Abstract
Background
This study aimed to identify exact anatomical landmarks and ideal injection volumes for safe adductor canal blocks (ACB).
Methods
Fifty thighs from 25 embalmed adult Korean cadavers were used. The measurement baseline was the line connecting the anterior superior iliac spine (ASIS) to the midpoint of the patellar base. All target points were measured perpendicular to the baseline. The relevant cadaveric structures were observed using ultrasound (US) and confirmed in living individuals. US-guided dye injection was performed to determine the ideal volume.
Results
The apex of the femoral triangle was 25.3 ± 2.2 cm distal to the ASIS on the baseline and 5.3 ± 1.0 cm perpendicular to that point. The midpoint of the superior border of the vasto-adductor membrane (VAM) was 27.4 ± 2.0 cm distal to the ASIS on the baseline and 5.0 ± 1.1 cm perpendicular to that point. The VAM had a trapezoidal shape and was connected as an aponeurosis between the medial edge of the vastus medialis muscle and lateral edge of the adductor magnus muscle. The nerve to the vastus medialis penetrated the muscle proximal to the superior border of the VAM in 70% of specimens. The VAM appeared on US as a hyperechoic area connecting the vastus medialis and adductor magnus muscles between the sartorius muscle and femoral artery.
The adductor canal, also known as the subsartorial canal, is an intermuscular passage that transmits the femoral vessels from the thigh to the knee [1]. Located in the middle third of the thigh [2], this canal starts proximally from the apex of the femoral triangle and ends distally in the adductor hiatus [1]. The posterior wall of the canal is formed mostly by the adductor longus muscle, its anterior wall is formed by the vastus medialis muscle, and the canal is roofed by the sartorius muscle [1]. In addition to the femoral vessels, the canal contains two nerves: the nerve to the vastus medialis (NVM) and the saphenous nerve (SN) [1,3].
Among various procedures performed in the middle of the thigh, the adductor canal block (ACB) has been effectively used to reduce postoperative pain related to total knee arthroplasty [2,4,5] and anterior cruciate ligament reconstruction [6], to manage lower leg pain caused by entrapment of the SN [7], and as a regional anesthetic technique, combined with a sciatic nerve block, for foot surgery [8]. This block has received attention because it can be adapted to early physical therapy to minimize pain without diminishing muscle strength after knee surgery [9]. However, this block has been clinically associated with unexpected motor weakness [10,11].
SN entrapment is an important concern for pain physicians when managing lower leg pain. Among various management modalities, a precise blockade has been a valuable diagnostic and therapeutic tool. Furthermore, the clinical effectiveness and safety of this block has been confirmed using ultrasound (US)-guided imaging [12,13]. However, for a precise and effective selective nerve blockade, an exact understanding of the topographical relationships related to nerve pathways is essential [14]. Until recently, physicians have attempted to block only the SN [15], a sensory nerve passing through the adductor canal, and to determine the exact location of the NVM in the canal to avoid unwanted side effects such as quadriceps paralysis. Cadaveric and clinical studies utilizing US have reported the morphological and morphometric characteristics of the adductor canal [16–24] and suggested a suitable volume of injectant through dye injection [25–30]. However, the precise landmark and optimal injectant volume for preventing potential side effects have not been definitively identified in the SN-only block. In addition, there are no definite data on this subject in the Korean population.
Thus, this study was performed to clarify the morphological and morphometric characteristics of the structures related to the adductor canal in the Korean population and to suggest exact anatomical landmarks and ideal injectant volumes for successful ACB that only include the SN.
This study was approved by the institutional review board of Wonkwang University (IRB ID No. WKIRB-202306-BR-035) for the cadavers and by Wonkwang University Hospital (IRB ID No. WKUH 2023-06-013) for the patients.
Fifty thighs from 25 embalmed Korean adult cadavers (14 males and 11 females; mean age at death, 74.1 years; age range at death, 50–93 years) were used in this study. All cadavers were legally donated to the University School of Medicine, and the study protocol was strictly conducted according to the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
None of the cadavers showed evidence of pathological changes, previous surgical procedures, or traumatic injuries to the target area. The fascia lata was exposed after the skin and subcutaneous tissue layers of the anterior and medial thighs were removed. The boundary of the femoral triangle was confirmed through careful reflection of the fascia lata. The sartorius muscle was cut at its midpoint and carefully examined to expose the vasto-adductor membrane (VAM). For the objective measurements, the baseline was determined as the line connecting the anterior superior iliac spine (ASIS) to the midpoint of the patellar base. All target points were measured perpendicular to the baseline (Fig. 1) using a digital caliper. During the measurements, the lower extremities of all cadavers were maintained in the anatomical position as much as possible. The Student’s t-test was used to compare all adductor canal measurements between the two sexes, with a statistical significance level of P < 0.05.
Before proceeding with the above steps, 14 thighs from 7 cadavers (4 males and 3 females; mean age, 72.6 years; range, 57–92 years) were subjected to US and dye injection based on the method introduced by Ham et al. [31], as follows.
Tap water was blended with gelatin (1:1 volume ratio) in a 200 mL beaker. The beaker was then placed in a water bath on a hot plate. The temperature of the solution was maintained at approximately 45°C, and the complete dissolution of the mixture was checked. The mixture was then blended with a moderate amount of blue ink.
The US transducer (3–12 MHz linear probe, Ecube INNO; Alpinion) was placed transversely at the lower part of the middle third of the anteromedial thigh based on the cadaveric dissection data. Additionally, a Tuohy needle (80 mm × 20 G echogenic Tuohy needle; Unisis) with a 10 mL syringe was used for a lateral to medial insertion with in-plane technique. The target point of the needle is within the adductor canal, at the portion of the VAM connecting between the vastus medialis and adductor magnus muscles, and the gelatin solution was slowly injected with US monitoring. The 10 mL injection was applied under the VAM in ten thighs from five cadavers, and the 5 mL injection was applied under the VAM in four thighs from two cadavers.
This study included the examination of ten thighs from five patients (3 males and 2 females; mean age, 53.8 years; range, 29–75 years) to confirm the structure of living individuals and verify the cadaveric observations. None of the patients had any history of trauma, surgery, or structural pathology in the target region. The patient's position was adjusted to a supine position with a slight flexion of the knee and external rotation of the leg. The US transducer (5–12 MHz Linear probe, Samsung HS 60; Samsung Medison) was placed transversely at the levels of the significant lines based on data from the cadaveric dissections. The US probe was meticulously scanned from the proximal to the distal aspect to observe the location of the femoral artery posterior to the sartorius muscle in a short-axis view. The sartorius muscle and the SN were observed as a hyperechoic structure which lies lateral to the artery and anterior to the vein. The vastus medialis muscle lies laterally to the SN, and the adductor longus and adductor magnus muscles are on its medial side. Careful attention was paid to the region where a thin membranous structure initially appeared and transitioned into a thicker structure, specifically at the point where VAM originated, as documented in the cadaver observation.
The measurements for each target point are listed in Table 1. The length of the baseline, the line from the ASIS to the midpoint of the patellar base, was 42.7 ± 2.1 cm (43.0 ± 2.5 cm in males, 42.3 ± 1.5 cm in females). The apex of the femoral triangle was 25.3 ± 2.2 cm distal to the ASIS on the baseline and 5.3 ± 1.0 cm perpendicular to that point. The midpoint of the superior border of the VAM was 27.4 ± 2.0 cm distal to the ASIS on the baseline and 5.0 ± 1.1 cm perpendicular to that point. The anterior foramen of the adductor canal was 33.8 ± 2.3 cm distal to the ASIS on the baseline and 5.5 ± 1.2 cm perpendicular to that point. The uppermost point of the adductor hiatus was 36.0 ± 2.3 cm distal to the ASIS on the baseline and 4.9 ± 1.1 cm perpendicular to that point.
The VAM was derived from the medial intermuscular septum of the thigh and distinctly identified in all specimens. The VAM had a trapezoidal shape, with a long upper border, and was connected as an aponeurosis between the medial edge of the vastus medialis muscle and the lateral edge of the adductor magnus muscle (Fig. 2). The mean ratio of the VAM to the adductor canal was 0.82; thus, the VAM covered the lower four-fifths of the entire length of the adductor canal. The upper fifth of the canal was covered by a connective tissue membrane under the sartorius muscle, and sparse tendinous fibers began to appear in the lower part. These fibers traversed the lower part of the adductor longus muscle and were attached to the medial edge of the vastus medialis muscle.
