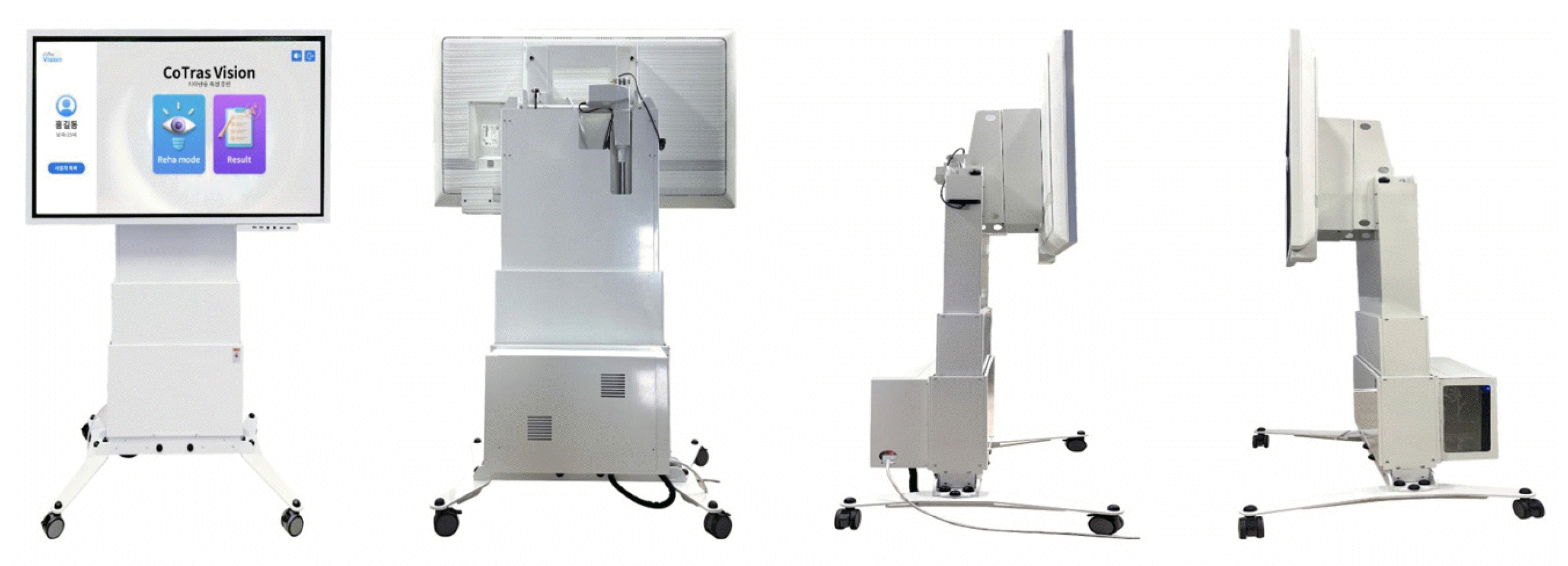
CoTras Vision (COTRAS) was developed for functional assessment and rehabilitation training for VFDs and spatial neglect. This system consists of a desktop computer and a large touch screen (Fig. 1) and is easy to transport because it has wheels. The height and angle of the touch screen can be adjusted between 128.5 and 193.5 cm and 0° and 90°, respectively, to accommodate the user’s height and arm length. Therefore, patients in wheelchairs can also use it.
CoTras Vision includes two functional assessments and four rehabilitation training modules. All assessments and training programs are conducted while the user sits 30–50 cm away from the screen and focuses on the center of the screen. Each assessment and training module is performed for a specified length of time, which can be modified in 30-second increments, from 30 seconds to 6 minutes, according to the condition of the patient. In visual tracking, the speed of the object on the screen can be adjusted in 10 steps.
Functional assessment using the system consists of the Albert’s test and the Star cancellation test, which are computerized versions of the traditional paper-and-pencil tests. The touch screen interface was constructed to closely mimic the traditional method of using a pen. In this study, a 5-minute time limit was set, at which point the test was terminated regardless of its completion (Fig. 2).
Rehabilitation training using the system consists of the visual tracking, visual perception training, central peripheral integration, and digit selection modules. In visual tracking, the patient’s gaze has to follow an object on the screen moving in different patterns, such as a star, curve, or line pattern, without head movement. In visual perception training, patients fix their gaze on a circle in the center of the screen and respond by touching the circle that randomly appears on the monitor. In central peripheral integration, patients are trained to touch a randomly appearing circle that is of the same color as the one in the center of the screen as quickly as possible while keeping their face and gaze fixed on the center of the screen. In digit selection, patients detect and touch the number that is the same as the one that appears in the top middle of the screen from six rows of 14 numbers presented sequentially from left to the right (Fig. 2).
The patients completed the modified Intrinsic Motivation Inventory (mIMI), Patient Satisfaction Questionnaire, and Simulator Sickness Questionnaire (SSQ) after performing functional assessment and rehabilitation training.
The Intrinsic Motivation Inventory, published in 1989 to assess patients’ subjective experience, uses a 7-point Likert scale (1=strongly disagree, 7=strongly agree) and consists of 45 items [19]. Scores are calculated for each area of interest/enjoyment, perceived competence, effort/importance, pressure/tension, perceived choice, value/usefulness, and relatedness. We extracted 19 relevant questions (total score of 133) to identify the level of motivation in terms of participating in CoTras Vision (Supplementary Table S1).
To assess the level of satisfaction with CoTras Vision, 11 questions (total 55 points) rated on a 5-point Likert scale (1=very dissatisfied, 5=very satisfied) were administered. The questions concerned the description, screen brightness, touch accuracy, size, and speed of the presented modules, their convenience, and their side effects (Supplementary Table S1) [20].
The SSQ published in 1993 measures cybersickness and is widely used to measure dizziness related to electronic devices. The CoTras Vision consists of a large touch screen with many modules with flickering, which is likely to result in cybersickness in some individuals [21]. Patients answered 16 questions after the intervention, rating each symptom on a scale from 0 to 3 (0=no symptoms, 3=severe symptoms). The collected scores were weighted to calculate the score for three major clusters: nausea, eye movement discomfort (oculomotor), and disorientation, for a total SSQ score. A higher total score indicates severe dizziness.
