Abstract
Transoral thyroidectomy is a promising technique because of its superior postoperative cosmesis. Although tract implantation after remote-access thyroidectomy is extremely rare, it can result in poor outcomes. A 45-year-old female presented with multiple palpable nodules in the anterior neck after a transoral thyroidectomy for which thyroid function tests, CT, ultrasonography, and fine-needle aspiration were performed. The thyroid function test results were normal. CT and ultrasonography revealed multiple, well-defined nodules at levels Ia and VI along the tracks left by the endoscopic instrument inserted during transoral thyroidectomy. Fineneedle aspiration revealed a microfollicular structure derived from thyroid tissue without evidence of atypia. Wash-out fluid from the nodules revealed elevated thyroglobulin levels. Initially, the patient was hesitant about surgical removal, but the nodules grew larger, so surgical removal was performed. Final histopathological examination revealed follicular thyroid carcinoma. The patient was recommended extensive resection but was lost to follow-up.
Transoral thyroidectomy has recently gained popularity and interest owing to its outstanding postoperative cosmesis compared to other remote-access approaches [1]. However, because these procedures are relatively new, remote-access thyroidectomies carry specific risks that are not encountered with open thyroidectomies. Several studies have reported thyroid tissue implantation in the operative tract after various remoteaccess thyroidectomies [2-9]. Herein, we report the case of a 45-year-old female who presented with palpable anterior neck masses after a transoral endoscopic thyroidectomy vestibular approach (TOETVA) in which tract implantation of the follicular thyroid carcinoma was confirmed. To the best of our knowledge, this is the first report of tract implantation following transoral thyroidectomy.
A 45-year-old female presented to a tertiary care center for evaluation of palpable non-tender nodules on the anterior neck. Physical examination revealed multiple palpable nodular lesions at levels Ia and VI. This patient had undergone TOETVA for a left thyroid nodule at another institution 4 years previously. According to the medical records of another institution, the final diagnosis was the presence of a 2.8×1.2 cm left follicular adenoma embedded in the unilateral thyroid lobe with a size of 5.0×2.2×1.5 cm. Thyroid function test results, including serum thyroid-stimulating hormone, triiodothyronine, free thyroxine, thyroglobulin, and anti-thyroglobulin antibody levels, were normal. Ultrasonography confirmed the presence of multiple nodules with a maximal diameter of 0.86 cm at levels Ia and VI. Each nodule had smooth margin and hypoechogenicity without vascularity (Fig. 1). Computed tomography revealed multiple scattered enhancing nodules of various sizes (Fig. 2). Fine-needle aspiration cytology showed a microfollicular structure derived from the thyroid tissue on hematoxylin and eosin staining (Fig. 3A). Papanicolaou-stained cytology specimens showed no significant atypia or evidence of malignancy (Fig. 3B). Thyroglobulin measurements in the washout fluid from fine-needle aspiration cytology specimen of the anterior neck nodules showed elevated thyroglobulin levels (1236 ng/mL and 1240 ng/mL at levels Ia and VI, respectively). According to previous hospital medical records, the possibility of malignancy was low; therefore, tract implantation of a benign thyroid tumor or normal thyroid tissue was suspected. The patient was advised to have a wide excision that included both the palpable nodules and the soft tissue used as the conduit for TOETVA. The patient refused additional surgical procedures and attended regular follow-up visits. However, the nodule gradually increased in size, and tenderness occurred. However, the patient refused wide excision surgery and only wanted to remove the palpable nodules under local anesthesia. Therefore, instead of wide excision, each palpable nodule was resected under local anesthesia. Palpable nodules were localized with ultrasound and excised by direct skin incision (Fig. 4). Final histopathology revealed follicular thyroid carcinoma in all nodules (Fig. 5). Immunohistochemical staining was positive for thyroid transcription factor-1, paired box gene 8, thyroglobulin, and CD56, and negative for galectin3. No BRAF mutations were detected.