In all specimens, the NVM arose from the posterior division of the femoral nerve under the sartorius muscle and proximal to the adductor canal. This nerve ran distally along the anteromedial border of the vastus medialis muscle and penetrated the muscle as one to three branches in the adductor canal. The relationship between this nerve and the superior border of VAM (SB) was categorized into three types based on the number of nerves, and each subtype was further classified into two or three subtypes based on the location where the nerve penetrated the muscle with respect to the SB (Fig. 3). The nerve penetrated the muscle proximal to the SB in 70% of the specimens and distal to the SB in 30%. All nerves were observed outside the VAM.
In all the specimens, the SN arose from the posterior division of the femoral nerve. This nerve was accompanied by femoral vessels in the adductor canal and exited through the anterior foramen of the canal. Most nerves passed through the midpoint of the superior border of the VAM. At that point, the femoral artery was lateral (52% of the specimens), posterior (32%), and medial (16%) to this nerve (Fig. 4).
During US imaging of the cadavers, the transducer was placed transversely at the lower part of the middle third of the anteromedial thigh. The VAM was observed as a hyperechoic area connecting the vastus medialis and adductor magnus muscles between the sartorius muscle and femoral artery (Fig. 5A). During the US scanning process in living humans, the area initially appearing as a thin membranous structure and transitioned into a thicker structure where the VAM began (Fig. 5B). Furthermore, these changes were similarly distinguishable in all subjects.
To screen for an adaptable injectant volume, we used US-guided dye injection. The 10 mL injection was applied under the VAM in 10 thighs of five cadavers, and the 5 mL injection was applied under the VAM in 4 thighs of two cadavers. After dissection, the gelatin mixture filled the full extent of the adductor canal; however, unwanted areas, such as the anteromedial side of the vastus medialis and posterior sartorius muscles, were also colored with the dye (Fig. 6A). After dissection, the gelatin mixture efficiently filled only the area covered by the VAM (Fig. 6B). The entire process of all injections was administered by a single practitioner, and a relatively lower pressure was noted in the 5 mL injection group when compared to the 10 mL group.
The location and boundary of the adductor canal have been similarly described in several anatomical studies. However, detailed explanations of the lower or deep parts of the canal are found in only a few studies [1,32,33]. The authors of these studies commonly mention the tendinous fiber, fascia, or aponeurosis running from the adductor longus and magnus muscles to the vastus medialis muscle. This structure was initially denominated as the fascia vasto-adductoria by Callander [32] and was later called the VAM. In regard to this structure, Woodburne and Burkel [33] stated that in the lower third of the thigh, tendinous fibers spread laterally from the rounded tendon of the adductor magnus muscle toward the vastus medialis muscle, end in the medial intermuscular septum of the thigh, and may be pierced by the SN and descending genicular artery. Rosse and Gaddum-Rosse [1] stated that deep in the sartorius muscle, there is a definite lamina of the fascia connecting the medial intermuscular septum to the deep fascia of the adductor muscles that intervenes between the sartorius muscle and the contents of the canal. The VAM has been morphologically described in detail by Tubbs et al. [16], who found this membrane in all specimens; the VAM originated from the medial intermuscular septum of the thigh, traversed from the medial edge of the vastus medialis muscle to the lateral edge of the adductor magnus muscle, and had a rhomboid shape with a wide proximal border. In this study, the VAM morphology was nearly similar. However, thin, sparse tendinous fibers were observed in the proximal VAM, which connected the lower part of the adductor longus and vastus medialis muscles. Additionally, the SN has a very long course, and multiple sites of entrapment are possible. Occasionally, SN entrapment can be difficult to diagnose because of the intimate relationship between the nerve and several other pain-generating structures. The SN joins the saphenous branch of the descending genicular artery, and both structures pierce the roof of the subsartorial canal just proximal to the adductor hiatus [34]. At this point, as well as within the adductor canal, the SN is most susceptible to entrapment. The pathophysiology of SN neuropathy may involve acute high-pressure or chronic intermittent compression, as well as posttraumatic or postsurgical scarring. As a result, pain can occur in various areas from the groin to the medial mid-thigh, medial knee, and big toe [35].
Recently, US-guided ACB have become a popular method for pain control after lower leg surgery. Therefore, the exact differentiation of the proximal boundary of the adductor canal on US images is necessary for a successful nerve block. Burckett-St Laurant et al. [19] and Nair et al. [23] stated that the proximal end of the canal was difficult to match with well-defined surface anatomical landmarks but could be easily identified on US images. In these studies, the adductor canal was identified by examining the site where the medial border of the sartorius muscles crossed the medial border of the adductor longus muscle or by following the femoral artery to directly below the middle of the sartorius muscle. Wong et al. [20] reported that US could identify the VAM in most subjects, and this could be used as additional confirmation of the canal location. In the present study, the proximal boundary of the canal and superior border of the VAM could also be easily detected in the middle third of the anteromedial thigh during US imaging from the proximal to distal direction. In particular, the VAM appeared as a distinct hyperechoic area connecting the vastus medialis and adductor magnus muscles between the sartorius muscle and femoral artery (Fig. 5).
Selective SN block at the adductor canal was first described by Romanoff et al. [7] to manage pain related to SN entrapment. Subsequently, various approaches for SN block with and without US have been introduced [36–38]; however, some problems remain, such as avoiding unwanted weakness of the quadriceps femoris muscle, which requires excessive recovery time for outpatients. This side effect can occur when injectants, including local anesthetics, invade the NVM. According to Burckett-St Laurant et al. [19], the NVM enters the adductor canal lateral to the femoral artery at the apex of the femoral triangle in all specimens and branches into three to four muscular branches a short distance into the canal. In the present study, all NVM were medial to the femoral artery, and had one to three branches (Fig. 3). In addition, the NVM penetrated the muscle distal to the superior border of the VAM in 30% of the specimens (Fig. 3). Therefore, even though physicians might succeed in accurately injecting the adductor canal by confirming the VAM through US imaging, quadriceps femoris muscle weakness could occur in one-fourth of patients.
Several researchers have attempted to determine the ideal injectant volume for ACB, including 30 mL [39,40], 20 mL [26,29,41], 15 mL [25], and 10 mL [28,30]. Among these, a proximal adductor canal injection of 10 mL was suggested, as it would likely preserve greater activation of the vastus medialis muscle by sparing the anterior branches of the NVM [30]. In the present study, the gelatin mixture filled the full extent of the adductor canal; however, unwanted areas were also invaded in the US-guided 10 mL injection group (Fig. 6A). However, the gelatin mixture efficiently filled only the area covered by the VAM in the 5 mL injection group (Fig. 6B). Based on our anatomical findings, additional clinical studies are warranted.
Our study shares some limitations with other cadaver studies. Understanding particular conditions or complications that may arise in real patients can be a challenging task. Therefore, a high degree of caution is necessary when interpreting the outcomes of cadaver studies and assessing their relevance to actual patient situations. Based on our findings of anatomical studies, future studies and additional clinical research is needed. In conclusion, our results can lead to insights for more accurate and effective ACB and indicate that avoiding unwanted weakness of the quadriceps femoris muscle may require several conditions. First, the superior border of the VAM can be easily confirmed using US. Second, the needle must be positioned obliquely downward, below the superior border of the VAM. And third, injection of only 5 mL might be more suitable than a large-volume injection for a selective SN block.
ACKNOWLEDGMENTS
The authors thank the body donors and their families. Without their unique donations, it would have been impossible for us to complete this study.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
REFERENCES
1. Rosse C, Gaddum-Rosse P. 1997. Hollinshead's textbook of anatomy. 5th ed. Lippincott-Raven.
2. Seo SS, Kim OG, Seo JH, Kim DH, Kim YG, Park BY. 2017; Comparison of the effect of continuous femoral nerve block and adductor canal block after primary total knee arthroplasty. Clin Orthop Surg. 9:303–9. DOI: 10.4055/cios.2017.9.3.303. PMID: 28861197. PMCID: PMC5567025.

