Abstract
Congenital factor V (FV) deficiency (FVD) is characterized by low serum FV levels. Because its’ clinical symptoms vary from asymptomatic to lifethreatening bleeding, accurate diagnosis is critical. We report a case of a patient with FVD and compound heterozygous variants, including a novel pathogenic variant. A 50-year-old man with a low FV activity underwent genetic analysis. The c.286G>C (p.Asp96His) and the novel variant, c.5403del (p.Lys1801AsnfsTer11), were confirmed. The c.286G>C (p.Asp96His) variant is located in the A1 domain of the F5 gene, affecting folding and interfering with intracellular trafficking and secretion. On the other hand, the novel c.5403del (p.Lys1801AsnfsTer11) variant is located in the C1 domain and is predicted to cause a frameshift, resulting in premature truncation. FV activity does not always correlate with clinical severity in patients with FVD. Thus, achieving a precise diagnosis of FVD is crucial to prevent unexpected bleeding accidents. Therefore, active genetic tests should be performed when FVD is clinically suspected.
초록
선천성 제V인자 결핍증(FVD)은 낮은 혈청 제V인자 수치가 특징인 질환으로 임상 증상이 무증상부터 치명적인 출혈까지 다양하기 때문에 정확한 진단이 중요하다. 저자들은 새로운 변이를 포함하는 FVD 환자 1예를 보고한다. 낮은 제V인자 활성도를 보이는 50세 남성에 대한 유전자 검사가 의뢰되었다. c.286G>C (p.Asp96His) 변이와 새로운 변이인 c.5403del (p.Lys1801AsnfsTer11)가 확인되었다. 문헌에 따르면 c.286G>C (p.Asp96His) 변이는 F5 유전자의 A1 도메인에 위치하며 단백질 접힘에 영향을 주어 세포 내 이동과 분비를 방해한다. 새로운 변이인 c.5403del (p.Lys1801AsnfsTer11)은 C1 도메인에 위치하며 프레임 이동을 유발하여 조기 절단을 유발할 것으로 예측된다. 제V인자 활성도가 FVD 중증도와 반드시 일치하는 것은 아니므로 정확한 진단을 통해 예상치 못한 출혈을 예방하기 위해서 임상적으로 FVD가 의심되는 경우 적극적인 유전자 검사가 필요하다.
Congenital factor V (FV) deficiency (FVD) is caused by variants in the F5 gene on chromosome 1q24.2 and accounts for 8% of rare bleeding disorders [1]. Clinical FVD manifestations range from asymptomatic to life-threatening bleeding, making diagnosis and prediction difficult. FVD is clinically suspected when there is a prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), which are corrected in the mixing test and confirmed by reduced FV activity. In this report, we describe a patient with hereditary FVD and compound heterozygous variants, including a novel pathogenic variant.
A 50-year-old male was referred to the hematology clinic because of prolonged PT and aPTT during a preoperative examination. He had a right parotid mass that needed excision. The patient had no medical or family history of bleeding. Prolonged PT (30.8 s; reference range, 10.8–14.0) and aPTT (51.8 s; reference range, 21.0–34.0) were corrected in the mixing test, and reduced FV activity (6%) was confirmed. Factor II and VII-XII assays showed no specific findings. Considering the discrepancy between the patient’s clinical symptoms and FV activity, as well as the presence of two children in his family tree, genetic studies and counseling were conducted.
Genomic DNA was extracted from the peripheral blood of the patient and his two children. A 108-gene panel by next-generation sequencing (NGS) for inherited coagulation disorders revealed that the patient had a compound heterozygous variant in the F5 gene (NM_000130.5): a missense variant c.286G>C (p.Asp96His) and a frameshift variant c.5403del (p.Lys1801AsnfsTer11), which is a novel variant. As this novel variant has not been reported before, we deposited it in the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar/; September 10, 2022) [2]. The variant was classified as pathogenic according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) guidelines: frame-shift null variant in the F5 gene for which loss-of-function is a known mechanism of disease (PVS1); not found in genome AD exomes of the Korean Reference Genome Database (PM2); International Society on Thrombosis and Hemostasis (ISTH) committee recently reports the variant as pathogenic (PP5); patient’s phenotype or family history is highly specific for a disease with a single genetic etiology (PP4). We further confirmed that the patient’s two children inherited the heterozygous c.286G>C (p.Asn96His) variant (Fig. 1). The patient’s FVD was confirmed through genetic study, and the biopsy performed simultaneously showed that the mass was a Warthin tumor with no possibility of malignancy, so the surgery was cancelled. Written informed consent was obtained from the patient for the publication of this case report.
The F5 gene consists of six domains: A1, A2, B, A3, C1, and C2. To date, 265 variants in F5 have been reported, mainly missense variants with high clustering in the A2 and C2 domains (Human Gene Mutation Database professional version (https://www.nihlibrary.nih.gov/resouces/tools/human-gene-mutation-databaseprofessional), last accessed on July 10, 2023). The c.286G>C (p.Asp96His) variant is located in the A1 domain and affects protein folding, thereby interfering with intracellular trafficking and secretion. This variant was previously described in four unrelated Chinese patients with FVD [3]. Since then, three cases have been reported in Korea, two of which were related (Table 1) [1, 4].
The patient in this case was a compound heterozygote with not only the c.286G>C (p.Asp96His) variant but also a novel variant, and therefore had a significantly lower FV activity than previously reported patients with only the c.286G>C (p.Asp96His) variant. This novel variant is located in the C1 domain of the FV protein, which is a mutational hot spot that is predicted to create a frameshift that results in a premature stop codon because of a 1-base pair deletion (Fig. 2), thus changing the protein length. The exact impact of this novel variant cannot be perfectly predicted; however, previously reported cases of compound heterozygous variants, including c.286G>C (p.Asp96His), can explain the very low FV activity observed in this patient [1, 4].
In rare bleeding disorders, the correlation between clinical severity and coagulation factor activity can vary [5]. Recent studies have highlighted the presence of FV in the platelet compartment, which may contribute to this diverse correlation [6]. In the present case, the patient exhibited very low FV activity but had no bleeding tendencies throughout his life; the deficiency was detected only during the preoperative examination. However, It is important to note that patients with FVD typically experience bleeding after invasive procedures. Therefore, without comprehensive genetic evaluation before surgery, there is a risk of massive bleeding. Furthermore, a familial genetic study confirmed that the patient’s children had inherited the variant, suggesting a potential bleeding tendency.
In conclusion, we present a case of FVD in a patient with no history of bleeding. The novel variant c.5403del (p.Lys1801AsnfsTer11) was identified through genetic testing. We suggest that this variant should be classified as a pathogenic variant. Additionally, this case emphasizes the clinical utility of genetic testing in FVD. The patient in this case was diagnosed with FVD through molecular genetic testing using an NGS-based 108-gene panel. Therefore, genetic testing might be an essential method for diagnosing patients with atypical presentation, particularly when there is a weak association between genotype and phenotype, as seen in FVD.
REFERENCES
1. Park CH, Yoo K, Lee KO, Kim SH, Sung KW, Kim HJ. 2016; Genetic confirmation of congenital factor V deficiency in Korean patients. Ann Lab Med. 36:182–4. DOI: 10.3343/alm.2016.36.2.182. PMID: 26709270. PMCID: PMC4713856.

2. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med [Internet]. 17:405–24. Available from: https://doi.org/10.1038/gim.2015.30. DOI: 10.1038/gim.2015.30. PMID: 25741868. PMCID: PMC4544753.

3. Liu HC, Shen MC, Eng HL, Wang CH, Lin TM. 2014; Asp68His mutation in the A1 domain of human factor V causes impaired secretion and ineffective translocation. Haemophilia. 20:318–26. DOI: 10.1111/hae.12476. PMID: 24893683.

4. Shim YJ, Jang KM. 2020; A novel mutation of congenital factor V deficiency in Henoch-Schönlein purpura. Pediatr Int. 62:1005–6. DOI: 10.1111/ped.14229. PMID: 32851759.

5. James P, Salomon O, Mikovic D, et al. 2014; Rare bleeding disorders - bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Haemophilia. 20:71–5. DOI: 10.1111/hae.12402. PMID: 24762279. PMCID: PMC4673660.

6. Duckers C, Simioni P, Spiezia L, Radu C, Dabrilli P, Gavasso S, et al. 2010; Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood. 115:879–86. DOI: 10.1182/blood-2009-08-237719. PMID: 19861681.

Fig. 1
(A) Results of molecular analyses of the F5 gene in a patient with congenital factor V deficiency along with his family members. Pedigree and results of coagulation test of the patient’s family. (B) Sanger sequencing of the F5 gene in proband and his children with congenital FV deficiency. Proband and his children were heterozygous for c.286G>C (p.Asp96His) (left panel). Proband was compound heterozygous for c.5403del (p.Lys1801AsnfsTer11), and the mutation was not detected in the children (right panel). The arrows indicate the mutations.
