Postherpetic neuralgia (PHN) has emerged as the most common and severe complication of acute herpes zoster. Compared with spinal nerve herpes zoster neuralgia, trigeminal neuralgia exhibits an increased propensity to transform into PHN, inflicting more intense pain [1,2]. The absence of prompt treatment may prolong the disease, and increase the risk of PHN, which remains a challenging condition to treat. There can be no overstatement of the importance of early and effective intervention to reduce the incidence of PHN.
In recent years, cardiac pacemaker implantation has seen an upsurge in popularity due to the fact that the elderly population is more likely to develop heart disease and to have cardiac implantable electronic devices than other populations of patients [3]. Simultaneously, the proportion of herpes zoster-related trigeminal neuralgia (AZTN) increases sharply with age [4]. Given the ineffectiveness of conservative treatment, the challenge of how to safely and efficaciously manage permanent cardiac pacemaker (PCP) patients with AZTN is escalating. Pulsed radiofrequency (PRF) is a neuroregulatory approach extensively employed for the minimally invasive management of herpes zoster neuralgia and PHN. It may be considered when medical therapy or other interventional procedures have proven ineffective. Recent investigations have demonstrated a correlation between PRF treatment efficacy and its parameters. Clinical research suggests that high-voltage, long-duration PRF delivers superior analgesic outcomes for peripheral neuralgia (e.g., herpes zoster neuralgia, chronic lumbosacral radicular pain, and pudendal neuralgia), without inducing a nerve thermal transection effect [5-7]. However, it is unclear whether the electromagnetic field of high-voltage, long-duration PRF therapy could interfere with medical implantable devices. No previous cases of high-voltage, long-duration PRF of the acute zoster neuralgia in the trigeminal V2 region in patients with a PCP have been reported.
An 83-year-old female patient presented with a PCP implanted 5 years prior to admission because of pathological sinoatrial syndrome. The patient’s pacemaker was operated in a dual-chamber, rate-modulated pacing (DDD) mode (60 bpm), and it was deemed stable following regular cardiology review. Twenty days before admission, she was diagnosed with AZTN because she had painful blisters with a numerical rating scale (NRS) score of 9 points on the second right trigeminal nerve dermatome (Fig. 1). During this period, acyclovir, mecobalamine, and pain relievers, (including 1,500 mg of gabapentin, 150 mg of tramadol and a lidocaine patch) were administered but had little effect. She had paroxysmal, sharp, and lancinating pain in her right maxillary area, which was aggravated by touching the right maxillary area, and was associated with talking. The intractable pain seriously affected her quality of life. After admission, we attempted maxillary block to control the zoster-related pain, but her facial pain only abated temporarily (for approximately 24 hours), before she began to experience excruciating pain and sleep disturbance again.
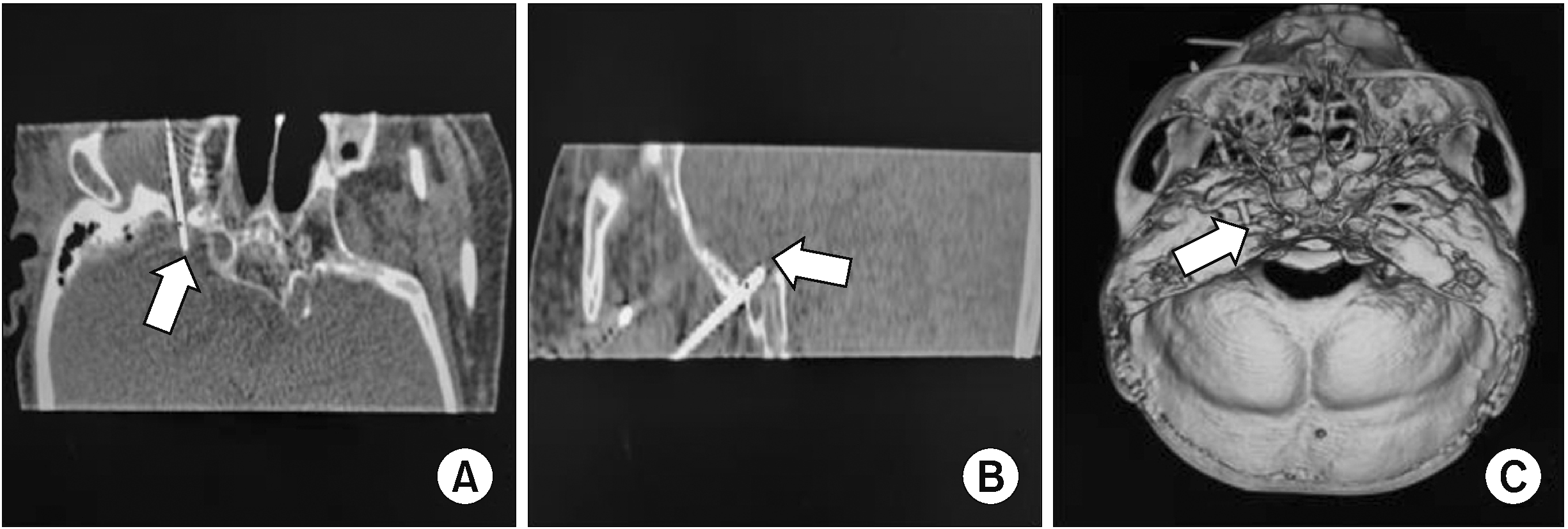
Considering the correlation between PRF treatment efficacy and its parameters, high-voltage, long-duration PRF was suggested to relieve the patient’s intractable pain and the patient signed the informed consent. To circumvent oversensing and other potential issues arising from the impact of pulsed electromagnetic fields on the pacemaker during the procedure, a professional electrophysiologist adjusted the pacing mode to DOO (asynchronous atrioventricular pacing mode, 80 bpm). In this mode, the atria and ventricles solely functioned for pacing and lacked sensing capabilities. In addition, we were taking some procedural precautions, including maintaining at least 15 cm between the grounding pad and PCP sensing leads and involving an electrocardiograph (ECG) and an electrophysiologist for the procedural or postprocedural monitoring. The Hartel anterior approach to access the Gasserian ganglion was adopted [8]. Following the administration of 0.5% lidocaine for ipsilateral anesthesia, a sterilized radiofrequency needle (20 gauge and 100-mm in length, with a 5-mm active tip) was guided along the established puncture angle until it reached the site of the foramen ovale. The needle was then guided to the Gasserian ganglion under the guidance of a three-dimensional CT. The electrode was subsequently connected for sensory testing (50 Hz, 0.1–0.3 V; Cosman G4 Radiofrequency Generator, Cosman Medical Inc.) to elicit paresthesia in the herpes area. Also, motor testing (2 Hz, 0.1 V) was performed, and there was no jaw tremor or jumping sensation. The depth and direction of the puncture needle were slightly adjusted corresponding to the feeling of the patient to ensure the accuracy of the puncture location. Three-dimensional CT imaging was performed to further clarify the path and position of the needle tip (Fig. 2). After sensorial and motor stimulation, PRF was administered at a pulse width of 20 ms and a controlled temperature of 42°C for 600 seconds, with the field intensity gradually increased to the maximum voltage the patient could bear without experiencing pain (90 V). Finally, the puncture point was compressed to stop bleeding after the puncture needle was withdrawn. The patient’s vital signs remained stable, and her pace rhythm was maintained at 80 bpm under DOO mode monitored by ECG throughout the PRF process. No malignant arrhythmia or electrocardiographic events occurred. After treatment, the pacemaker was interrogated, showing no change in its variables, and reprogrammed to its original settings (DDD).
This procedure reduced the patient’s pain immediately, and her pain severity was 0/10 points on the NRS, remaining so for 6 months of follow-up. No residual discomfort, numbness, or hypoesthesia were noted. The dosage of gabapentin was decreased gradually to 300 mg per day 6 months post-procedure, without the need for additional painkillers.
To our knowledge, this case firstly demonstrates that high-voltage, long-duration PRF of the trigeminal Gasserian ganglion can be an effective and safe treatment for PCP patients with intractable AZTN in the V2 area. And no electrical problems occurred during or after the procedure. However, this treatment necessitates a comprehensive preoperative evaluation and meticulous surgical planning, taking into account each patient’s specific circumstances. Based on our long-term experience, PCP patients can benefit from high-voltage, long-duration PRF pain-relieving procedures without any adverse events by adding some preventive measures. But for large-scale promotion and application, further randomized controlled studies will be needed to demonstrate the safety and efficacy of this treatment.
Notes
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed for this paper.
REFERENCES
1. Bouhassira D, Chassany O, Gaillat J, Hanslik T, Launay O, Mann C, et al. 2012; Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain. 153:342–9. DOI: 10.1016/j.pain.2011.10.026. PMID: 22138256.