3. Romanes GJ. 1981. Cunningham's textbook of anatomy. 20th ed. Oxford University Press.
4. Dong CC, Dong SL, He FC. 2016; Comparison of adductor canal block and femoral nerve block for postoperative pain in total knee arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore). 95:e2983. DOI: 10.1097/MD.0000000000002983. PMID: 27015172. PMCID: PMC4998367.
5. You D, Qin L, Li K, Li D, Zhao G, Li L. 2021; A meta-analysis on advantages of peripheral nerve block post-total knee arthroplasty. Korean J Pain. 34:271–87. DOI: 10.3344/kjp.2021.34.3.271. PMID: 34193634. PMCID: PMC8255149.

6. Runner RP, Boden SA, Godfrey WS, Premkumar A, Samady H, Gottschalk MB, et al. 2018; Quadriceps strength deficits after a femoral nerve block versus adductor canal block for anterior cruciate ligament reconstruction: a prospective, single-blinded, randomized trial. Orthop J Sports Med. 6:2325967118797990. DOI: 10.1177/2325967118797990. PMID: 30276220. PMCID: PMC6158619.

7. Romanoff ME, Cory PC Jr, Kalenak A, Keyser GC, Marshall WK. 1989; Saphenous nerve entrapment at the adductor canal. Am J Sports Med. 17:478–81. DOI: 10.1177/036354658901700405. PMID: 2782531.

8. Joe HB, Choo HS, Yoon JS, Oh SE, Cho JH, Park YU. 2016; Adductor canal block versus femoral nerve block combined with sciatic nerve block as an anesthetic technique for hindfoot and ankle surgery: a prospective, randomized noninferiority trial. Medicine (Baltimore). 95:e5758. DOI: 10.1097/MD.0000000000005758. PMID: 28033291. PMCID: PMC5207587.
9. Wang CG, Ding YL, Wang YY, Liu JY, Zhang Q. 2020; Comparison of adductor canal block and femoral triangle block for total knee arthroplasty. Clin J Pain. 36:558–61. DOI: 10.1097/AJP.0000000000000833. PMID: 32271182.

10. Chen J, Lesser JB, Hadzic A, Reiss W, Resta-Flarer F. 2014; Adductor canal block can result in motor block of the quadriceps muscle. Reg Anesth Pain Med. 39:170–1. DOI: 10.1097/AAP.0000000000000053. PMID: 24553306.

11. Veal C, Auyong DB, Hanson NA, Allen CJ, Strodtbeck W. 2014; Delayed quadriceps weakness after continuous adductor canal block for total knee arthroplasty: a case report. Acta Anaesthesiol Scand. 58:362–4. DOI: 10.1111/aas.12244. PMID: 24372058.

12. Ahuja V, Thapa D, Chander A, Gombar S, Gupta R, Gupta S. 2020; Role of dexmedetomidine as adjuvant in postoperative sciatic popliteal and adductor canal analgesia in trauma patients: a randomized controlled trial. Korean J Pain. 33:166–75. DOI: 10.3344/kjp.2020.33.2.166. PMID: 32235017. PMCID: PMC7136291.

13. Ming LH, Chin CS, Yang CT, Suhaimi A. 2022; Adductor canal block versus intra-articular steroid and lidocaine injection for knee osteoarthritis: a randomized controlled study. Korean J Pain. 35:191–201. DOI: 10.3344/kjp.2022.35.2.191. PMID: 35354682. PMCID: PMC8977207.