Patients in this study underwent the following procedures. Initially, paper-and-pencil tests, including Albert’s test and the Star cancellation test, were conducted. For these tests, the evaluation concluded when patients declared their completion, even if they did not perform them perfectly. Additionally, if a patient took more than 5 minutes to complete the tests, the evaluation was terminated. Subsequently, assessments using the CoTras Vision were carried out, also imposing a 5-minute time limit [9,22]. Following the assessment, rehabilitation training using the CoTras Vision was administered, incorporating the visual tracking, visual perception training, central peripheral integration, and digit selection modules. Each training session was allotted 1 minute, and a total of 3 sessions were conducted per module. The overall duration of the training was approximately 15–20 minutes. Surveys were conducted after both the evaluation and training. All procedures were administered by the same trained researchers across both assessments and training sessions.
Baseline demographics of patients (sex, age, stroke type, lesion side, and lesion location) were analyzed using descriptive statistics. To evaluate the agreement of functional assessment between CoTras Vision and the paper-and-pencil test, a Bland–Altman plot was used. We performed frequency analysis and descriptive statistics to analyze the results of the patient-reported questionnaires. IBM SPSS Statistics 27.0 (IBM Corp.) was used for all statistical analyses, and the level of statistical significance was set at 0.05.
Among the 12 patients enrolled in the study, two dropped out, and 10 completed the assessments and rehabilitation training. The baseline demographics of the patients are presented in Table 1. Of the 10 patients, 7 were males and 9 had ischemic stroke. The mean±standard deviation age was 64.9±10.3 years.
The CoTras Vision exhibited good agreement with the paper-and-pencil test. The Bland–Altman plot revealed a mean difference of -0.3±4.5, and the results of all patients except one fell within the 95% confidence interval in Albert’s test. The Star cancellation test also exhibited good agreement, with a mean difference of 0.3±0.7, and the results of all 10 patients fell within the confidence interval (Fig. 3, Supplementary Table S2).
The mean mIMI score was 84.7±30.6. The item with the highest score was item 1: “I enjoyed doing this training very much.” The item with the lowest score was item 19: “I did not put much energy into this.” (Supplementary Table S3). The mean score for the Patient Satisfaction Questionnaire was 40.5±7.9 points. The item with the highest mean score (4.2 points) was item 3: “Understanding of the description displayed at the beginning of the module.” The size of the monitor, distance between the equipment and the patient, and convenience of use without any physical side effects (items 1, 2, and 11) exhibited the lowest mean score of 3.4 (Supplementary Table S4). The mean SSQ score was 34.0±34.5. In terms of the total score, the extent of cybersickness was substantial in four patients (patient 1, 78.54; patient 2, 44.88; patient 4, 108.46; patient 9, 48.62; Fig. 4, Supplementary Table S5).
In this study, we developed CoTras Vision, a computer-based visuomotor integration system for patients with poststroke VFDs and spatial neglect. The overall validity, side effects, patient satisfaction, and usability of this system were assessed in the entire cohort of poststroke patients. Two functional assessments, Albert’s test and Star cancellation test, exhibited a high level of agreement with the CoTras Vision. The questionnaires for internal motivation and satisfaction revealed results that were above moderately positive. Although no serious side effects were observed, some patients complained of dizziness.