Tract implantation after the previous TOETVA was suspected, and an additional wide surgical excision, including levels Ia and VI, which were possible in the previous surgical field, was strongly recommended. However, the patient was lost to follow-up after a consultation for further resection.
The patient presented with multiple tract implantations after a transoral thyroidectomy. In contrast to other institutions’ diagnoses of follicular adenoma for thyroid nodules after TOETVA, our institution’s diagnosis of all tumors implanted in the preceding surgical tract found follicular cancer. Although malignant transformation from all implanted tissues is possible, it is more likely that the preceding hospital’s diagnosis of follicular adenoma following TOETVA was a misdiagnosis. Since the histopathology at the previous institution revealed follicular adenoma, more aggressive treatment was delayed at our institution. Given that it is difficult to distinguish follicular adenoma from follicular carcinoma with only fine-needle aspiration, more aggressive diagnostic procedures, such as excisional biopsy, should have been considered sooner for an accurate diagnosis. Due to distance issues, the patient found it difficult to visit the former hospital. She was unable to bring histopathological slides following the TOETVA surgery, thus a review of those slides was not possible. Furthermore, the surgical technique was not specified in the medical records of the prior hospital; hence, tumor rupture could not be confirmed. As a result, there is a limitation in that the cause of tract implantation cannot be determined. Furthermore, because only separated resection was conducted with the patient’s wish in mind, additional surgery was required. Ultimately, the patient was lost to follow-up, and curative treatment could not be performed at our institution; therefore, the final outcome could not be confirmed.
Transoral thyroidectomy has recently been emphasized and adopted by many surgeons worldwide because of its significant advantages, such as low surgical morbidity rates, good postoperative aesthetics, and good functional and voice outcomes [10]. Since the first report of TOETVA in humans in 2014, the efficacy and safety of transoral thyroidectomy have been evaluated in many studies. Tae [10] reviewed the complications of transoral thyroidectomy and reported similar complication rates of vocal fold paralysis, hypoparathyroidism/hypocalcemia, hematoma, and seroma between transoral and conventional thyroidectomies. Unusual complications of transoral thyroidectomy include CO2 embolism, mental nerve injury, surgical site infection, skin perforation, skin burns, and trauma [10]. Another systematic review described various postoperative complications comparable to those of conventional thyroidectomy, including recurrent laryngeal nerve, superior laryngeal nerve, mental nerve injuries, hypoparathyroidism/hypocalcemia, skin complications (skin flap perforation, skin bruise/injury/dimpling), seroma, hematoma, subcutaneous emphysema, infection, CO2 embolism, painful pulling sensation, swallowing discomfort, Horner syndrome, and tracheal injury [11]. Tract implantation of thyroid tumor tissue is extremely rare. Needle tract implantation after thyroid fine-needle aspiration cytology can also occur, with incidences of 0.15% and 0.37% at 5 and 10 years after fine-needle aspiration, respectively [12]. However, the incidence of tract implantation after remote-access thyroidectomy remains unknown. To the best of our knowledge, no previous studies have described tissue implantation after transoral thyroidectomy. Only a few case reports have described tissue implantation after various remote-access thyroidectomies (Table 1) [2-9]. Piecemeal resection or tumor spillage was experienced in three of the four cases described despite en bloc resection. Owing to the relatively large tumor size (mean diameter, 4.2 cm; range, 2.8-6.0 cm), piecemeal resection might be required or occur unintentionally, resulting in tract implantation. Although the exact etiology of local implantation after remote-access thyroid surgery remains unknown, there are multiple probable factors associated with local recurrence or port-site metastases, including direct wound implantation, instrument contamination, tumor cell aerosolization, chimney effect, surgical technique, and excessive tumor manipulation, etc [3]. There have been no studies on treatment