14. Kim YD. 2021; Necessity of an exact anatomical understanding for the better pain practice. Korean J Pain. 34:373–4. DOI: 10.3344/kjp.2021.34.4.373. PMID: 34593655. PMCID: PMC8494956.

15. Bouaziz H, Benhamou D, Narchi P. 1996; A new approach for saphenous nerve block. Reg Anesth. 21:490.
16. Tubbs RS, Loukas M, Shoja MM, Apaydin N, Oakes WJ, Salter EG. 2007; Anatomy and potential clinical significance of the vastoadductor membrane. Surg Radiol Anat. 29:569–73. DOI: 10.1007/s00276-007-0230-4. PMID: 17618402.

17. Kapoor R, Adhikary SD, Siefring C, McQuillan PM. 2012; The saphenous nerve and its relationship to the nerve to the vastus medialis in and around the adductor canal: an anatomical study. Acta Anaesthesiol Scand. 56:365–7. DOI: 10.1111/j.1399-6576.2011.02645.x. PMID: 22335278.

18. Bendtsen TF, Moriggl B, Chan V, Pedersen EM, Børglum J. 2014; Defining adductor canal block. Reg Anesth Pain Med. 39:253–4. DOI: 10.1097/AAP.0000000000000052. PMID: 24747312.

19. Burckett-St Laurant D, Peng P, Girón Arango L, Niazi AU, Chan VW, Agur A, et al. 2016; The nerves of the adductor canal and the innervation of the knee: an anatomic study. Reg Anesth Pain Med. 41:321–7. DOI: 10.1097/AAP.0000000000000389. PMID: 27015545.
20. Wong WY, Bjørn S, Strid JM, Børglum J, Bendtsen TF. 2017; Defining the location of the adductor canal using ultrasound. Reg Anesth Pain Med. 42:241–5. DOI: 10.1097/AAP.0000000000000539. PMID: 28002228. PMCID: PMC5318152.

21. Kavolus JJ, Sia D, Potter HG, Attarian DE, Lachiewicz PF. 2018; Saphenous nerve block from within the knee is feasible for TKA: MRI and cadaveric study. Clin Orthop Relat Res. 476:30–6. DOI: 10.1007/s11999.0000000000000006. PMID: 29529612. PMCID: PMC5919220.

22. Kendir S, Torun Bİ, Akkaya T, Comert A, Tuccar E, Tekdemir I. 2018; Re-defining the anatomical structures for blocking the nerves in adductor canal and sciatic nerve through the same injection site: an anatomical study. Surg Radiol Anat. 40:1267–74. DOI: 10.1007/s00276-018-2094-1. PMID: 30167824.

23. Nair A, Dolan J, Tanner KE, Kerr CM, Jones B, Pollock PJ, et al. 2018; Ultrasound-guided adductor canal block: a cadaver study investigating the effect of a thigh tourniquet. Br J Anaesth. 121:890–8. DOI: 10.1016/j.bja.2018.04.044. PMID: 30236251.

24. Thiayagarajan MK, Kumar SV, Venkatesh S. 2019; An exact localization of adductor canal and its clinical significance: a cadaveric study. Anesth Essays Res. 13:284–6. DOI: 10.4103/aer.AER_35_19. PMID: 31198246. PMCID: PMC6545962.

25. Andersen HL, Andersen SL, Tranum-Jensen J. 2015; The spread of injectate during saphenous nerve block at the adductor canal: a cadaver study. Acta Anaesthesiol Scand. 59:238–45. DOI: 10.1111/aas.12451. PMID: 25496028.

26. Goffin P, Lecoq JP, Ninane V, Brichant JF, Sala-Blanch X, Gautier PE, et al. 2016; Interfascial spread of injectate after adductor canal injection in fresh human cadavers. Anesth Analg. 123:501–3. DOI: 10.1213/ANE.0000000000001441. PMID: 27442773.

27. Pepper AM, North TW, Sunderland AM, Davis JJ. 2016; Intraoperative adductor canal block for augmentation of periarticular injection in total knee arthroplasty: a cadaveric study. J Arthroplasty. 31:2072–6. DOI: 10.1016/j.arth.2016.02.030. PMID: 26996675.

28. Runge C, Moriggl B, Børglum J, Bendtsen TF. 2017; The spread of ultrasound-guided injectate from the adductor canal to the genicular branch of the posterior obturator nerve and the popliteal plexus: a cadaveric study. Reg Anesth Pain Med. 42:725–30. DOI: 10.1097/AAP.0000000000000675. PMID: 28937534.
29. Johnston DF, Black ND, Cowden R, Turbitt L, Taylor S. 2019; Spread of dye injectate in the distal femoral triangle versus the distal adductor canal: a cadaveric study. Reg Anesth Pain Med. 44:39–45. DOI: 10.1136/rapm-2018-000002. PMID: 30640651.

30. Tran J, Chan VWS, Peng PWH, Agur AMR. 2020; Evaluation of the proximal adductor canal block injectate spread: a cadaveric study. Reg Anesth Pain Med. 45:124–30. DOI: 10.1136/rapm-2019-101091. PMID: 31879373.

31. Ham HD, Kim YD, Won HS. 2021; Introduction of a reasonable manner for injection studies using cadavers. Braz J Anesthesiol. 71:188–9. DOI: 10.1016/j.bjane.2020.10.016. PMID: 33894863. PMCID: PMC9373090.

32. Callander CL. 1939. Surgical anatomy. 2nd ed. W.B. Saunders.
33. Woodburne RT, Burkel WE. 1994. Essentials of human anatomy. 9th ed. Oxford University Press.
34. Luerssen TG, Campbell RL, Defalque RJ, Worth RM. 1983; Spontaneous saphenous neuralgia. Neurosurgery. 13:238–41. DOI: 10.1227/00006123-198309000-00004. PMID: 6621837.

35. Imani F, Rahimzadeh P, Abolhasan Gharehdag F, Faiz SH. 2013; Sonoanatomic variation of pes anserine bursa. Korean J Pain. 26:249–54. DOI: 10.3344/kjp.2013.26.3.249. PMID: 23861998. PMCID: PMC3710938.

36. Benzon HT, Sharma S, Calimaran A. 2005; Comparison of the different approaches to saphenous nerve block. Anesthesiology. 102:633–8. DOI: 10.1097/00000542-200503000-00023. PMID: 15731603.

37. Orebaugh SL, Moreno M, Breneman SM, Bigeleisen PE. Bigeleisen PE, Gofeld M, Orebaugh SL, editors. 2009. Ultrasound-guided saphenous nerve block. In: Ultrasound-guided regional anesthesia and pain medicine. Lippincott Williams & Wilkins;p. 108–13.
38. Saranteas T, Anagnostis G, Paraskeuopoulos T, Koulalis D, Kokkalis Z, Nakou M, et al. 2011; Anatomy and clinical implications of the ultrasound-guided subsartorial saphenous nerve block. Reg Anesth Pain Med. 36:399–402. DOI: 10.1097/AAP.0b013e318220f172. PMID: 21697687.