Existing functional assessments for spatial neglect using paper-and-pencil tests have necessitated usage of the same size of test paper to be placed on the desk and to be completed using a pen or pencil. Regarding the CoTras Vision system, the components of the original tests were faithfully reproduced, albeit on a larger screen size, and patients could use the touch function rather than a pen or pencil. The test needed to be completed on a screen at eye level rather than on a desk, which might have restricted its use by patients with weak upper limb strength. Despite these differences, the CoTras Vision system had high agreement with the original tests. The CoTras Vision system had several strengths. The assessment was conducted on a high-resolution display; therefore, the patient could have focused better during the test. The modules were designed for a computer and the same items could be implemented in alternative ways on the screen. This could potentially reduce the learning effect that may have occurred when the same test was taken by the same patient. Thus, if the reliability of the alternative test could be maintained, functional improvement in patients could be evaluated with higher precision. Furthermore, the size of the screen could be reduced by adjusting the resolution, and the height could be adjusted vertically. Therefore, this system has flexibility for use by children. Moreover, since it can be implemented on a web-based platform, the tests can be conducted remotely; we expect eventual widespread adoption of this system for tele-rehabilitation.
The mean mIMI score of 84.7±30.6 out of 133, which was markedly higher than the median value of 66.5, indicated that patients were sufficiently motivated to use the CoTras Vision system. Specifically, the high score for the item “I enjoyed doing this training very much” confirmed that patients were satisfied to perform the test and training even when they were not familiar with the equipment.
The mean satisfaction score was 40.5±7.9 out of 55, which was mostly positive. However, in line with previous studies about computer-based rehabilitation treatments, dissatisfaction with the size of the monitor, the distance from the monitor, and visual fatigue due to the brightness of the screen were also identified in this study [23].
Finally, the mean SSQ score was 34.0±34.5 out of 235.6. SSQ was originally proposed by Kennedy et al. [21] in 1993, and it has been widely used to assess sickness symptoms associated with medical devices. A total score of SSQ exceeding 20 is generally considered to be categorized as a “bad” simulator [24]. However, this severity classification has been criticized for being a potential overestimation, because the majority of the dataset of SSQ is derived from a military population [25]. All but one patient scored below 80, a relative low score compared to the total score of 235.6, which was not associated with severe cyber sickness. We conducted a detailed evaluation of scores for each question and analyzed the subjective responses in four patients with relatively high scores. This analysis revealed a tendency to complain of difficulties in visually focusing on the screen, which corresponded with responses to the satisfaction questionnaire.
The rehabilitation training modules of the CoTras Vision system were developed based on rehabilitative devices and software that yielded positive clinical outcomes in previous studies [11,26-28]. A representative rehabilitation training device is the Dynavision system [11,15]. The Dynavision system has 64 flashing physical buttons arranged radially on a 165×120 cm panel and is used by pressing buttons randomly or in a specific sequence within a given time limit. It is widely used to improve visual perception function and reaction rates in athletes, and its efficacy has been demonstrated in the treatment of visual perception disorders in patients who have had a stroke [2,11,29]. The rehabilitation training of the CoTras Vision system was similar to that of the Dynavision system, but a wider variety of training modules can and have been developed and implemented with the latter, and usage of a touch screen allows patient-customized treatment with higher levels of motivation. Another advantage is that the real-time feedback provided during training may increase the focus and invoke a sense of achievement in the patient.