methods for tract implantation, and only a few cases have been reported previously described. Some patients underwent separate resections of the confirmed nodules along with the surrounding soft tissue. One underwent wide en bloc resection including the confirmed nodules and the soft tissue used as a surgical route among the nodules [9]. Compared to other remote-access thyroidectomies, transoral thyroidectomies have a shorter and narrower surgical path. When tract implantation is identified after transoral thyroidectomy, wide en bloc resection may be performed more easily, and fewer cosmetic defects may be expected. Further research is required to determine the surgical method for tract implantation with the best outcomes.
Remote-access thyroidectomy is widely used for the treatment of thyroid diseases. Tract implantation after surgery is uncommon; however, the reported cases reiterate the need for caution when considering endoscopic or robotic surgery for thyroid diseases, especially thyroid cancer. A potential cause of tract implantation, in this case, could have been the selection of surgical instruments (endoscope or surgical robot), gas insufflation, tumor spillage or piecemeal resection, large mass size, or lack of use of a retrieval bag. The risk of seeding tumor cells along the incision, although very low, must be considered. The risk may be higher in the presence of a very large nodule, fragmented resection, extrathyroidal extension, perineural invasion, or tumor emboli [4]. In an experimental study using cultured cancer cells, Ludemann, et al. [13] suggested that tumor implantation was independent of gas insufflation. To date, no reports have compared the selection of robotic or endoscopic assistance with tract implantation. One metaanalysis reported that the addition of a surgical robot was associated with a lower risk of temporary recurrent laryngeal nerve injury and a shorter length of hospital stay [14]. However, Chen, et al. [15] reported no difference in postoperative outcomes between transoral robotic thyroidectomy and TOETVA. Further studies are required to elucidate the role of surgical robot in tract implantation.
Tumor size is also a significant factor in tract implantation. According to the American Thyroid Association, robotic thyroidectomy should be performed with caution by experienced surgeons in reference centers for well-circumscribed nodules not larger than 3 cm in the thyroid lobe and not exceeding 5-6 cm in the largest dimension. Contraindications for robotic thyroidectomy include thyroiditis (essentially, Graves’ disease), previous neck surgery, substernal extension, and suspected cancer with extrathyroidal extension or lymph node involvement [4].
The number of patients undergoing transoral thyroidectomy is rapidly increasing because of its excellent cosmetic results, rapid recovery, and reduced tissue trauma compared with other remote-access thyroidectomies. However, it is important to recognize that remote-access thyroidectomy may lead to thyroid cell implantation, a common complication. Remote-access thyroidectomy aims to reduce the extent of the incision and hide it; however, this increases the incidence of tract implantation. Thus, a wide and destructive ablative tract surgery may be required for cancer cell implantation. Following strict indications for remote-access thyroidectomy, accurate preoperative evaluation, meticulous surgical handling, and effective protective measures, the incidence of locoregional implantation or recurrence can be dramatically reduced. Every effort should be made to avoid tumor spillage during surgery, and the extraction of the resected specimen using a retrieval bag is desirable to avoid tumor seeding.
ACKNOWLEDGMENTS
This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.
Notes
Author Contribution
Conceptualization: Minhyung Lee, Jin-Choon Lee. Data curation: Minhyung Lee. Funding acquisition: Minhyung Lee. Investigation: Minhyung Lee, Jin-Choon Lee. Resources: Minhyung Lee, Dong Hoon Shin, Hyun Jung Lee. Software: Dong Hoon Shin, Hyun Jung Lee. Supervision: Minhyung Lee, Jin-Choon Lee. Visualization: Minhyung Lee, Dong Hoon Shin, Hyun Jung Lee. Writing—original draft: Minhyung Lee, Jin-Choon Lee. Writing—review & editing: Minhyung Lee, Jin-Choon Lee.
REFERENCES
1. Tae K, Ji YB, Song CM, Ryu J. Robotic and endoscopic thyroid surgery: Evolution and advances. Clin Exp Otorhinolaryngol. 2019; 12(1):1–11.