2. Sim WS, Choi JH, Han KR, Kim YC. 2008; Treatment of herpes zoster and postherpetic neuralgia. Korean J Pain. 21:93–105. DOI: 10.3344/kjp.2008.21.2.93.

3. White WB, Berberian JG. 2022; Pacemaker malfunction-review of permanent pacemakers and malfunctions encountered in the emergency department. Emerg Med Clin North Am. 40:679–91. DOI: 10.1016/j.emc.2022.06.007. PMID: 36396215.

4. Kaufman AR, Myers EM, Moster ML, Stanley J, Kline LB, Golnik KC. 2018; Herpes zoster optic neuropathy. J Neuroophthalmol. 38:179–89. DOI: 10.1097/WNO.0000000000000607. PMID: 29266031.

5. Zhang E, Fei Y, Xu L, Huang B, Yao M. 2022; Effect of repeated high-voltage long-duration pulsed radiofrequency on herpetic neuralgia. Pain Physician. 25:E1047–55.
6. Erken B, Edipoglu IS. 2024; Efficacy of high-voltage pulsed radiofrequency of the dorsal root ganglion for treatment of chronic lumbosacral radicular pain: a randomized clinical trial. Neuromodulation. 27:135–40. DOI: 10.1016/j.neurom.2022.10.056. PMID: 36463027.
7. Ji F, Zhou S, Li C, Zhang Y, Xu H. 2021; Therapeutic efficacy of ultrasound-guided high-voltage long-duration pulsed radiofrequency for pudendal neuralgia. Neural Plast. 2021:9961145. DOI: 10.1155/2021/9961145. PMID: 34373690. PMCID: PMC8349273.