Various therapeutic strategies have been considered for treating VFDs, including optical substitution, visual search or exploration training, restorative training, and blind spot stimulation [30]. Mueller et al. [31] reported significant improvements in the detection of super-threshold stimuli in patients with VFDs caused by various central nervous system disorders following vision restoration therapy (NovaVision AG). This therapy involves patients focusing on a point in the center of the screen and responding every time they see light stimuli that appear elsewhere on the screen. The “visual perception training” and “central peripheral integration training” approaches in our study aligned with these approaches. Aimola et al. [32] demonstrated objective benefits in patients with homonymous VFD using unsupervised compensatory computer-based training. This intervention consists of a visual exploration task, in which patients try to find specific feature among the distractors, and a reading task, during which patients define a nonword target among the words. These training routines share similar treatment mechanisms with the “visual tracking” and “digit selection” approaches used in our study. Another study used a desktop computer and projector to show positive effects in patients experiencing poststroke neglect [33].
This study had some limitations. First, the patient population recruited may not have been representative of the target patient population for this system. CoTras Vision was specifically developed for evaluation and treatment of VFDs and hemineglect symptoms in poststroke patients. Nevertheless, our study included all stroke patients, irrespective of these symptoms, to assess its broader applicability. To use CoTras Vision in clinical practice, future studies are necessary to validate the effectiveness of its evaluation and treatment in poststroke patients who do have symptoms of VFDs and hemineglect. Second, the small sample size might not have been sufficient to compare its validity to that of the original tests. The CoTras Vision system was a computer-based assessment tool that had advantages in terms of patient data management and could provide reliable measurements in repeated tests. However, much larger sample sizes are required to prove its reliability. Third, the effect of rehabilitation training using the CoTras Vision system needs to be verified in patients with stroke. Our results indicated that patients demonstrate substantial motivation and minimal resistance when using the CoTras Vision system. Nevertheless, to corroborate its efficacy in the clinical setting, additional clinical trials are imperative.
In this study, we verified that the CoTras Vision system was feasible and safe in patients with stroke. Positive results in terms of motivation and satisfaction with the CoTras Vision system were confirmed by most of the patients. Further investigation is necessary in patients with specific symptoms of VFDs and hemineglect. Furthermore, additional randomized clinical trial is required to determine the clinical effects of rehabilitation training using the CoTras Vision system.
Notes
FUNDING INFORMATION
This research was supported by a grant from the Daegu-Gyeongbuk Medical Innovation Foundation, funded by the Daegu Metropolitan City of the Republic of Korea (No. B-A-K-22-01).
AUTHOR CONTRIBUTION
Conceptualization: Hong HT, Jeong MG, Kim KT. Methodology: Hong HT, Jeong MG, Kim KT. Formal analysis: Hong HT, Jeong MG, Kim KT. Funding acquisition: Kim KT. Project administration: Kim KT. Visualization: Hong HT, Kim KT. Writing – original draft: Hong HT. Writing – review and editing: Hong HT, Jeong MG, Kim KT. Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5535/arm.230028.
Supplementary Table S2.
Comparison of the CoTras Vision (COTRAS) and original paper-and-pencil test
Supplementary Table S3.
Results of modified Intrinsic Motivation Inventory of CoTras Vision (COTRAS)
Supplementary Table S4.
Results of Patient Satisfaction Questionnaire of CoTras Vision (COTRAS)
Supplementary Table S5.
Results of Simulator Sickness Questionnaire of CoTras Vision (COTRAS)
REFERENCES
2. Rowe FJ, Hepworth LR, Howard C, Hanna KL, Currie J. Impact of visual impairment following stroke (IVIS study): a prospective clinical profile of central and peripheral visual deficits, eye movement abnormalities and visual perceptual deficits. Disabil Rehabil. 2022; 44:3139–53.