2. Bakkar S, Frustaci G, Papini P, Fregoli L, Matteucci V, Materazzi G, et al. Track recurrence after robotic transaxillary thyroidectomy: A case report highlighting the importance of controlled surgical indications and addressing unprecedented complications. Thyroid. 2016; 26(4):559–61.

3. Beninato T, Kleiman DA, Scognamiglio T, Fahey TJ, Zarnegar R. Tract recurrence of a follicular thyroid neoplasm following transaxillary endoscopic thyroidectomy. Thyroid. 2012; 22(2):214–7.

4. Chabrillac E, Zerdoud S, Fontaine S, Sarini J. Multifocal recurrence on the transaxillary robotic thyroidectomy incision. Eur Ann Otorhinolaryngol Head Neck Dis. 2020; 137(1):59–60.

5. Kim JH, Choi YJ, Kim JA, Gil WH, Nam SJ, Oh YL, et al. Thyroid cancer that developed around the operative bed and subcutaneous tunnel after endoscopic thyroidectomy via a breast approach. Surg Laparosc Endosc Percutan Tech. 2008; 18(2):197–201.

6. Koh KW, Lee TH, Cho SY, Lee SS, Kim JM, Yi KH, et al. Subcutaneous implantation of adenomatous goiter: An unpredicted complication of endoscopic thyroid surgery. Thyroid. 2010; 20(4):441–3.

7. Lee YS, Yun JS, Jeong JJ, Nam KH, Chung WY, Park CS. Soft tissue implantation of thyroid adenomatous hyperplasia after endoscopic thyroid surgery. Thyroid. 2008; 18(4):483–4.

8. Li S, Zhang F, Zhang Y, Liang Y, Qi X, Yang X, et al. Implantation at sternocleidomastoid and chest wall after endoscopic thyroid carcinoma surgery. Surg Laparosc Endosc Percutan Tech. 2012; 22(4):e239–42.

9. Hur SM, Kim SH, Lee SK, Kim WW, Choi JH, Kim JH, et al. Is a thyroid follicular neoplasm a good indication for endoscopic surgery? Surg Laparosc Endosc Percutan Tech. 2011; 21(3):e148–51.

10. Tae K. Complications of transoral thyroidectomy: Overview and update. Clin Exp Otorhinolaryngol. 2021; 14(2):169–78.

11. Akritidou E, Douridas G, Spartalis E, Tsourouflis G, Dimitroulis D, Nikiteas NI. Complications of trans-oral endoscopic thyroidectomy vestibular approach: A systematic review. In Vivo. 2022; 36(1):1–12.

12. Hayashi T, Hirokawa M, Higuchi M, Kudo T, Ito Y, Miyauchi A. Needle tract implantation following fine-needle aspiration of thyroid cancer. World J Surg. 2020; 44(2):378–84.

13. Ludemann R, Watson DI, Smith E, Ellis T, Jamieson GG. Tumor implantation during laparoscopy using different insufflation gases - an experimental study using cultured cancer cells. Minim Invasive Ther Allied Technol. 2003; 12(6):310–4.