8. Ding Y, Hong T, Li H, Yao P, Zhao G. 2019; Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front Neurosci. 13:708. DOI: 10.3389/fnins.2019.00708. PMID: 31354417. PMCID: PMC6630731.

Fig. 1
Distribution area of the patient’s herpes lesion. The patient’s painful herpes lesion was present on the second right trigeminal nerve dermatome (maxillary branch). Permission to use this photo in the case report was obtained from the patient.
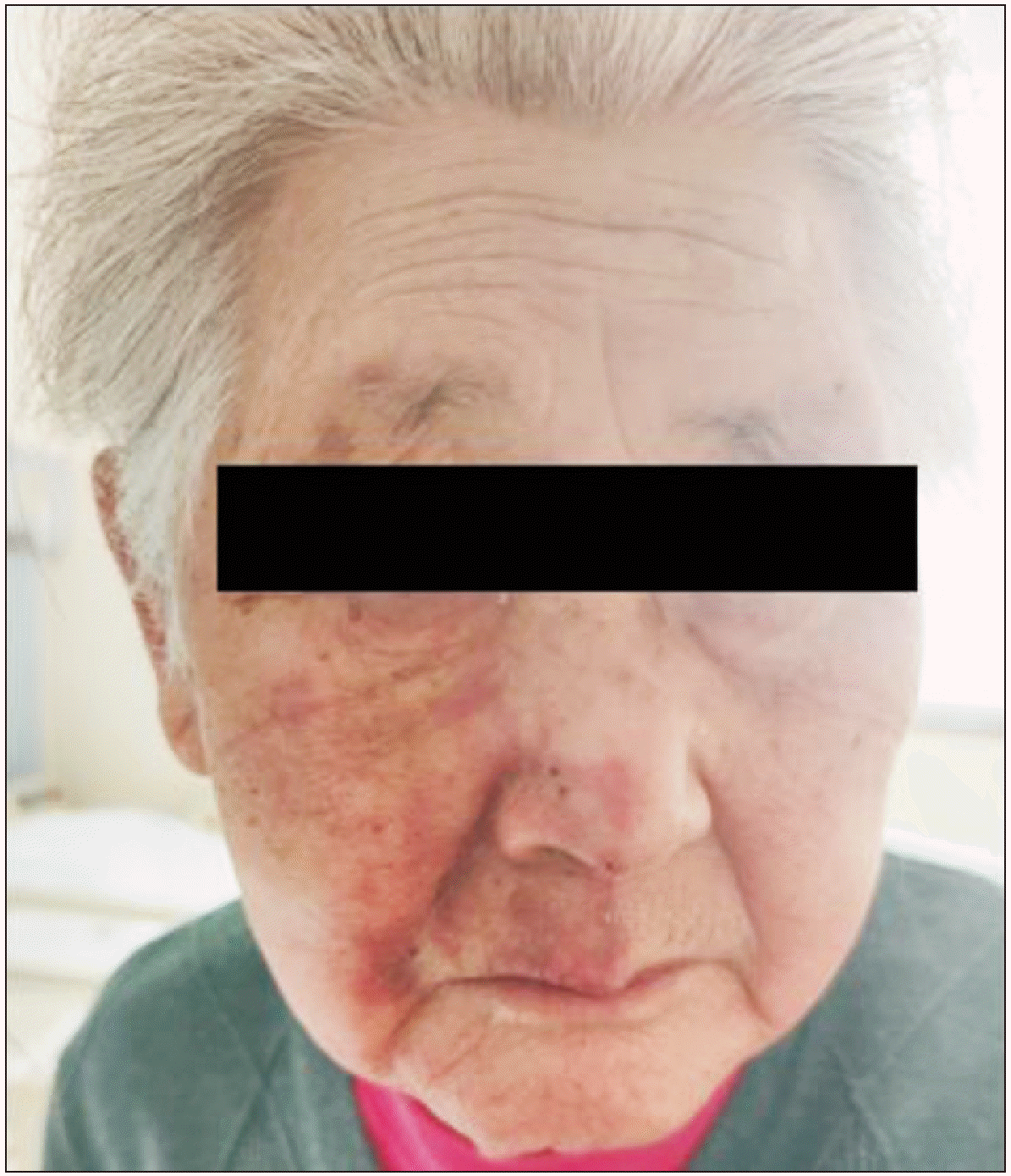
Fig. 2
CT-guided puncture of the Gasserian ganglion. (A) CT coronal view illustrating the RF needle tip entry into the foramen ovale; (B) CT sagittal view illustrating the radiofrequency needle tip entry into the foramen ovale; (C) Three-dimensional CT reconstruction showing the position of the needle tip in the foramen ovale.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download