3. Bouwmeester L, Heutink J, Lucas C. The effect of visual training for patients with visual field defects due to brain damage: a systematic review. J Neurol Neurosurg Psychiatry. 2007; 78:555–64.

4. Matteo BM, Viganò B, Cerri CG, Perin C. Visual field restorative rehabilitation after brain injury. J Vis. 2016; 16:11.

5. Urbanski M, Thiebaut de Schotten M, Rodrigo S, Oppenheim C, Touzé E, Méder JF, et al. DTI-MR tractography of white matter damage in stroke patients with neglect. Exp Brain Res. 2011; 208:491–505.

7. Hepworth L, Rowe F. Visual Impairment following stroke - the impact on quality of life: a systematic review. Ophthalmol Res Int J. 2016; 5:1–15.

8. Berger S, Kaldenberg J, Selmane R, Carlo S. Effectiveness of interventions to address visual and visual-perceptual impairments to improve occupational performance in adults with traumatic brain injury: a systematic review. Am J Occup Ther. 2016; 70:7003180010p1-7.

9. Zeltzer L, Menon A. Albert’s test [Internet]. STROKE ENGINE team;2010 [cited 2023 Apr 16]. Available from: https://strokengine.ca/en/assessments/alberts-test/.
10. Zeltzer L, Menon A. Line bisection test [Internet]. STROKE ENGINE team;2008 [cited 2023 Apr 16]. Available from: https://strokengine.ca/en/assessments/line-bisection-test/.
11. Anderson L, Cross A, Wynthein D, Schmidt L, Grutz K. Effects of Dynavision training as a preparatory intervention status postcerebrovascular accident: a case report. Occup Ther Health Care. 2011; 25:270–82.

12. Jutai JW, Bhogal SK, Foley NC, Bayley M, Teasell RW, Speechley MR. Treatment of visual perceptual disorders post stroke. Top Stroke Rehabil. 2003; 10:77–106.

13. El Nahas N, Elbokl AM, Abd Eldayem EH, Roushdy TM, Amin RM, Helmy SM, et al. Navigated perilesional transcranial magnetic stimulation can improve post-stroke visual field defect: a double-blind sham-controlled study. Restor Neurol Neurosci. 2021; 39:199–207.

14. Gartz R, Dickerson A, Radloff JC. Comparing component-based and occupation-based interventions of a person with visual deficits’ performance. Occup Ther Health Care. 2021; 35:40–56.

15. Pratviel Y, Deschodt-Arsac V, Larrue F, Arsac LM. Reliability of the Dynavision task in virtual reality to explore visuomotor phenotypes. Sci Rep. 2021; 11:587.

16. Jang HJ, Choi YD, Kim NH. Effects and satisfaction of medical device safety information reporting system using electronic medical record. Healthc Inform Res. 2017; 23:94–100.

17. Sahraie A, Smania N, Zihl J. Use of NeuroEyeCoach™ to improve eye movement efficacy in patients with homonymous visual field loss. Biomed Res Int. 2016; 2016:5186461.

18. Varela-Aldás J, Buele J, Ramos Lorente P, García-Magariño I, Palacios-Navarro G. A virtual reality-based cognitive telerehabilitation system for use in the COVID-19 pandemic. Sustainability. 2021; 13:2183.

19. King M, Hijmans JM, Sampson M, Satherley J, Hale L. Home-based stroke rehabilitation using computer gaming. NZ J Physiother. 2012; 40:128–34.
20. Beattie PF, Pinto MB, Nelson MK, Nelson R. Patient satisfaction with outpatient physical therapy: instrument validation. Phys Ther 2002;82:557-65. Erratum in: Phys Ther. 2002; 82:827.
21. Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG. Simulator Sickness Questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol. 1993; 3:203–20.