Fig. 1.
Ultrasonography images. Several hypoechoic solid round nodules with smooth margins presented at anterior neck levels Ia and VI.
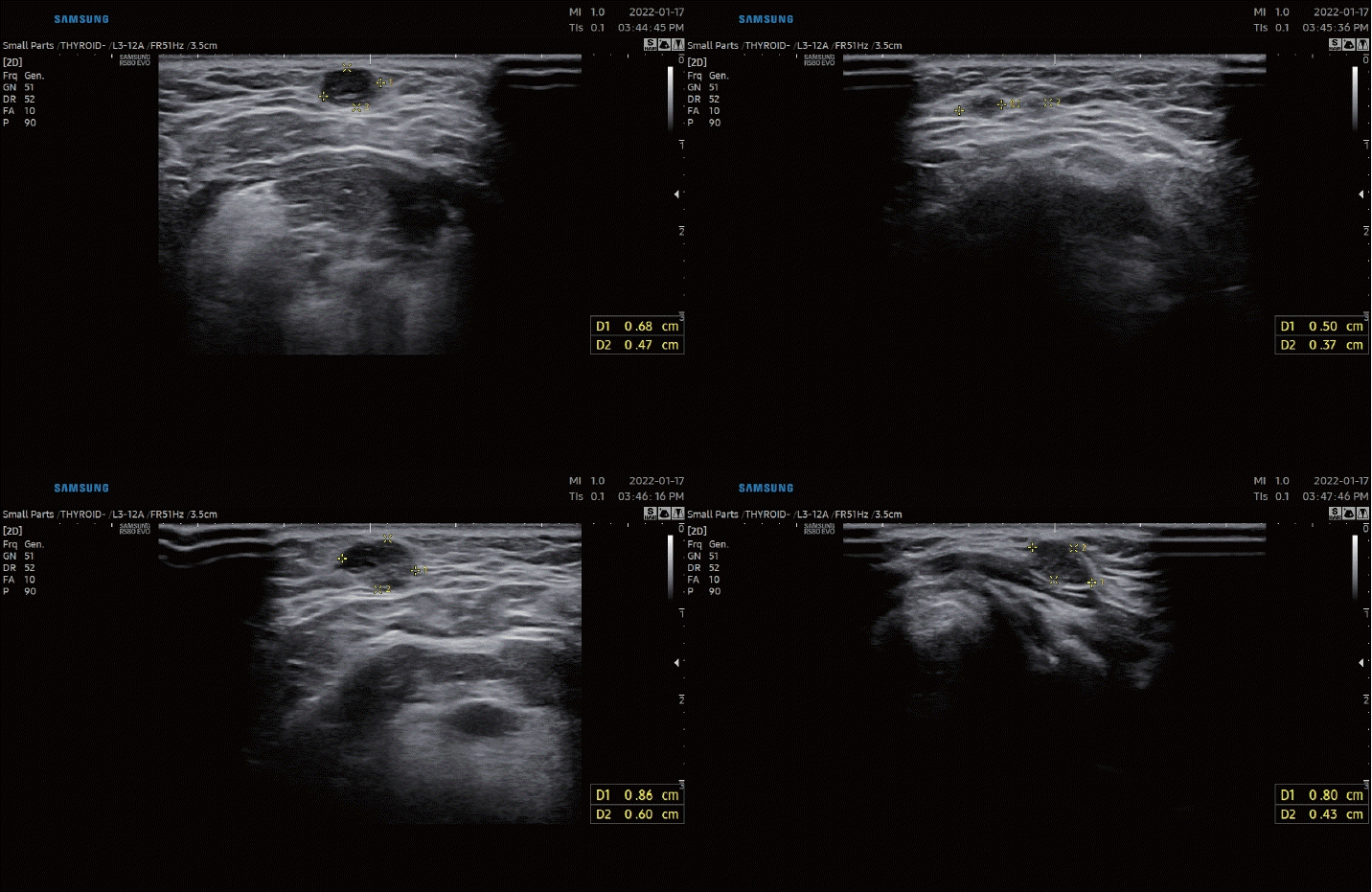
Fig. 2.
CT images. Several enhancing nodules with subtle perilesional fat infiltration presented at anterior neck levels Ia and VI (red arrows).
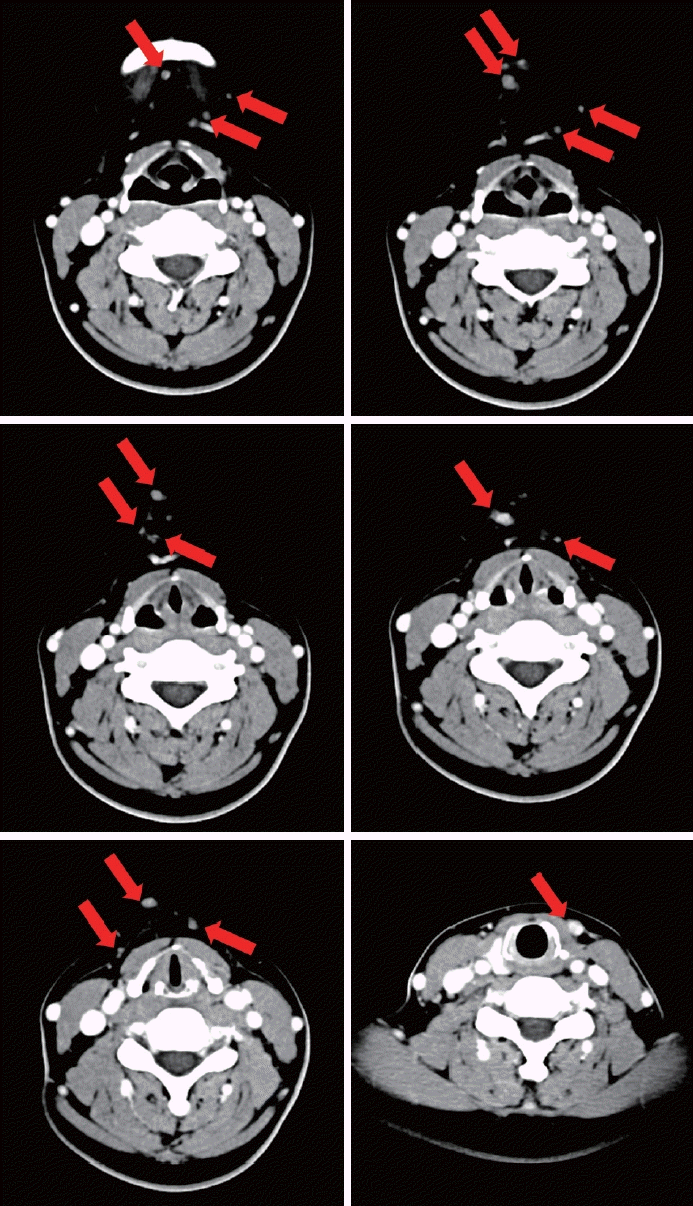
Fig. 3.
Cytopathology from fine needle aspiration biopsy. A: On a low-power view, microfollicular structures that are thought to be derived from the remnant thyroid tissue are visible. Naked nuclei of follicular cells are seen singly scattered in the background (hematoxylin and eosin stain, ×40). B: The follicular cells show no significant atypia or evidence of malignancy (Papanicolaou stain, ×400).
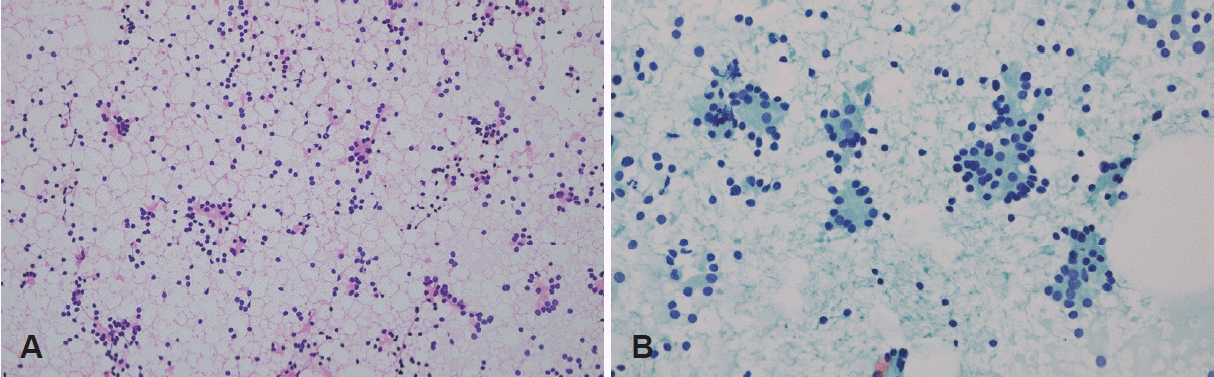
Fig. 4.
Intraoperative photos. A: Photo of surgical fields. The circles marked by surgical pen indicates the location of the nodules confirmed by ultrasound. B: Photo of gross specimens.