39. Davis JJ, Bond TS, Swenson JD. 2009; Adductor canal block: more than just the saphenous nerve? Reg Anesth Pain Med. 34:618–9. DOI: 10.1097/AAP.0b013e3181bfbf00. PMID: 19901788.
40. Lund J, Jenstrup MT, Jaeger P, Sørensen AM, Dahl JB. 2011; Continuous adductor-canal-blockade for adjuvant post-operative analgesia after major knee surgery: preliminary results. Acta Anaesthesiol Scand. 55:14–9. DOI: 10.1111/j.1399-6576.2010.02333.x. PMID: 21039357.

41. Gautier PE, Hadzic A, Lecoq JP, Brichant JF, Kuroda MM, Vandepitte C. 2016; Distribution of injectate and sensory-motor blockade after adductor canal block. Anesth Analg. 122:279–82. DOI: 10.1213/ANE.0000000000001025. PMID: 26678473.

Fig. 1
A schematic drawing showing each target point. The baseline is the red line connecting the anterior superior iliac spine (ASIS) to the midpoint of the patellar base (PB). Points a, b, c, and d indicate the apex of the femoral triangle, the midpoint of the superior border of the VAM, the anterior foramen of the adductor canal, and the uppermost point of the adductor hiatus, respectively. VAM: vasto-adductor membrane.
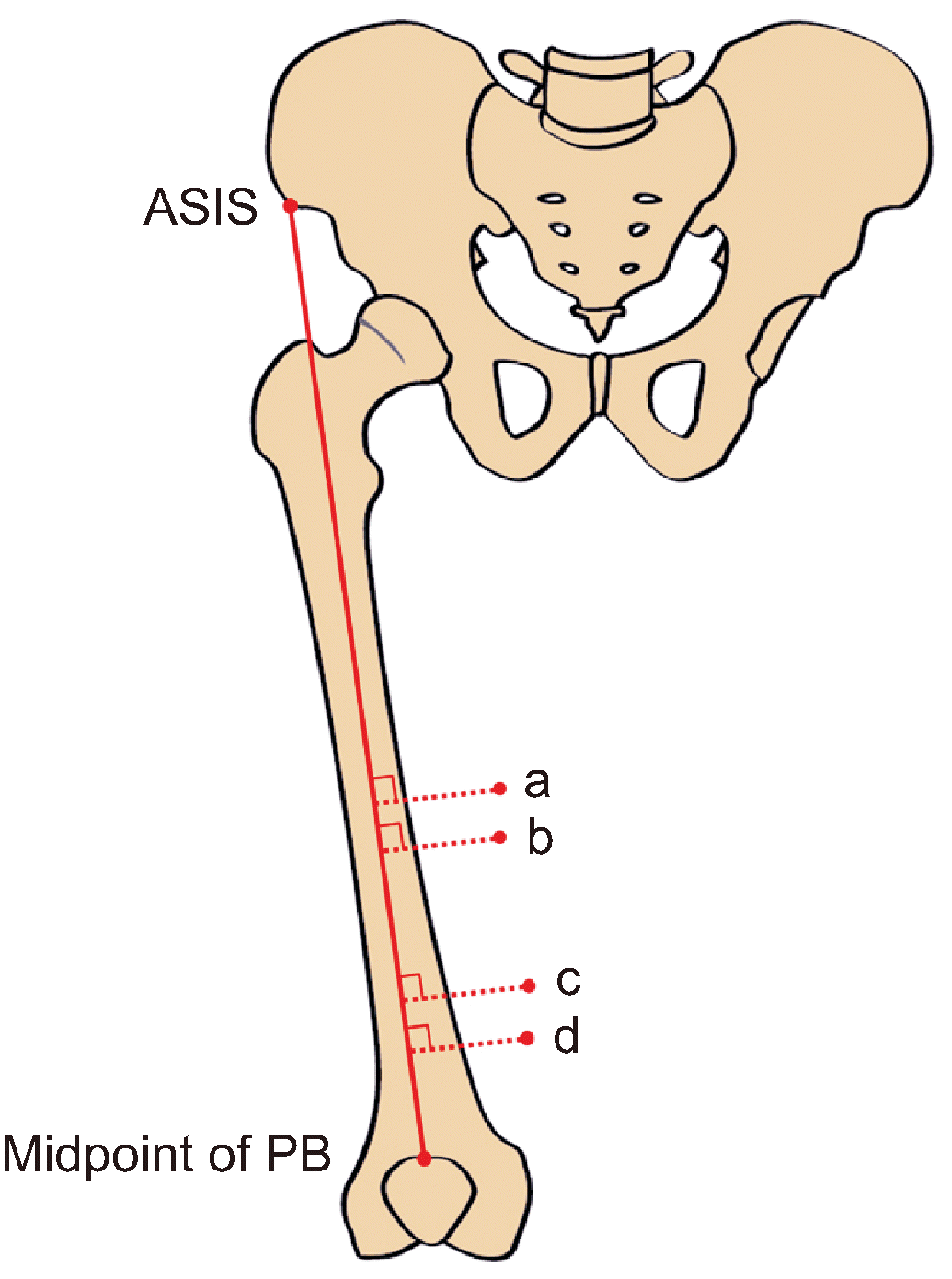
Fig. 2
The VAM exposed by fine dissection. The asterisk indicates the VAM. NVM: nerve to vastus medialis, SN: saphenous nerve, FA: femoral artery, S: sartorius muscle, VMe: vastus medialis muscle, FL: fascia lata, VAM: vasto-adductor membrane, Cra: cranial side, Cau: caudal side, Med: medial side, Lat: lateral side.
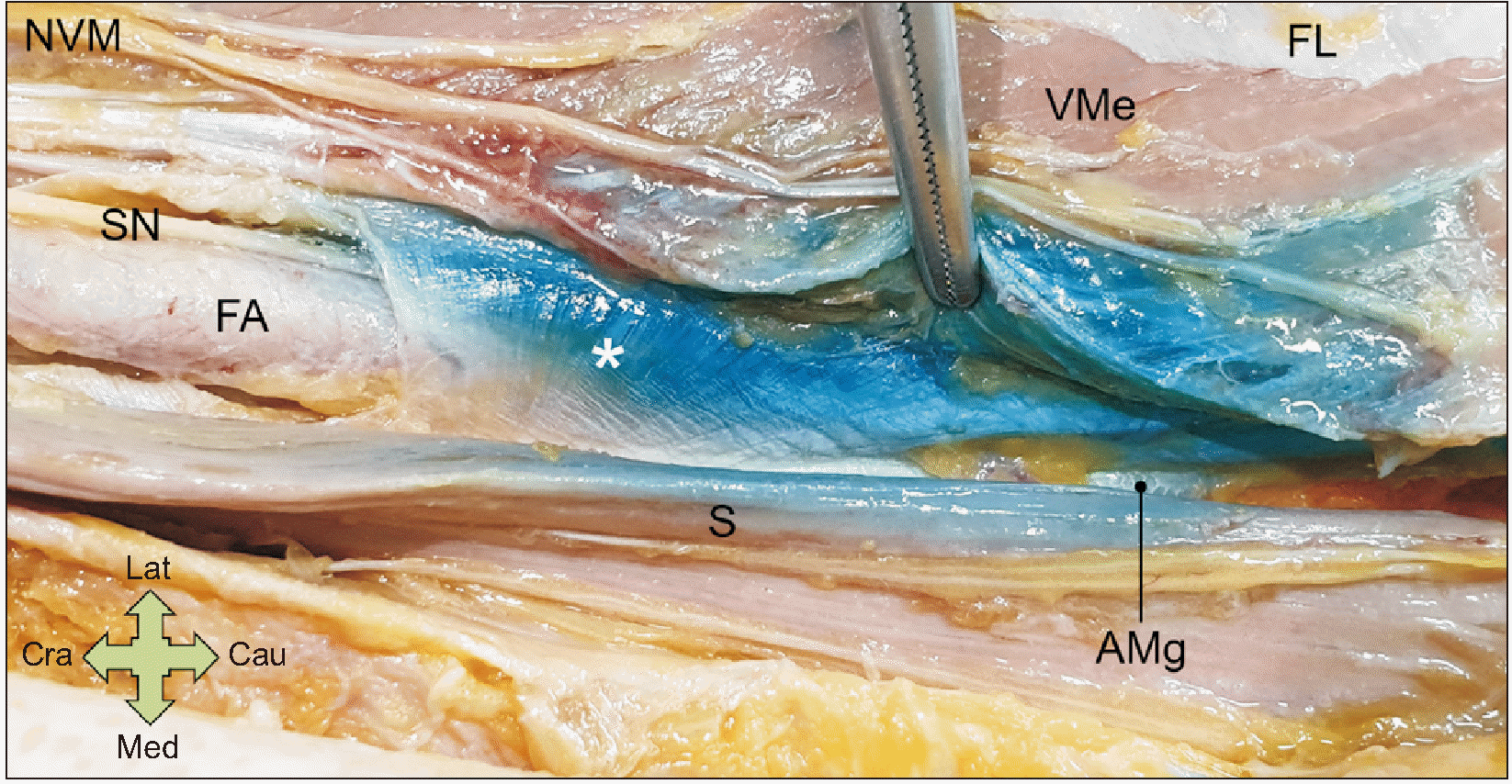
Fig. 3
Relationship between the NVM and superior border of VAM (SB). The red lines indicate the SB. NVM: nerve to the vastus medialis, VAM: vasto-adductor membrane, Med: medial side, Lat: lateral side.
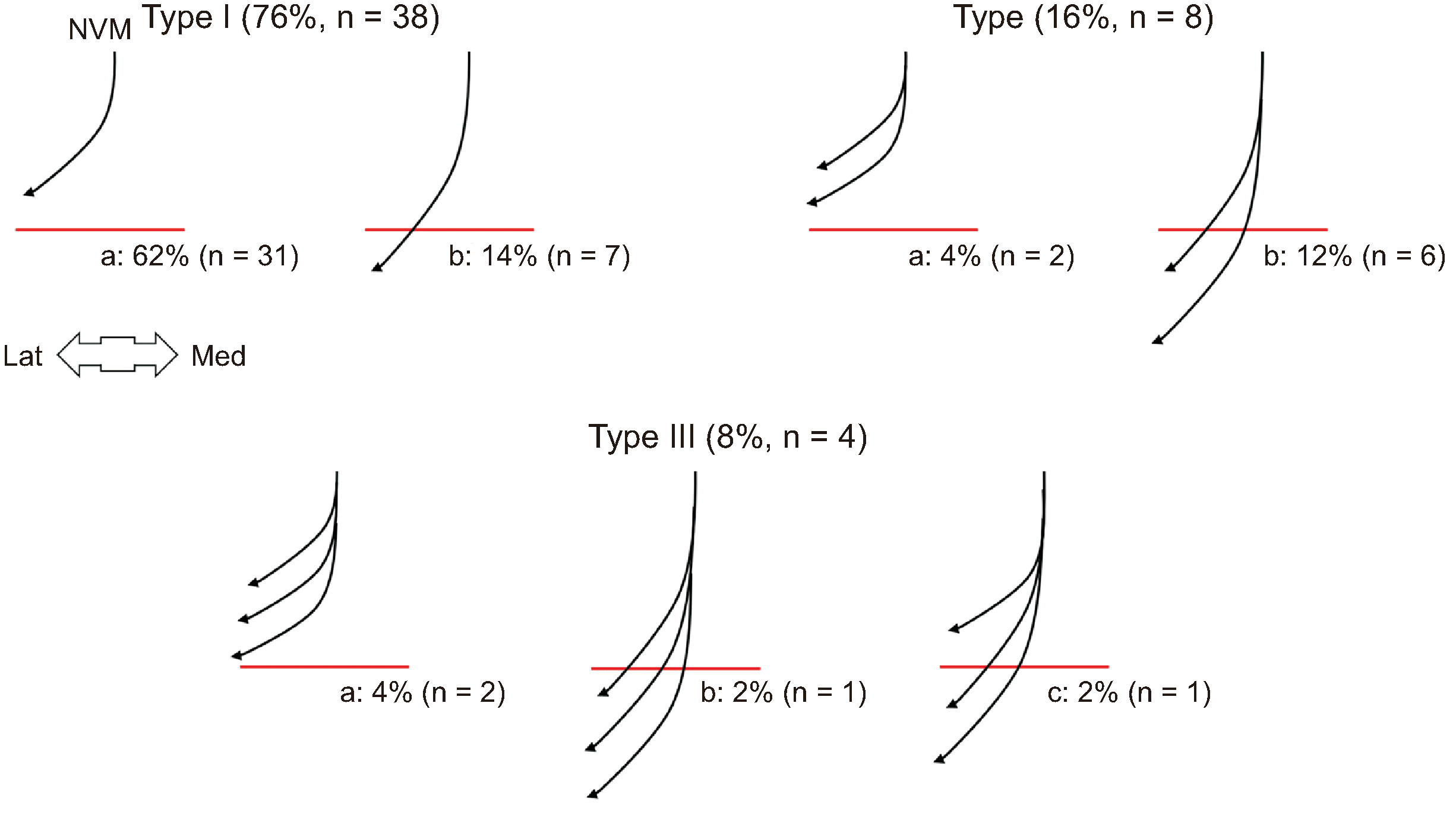
Fig. 4