22. Zeltzer L, Menon A. Star cancellation test [Internet]. STROKE ENGINE team;2008 [cited 2023 Apr 16]. Available from: https://strokengine.ca/en/assessments/star-cancellation-test/.
23. Mannan MMN, Kamran MA, Kang S, Choi HS, Jeong MY. A hybrid speller design using eye tracking and SSVEP brain-computer interface. Sensors (Basel). 2020; 20:891.

24. Stanney KM, Kennedy RS, Drexler JM. Cybersickness is not simulator sickness. Proc Hum Factors Ergon Soc Annu Meet. 1997; 41:1138–42.

25. Bimberg P, Weissker T, Kulik A. On the usage of the Simulator Sickness Questionnaire for virtual reality research. Paper presented at: 2020 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW); 2020 Mar 22-26; Atlanta, USA.
26. Circle Peripheral Awareness. Eye Cam Learn. Tracking: central-peripheral integration [Internet]. Circle Peripheral Awareness;2014 [cited 2023 Apr 16]. Available from: https://eyecanlearn.com/tracking/peripheral/.
27. Rusconi ML, Meinecke C, Sbrissa P, Bernardini B. Different cognitive trainings in the rehabilitation of visuo-spatial neglect. Eur J Phys Rehabil Med. 2002; 38:159–66.
28. Moon SJ, Park CH, Jung SI, Yu JW, Son EC, Lee HN, et al. Effects of an eye-tracking linkage attention training system on cognitive function compared to conventional computerized cognitive training system in patients with stroke. Healthcare (Basel). 2022; 10:456.

29. Choi SY, Han SW, Lee JS. The effect of Dynavision training on visual attention during task after stroke: single subject research design. Korean J Occup Ther. 2015; 23:1–13.
30. Pouget MC, Lévy-Bencheton D, Prost M, Tilikete C, Husain M, Jacquin-Courtois S. Acquired visual field defects rehabilitation: critical review and perspectives. Ann Phys Rehabil Med. 2012; 55:53–74.