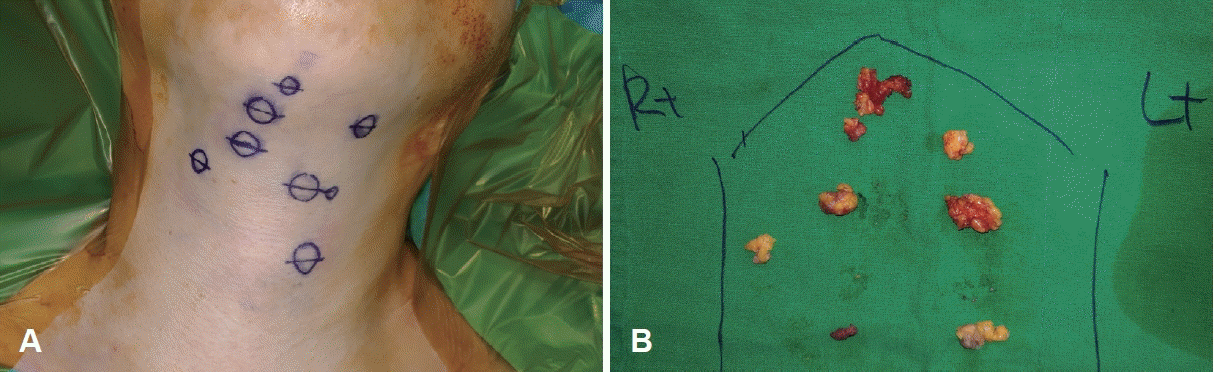
Fig. 5.
Histopathologic findings from surgical excision. A: The tumor shows a microfollicular pattern with hyalinized stroma (hematoxylin and eosin [H&E] stain, ×100). B: The nuclei are round and have coarse chromatin (H&E stain, ×200).
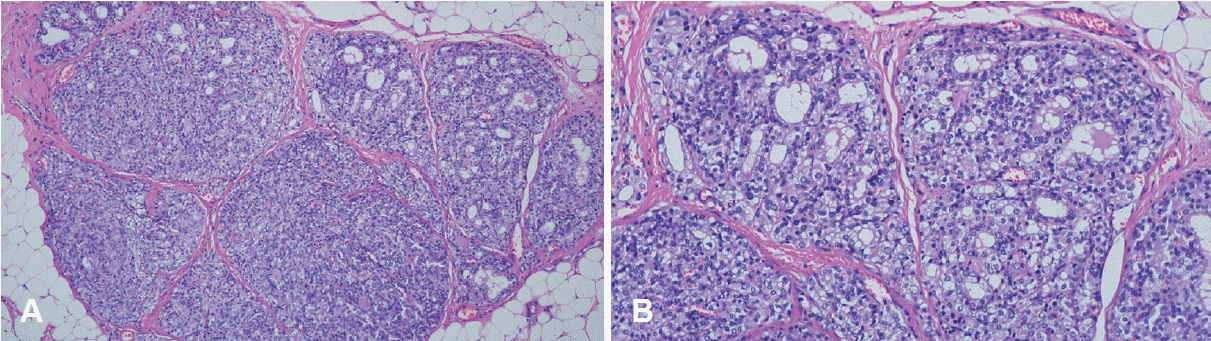
Table 1.
Clinical characteristics of tract implantation cases after endoscopic/robotic thyroidectomy
| Case | Reference number | Age | Sex | Approach | Endoscopic/robotic | Gas insufflation | Excision | Tumor size (cm) | Histopathology |
|---|---|---|---|---|---|---|---|---|---|
| Kim, et al., [5] 2008 | 11 | 25 | Female | Axillo–breast | Endoscopic | Yes | Tumor spillage | 3.1 | Thyroid adenomatous hyperplasia |
| Lee, et al., [7] 2008 | 13 | 17 | Female | Transcervical 4-port | Endoscopic | Yes | ND | 5.5 | Thyroid adenomatous hyperplasia |
| Koh, et al., [6] 2010 | 12 | 22 | Female | Chest–breast | Endoscopic | Yes | ND | 4.0 | Thyroid adenomatous hyperplasia |
| Hur, et al., [9] 2011 | 15 | 61 | Female | Axillo–breast | Endoscopic | Yes | ND | 4.4 | Thyroid adenomatous hyperplasia |
| Li, et al., [8] 2012 | 14 | 39 | Female | Breast | Endoscopic | Yes | ND | 2.9 | Papillary thyroid carcinoma |
| Beninato, et al., [3] 2012 | 9 | 31 | Female | Transaxillary | Endoscopic | Yes | Piecemeal | 3.4 | Follicular adenoma |
| Bakker, et al., [2] 2016 | 8 | 65 | Male | Transaxillary | Robotic | No | Piecemeal | 6.0 | Follicular variant papillary thyroid carcinoma |
| Chabrillac, et al., [4] 2020 | 10 | 50 | Male | Transaxillary | Robotic | No | En bloc | 6.0 | Follicular thyroid carcinoma |
| Present case | 45 | Female | Transoral | Endoscopic | Yes | ND | 2.8 | Follicular adenoma |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download