Relationships between the femoral artery and saphenous nerve at the superior border of the VAM (SB). The horizontal line in the circle indicates the SB, and the vertical line is a line passing through the midpoint of the SB. VAM: vasto-adductor membrane, Med: medial side, Lat: lateral side.
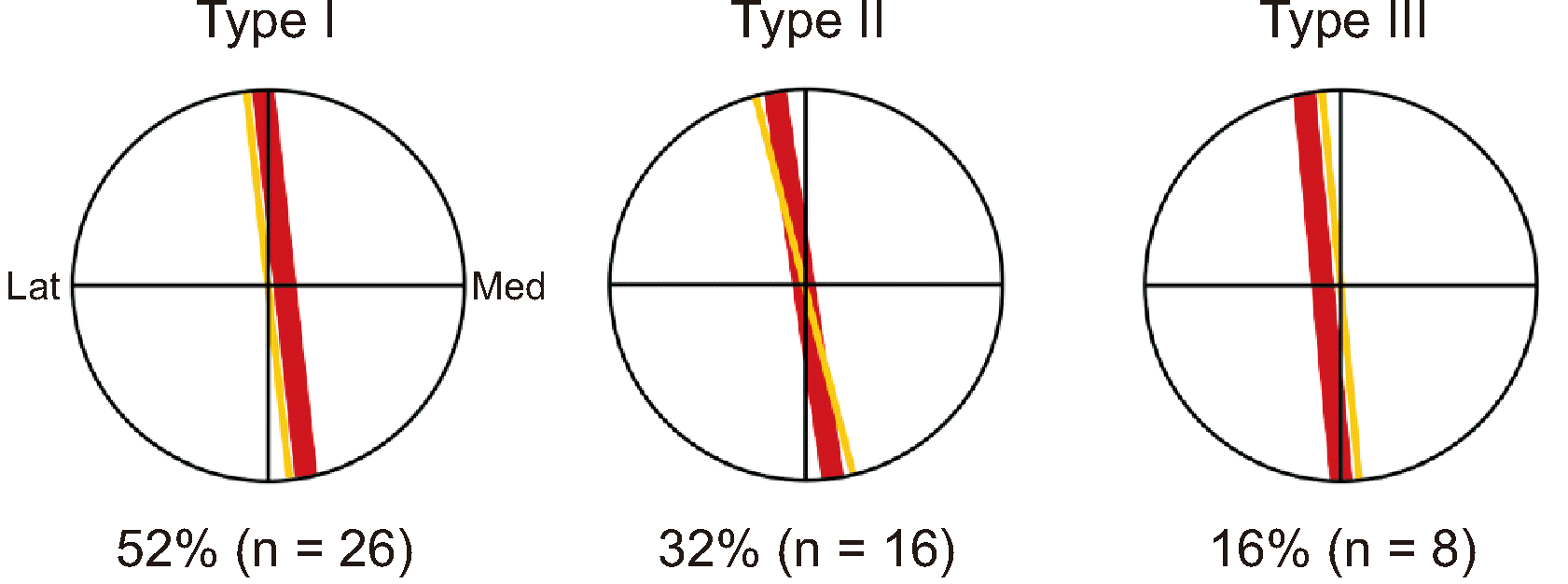
Fig. 5
The VAM shown on US images of a cadaver (A) and living individual (B). The yellow asterisk indicates the VAM. S: sartorius muscle, AMg: adductor magnus muscle, FA: femoral artery, FV: femoral vein, VMe: vastus medialis muscle, VAM: vasto-adductor membrane, US: ultrasound, Med: medial side, Lat: lateral side.
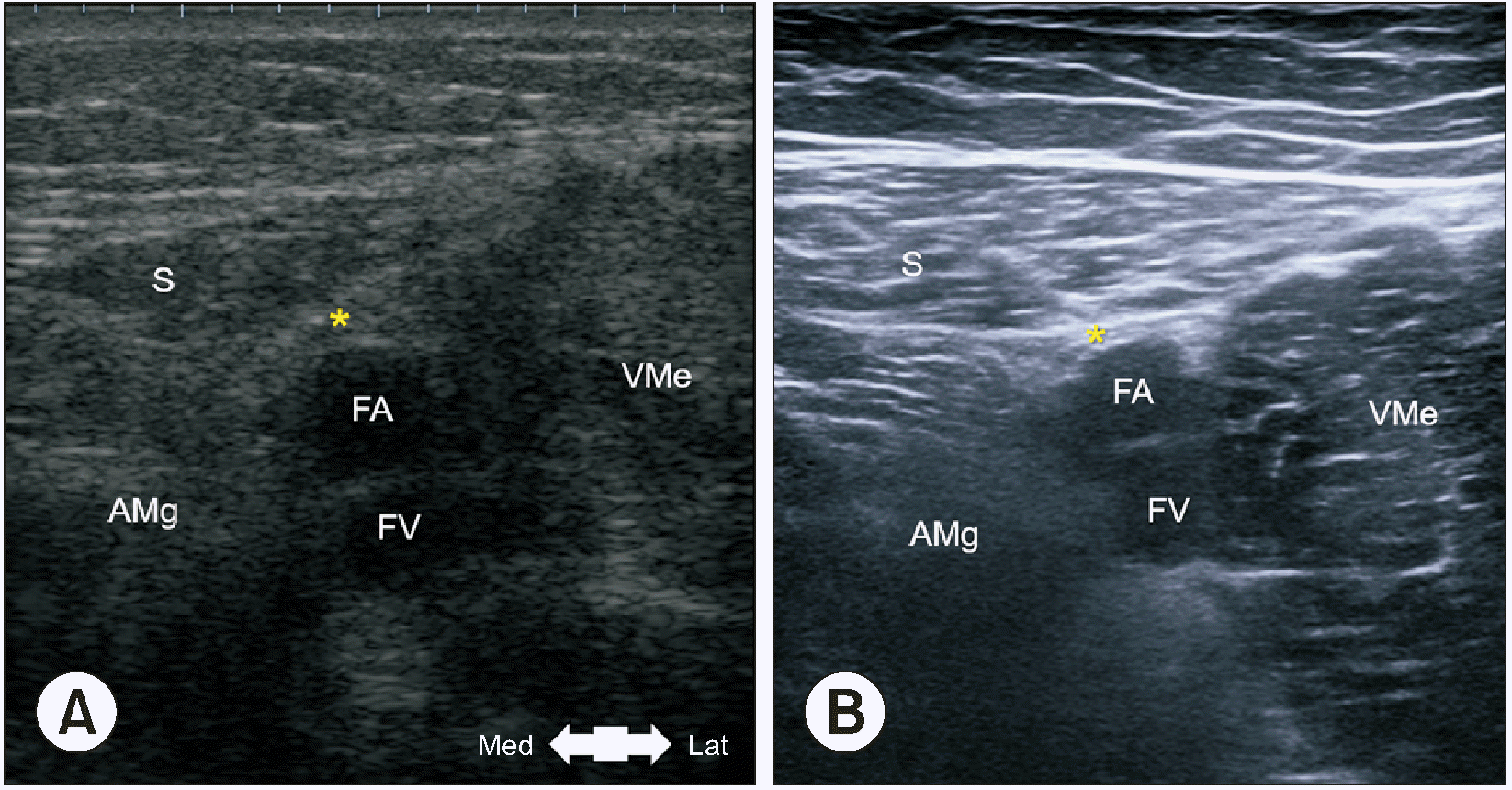
Fig. 6
Photographs showing the thigh injected with 10 mL (A) and 5 mL (B) of the mixture. The asterisks indicate the VAM. SN: saphenous nerve, FA: femoral artery, S: sartorius muscle, VMe: vastus medialis muscle, NVM: nerve to the vastus medialis, AL: adductor longus muscle, G: gracilis muscle, AMg: adductor magnus muscle, VAM: vasto-adductor membrane, Cra: cranial side, Cau: caudal side, Med: medial side, Lat: lateral side.