31. Mueller I, Mast H, Sabel BA. Recovery of visual field defects: a large clinical observational study using vision restoration therapy. Restor Neurol Neurosci. 2007; 25:563–72.
Fig. 2.
Functional assessments and rehabilitation training modules of the CoTras Vision system (COTRAS).
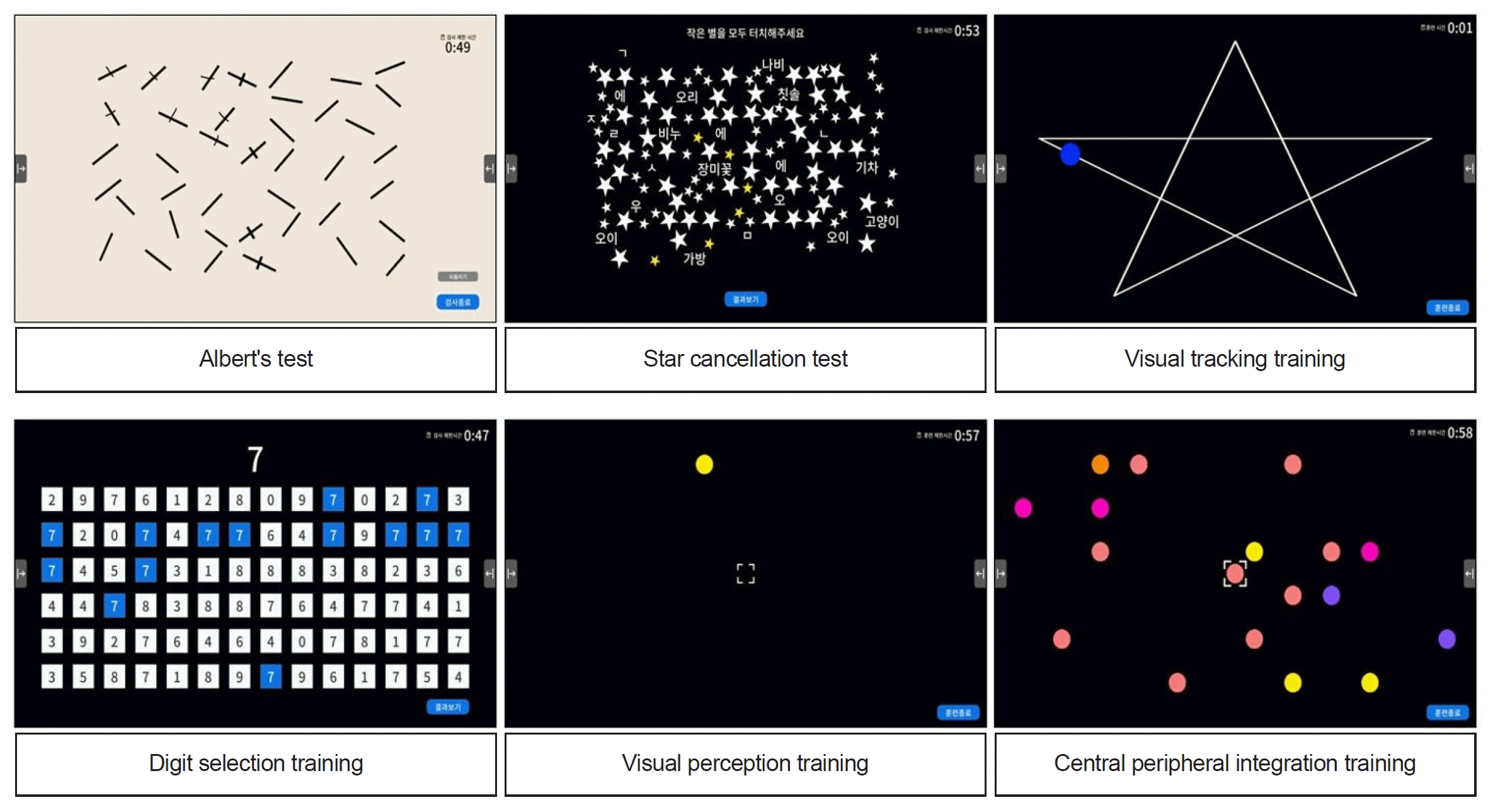
Fig. 3.