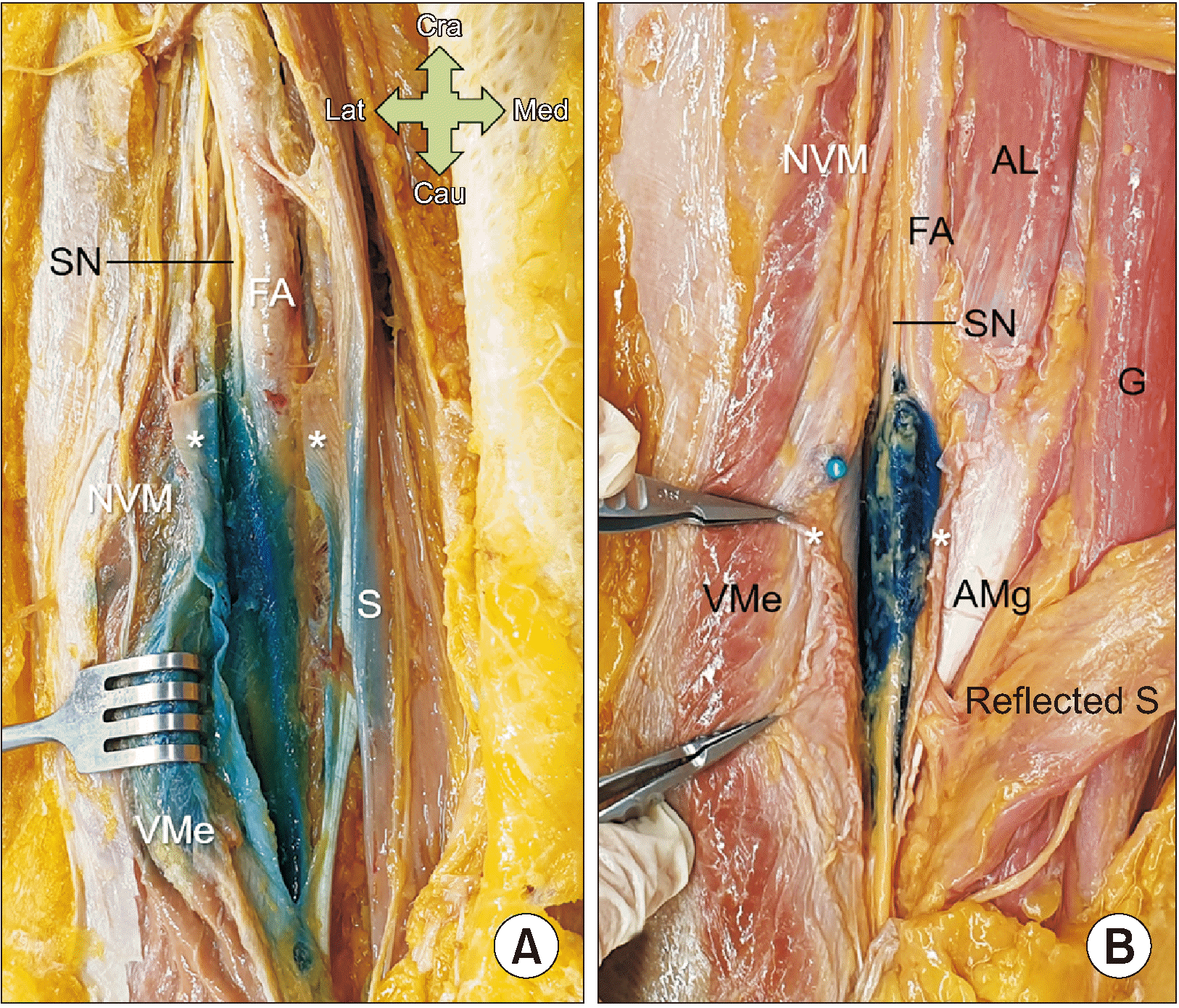
Table 1
Measurement of each target point
| Measurement point | Distance from ASIS on the baseline (α) (cm) | Perpendicular distance from α (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Specimens (number) | Male (28) | Female (22) | Total (50) | Male (28) | Female (22) | Total (50) | ||
| Apex of FT | 24.1 ± 2.0 | 26.8 ± 1.3* | 25.3 ± 2.2 | 5.7 ± 1.1* | 4.8 ± 0.5 | 5.3 ± 1.0 | ||
| Midpoint of SB of VAM | 27.8 ± 2.0 | 27.0 ± 1.9 | 27.4 ± 2.0 | 5.4 ± 0.8* | 4.6 ± 1.2 | 5.0 ± 1.1 | ||
| Uppermost point of AF | 34.4 ± 2.4* | 33.0 ± 1.9 | 33.8 ± 2.3 | 5.9 ± 0.8* | 5.1 ± 1.3 | 5.5 ± 1.2 | ||
| Uppermost point of AH | 36.8 ± 2.4* | 35.1 ± 1.8 | 36.0 ± 2.3 | 5.2 ± 0.9* | 4.5 ± 1.3 | 4.9 ± 1.1 | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download