Bland–Altman plots of comparison between the CoTras Vision (COTRAS) and paper-and-pencil test. (A) Albert’s test. (B) Star cancellation test.
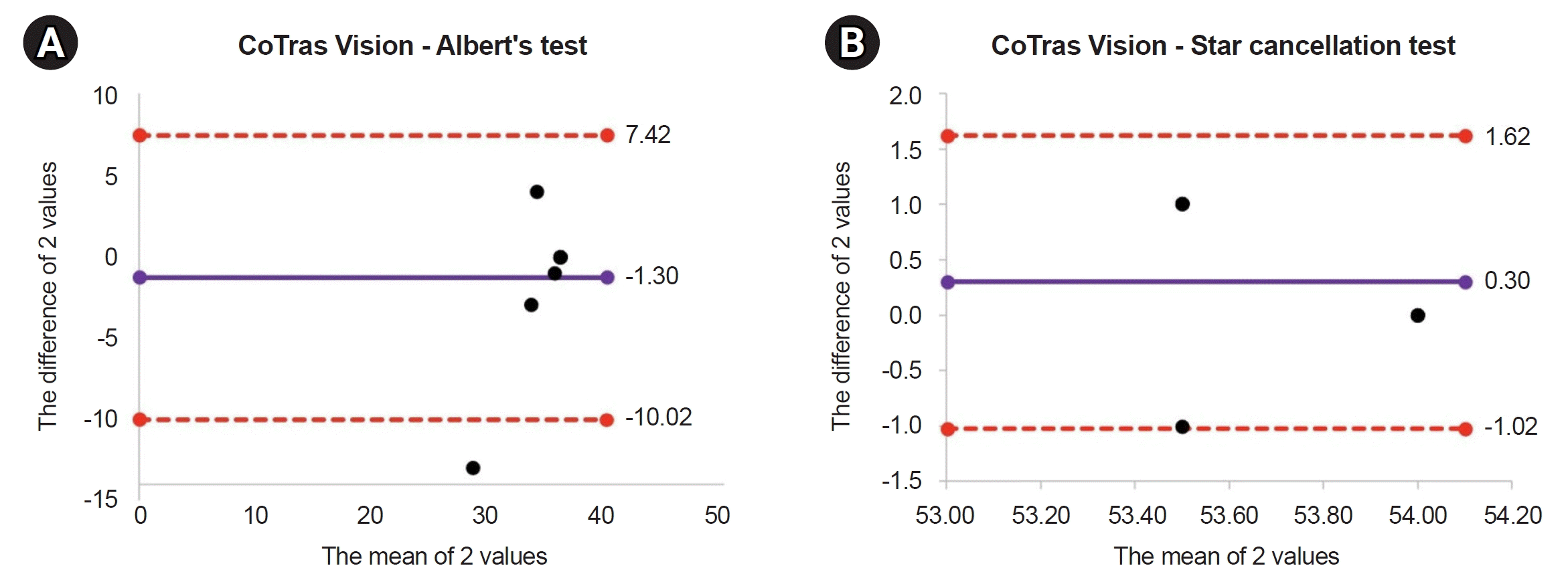
Fig. 4.
Total and symptom cluster score of Simulator Sickness Questionnaire. (A) Total score. (B) Symptom cluster score.
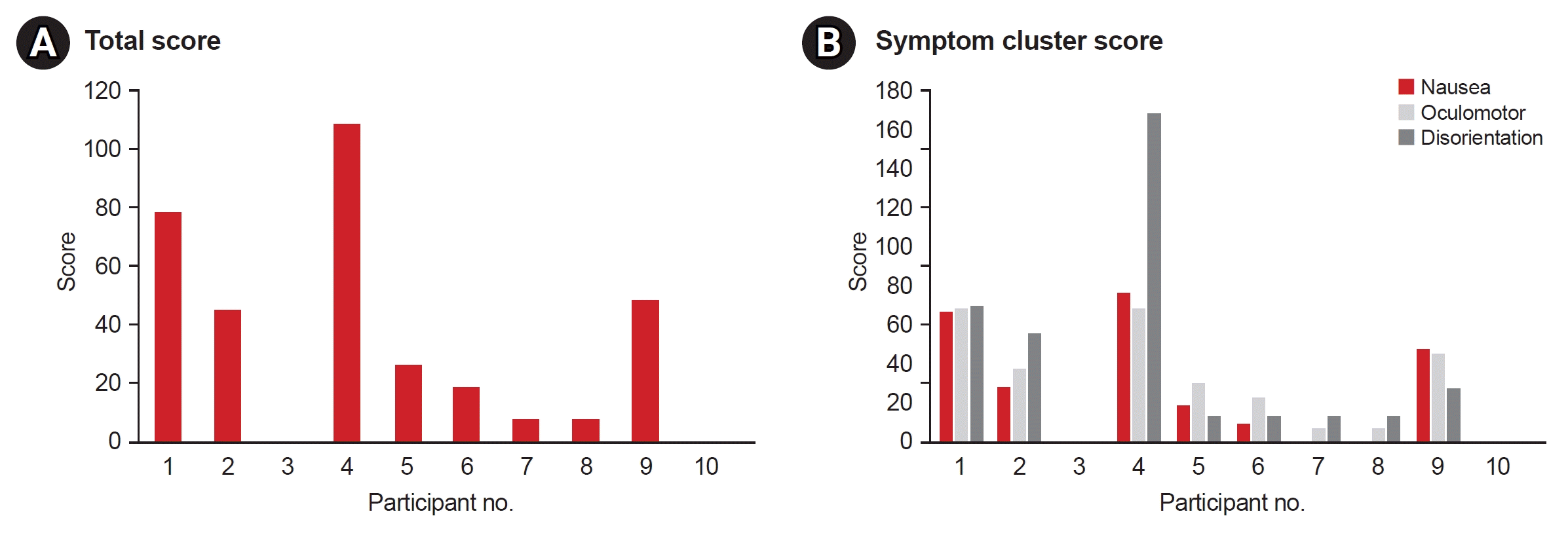
Table 1.
Demographic of patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download