Abbreviations: PT, prothrombin time; aPTT, activated partial thromboplastin time.
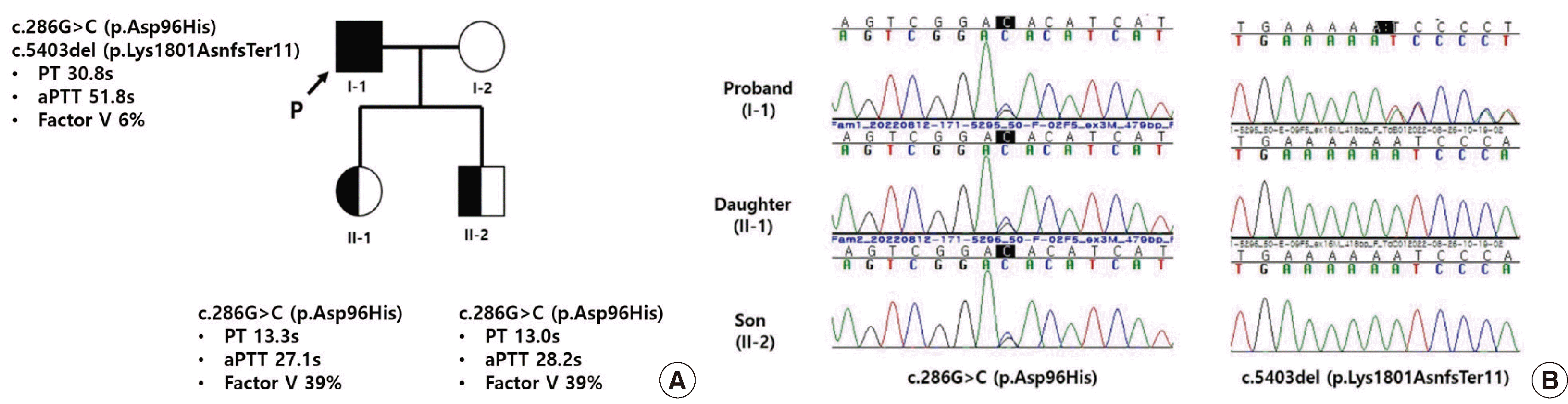
Fig. 2
The structure of the Factor V protein. The arrows indicate the mutation in the present case, including the novel variant (c.5403del: p.Lys1801AsnfsTer11). The arrowheads indicate the cleavage sites of activated protein C (APC) and thrombin.
Abbreviation: APC, activated protein C.
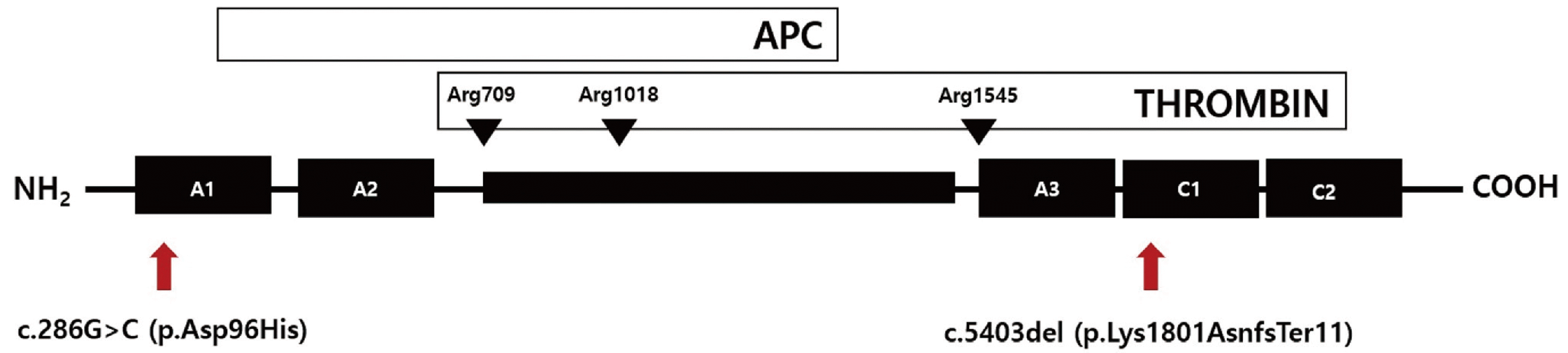
Table 1
Clinical features and laboratory findings of patients with the F5 (NM_000130.5):c.286G>C (p.Asp96His) variant in Korea
| Case | Relationship | Age/Sex | Bleeding episode | PT (sec) (N/I) | aPTT (sec) (N/I) | FV Activity (%) | Variants | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Proband | 50/M | Asymptomatic | 30.8 (I) | 51.8 (I) | 6 | c.286G>C (p.Asp96His); c.5403del (p.Lys1801AsnfsTer11) | Present study |
| 1-1 | Daughter | 21/F | Asymptomatic | 13.3 (N) | 27.1 (N) | 39 | c.286G>C (p.Asp96His) | Present study |
| 1-2 | Son | 17/M | Asymptomatic | 13.0 (N) | 28.2 (N) | 39 | c.286G>C (p.Asp96His) | Present study |
| 2 | Proband | 14/F | Hematuria Menorrhagia | 15.4 (I) | 48.1 (I) | 41 | c.286G>C (p.Asp96His) | 2016 [1] |
| 2-1 | Mother | 40/F | Asymptomatic | 14.1 (N) | 37.9 (N) | 44 | c.286G> C(p.Asp96His) | 2016 [1] |
| 3 | Proband with HSP | 15/F | Microscopic hematuria | 19.0 (I) | 42.0 (I) | 4 | c.286G>C (p.Asp96His); c.5030A>G(p.Tyr1677Cys) | 2020 [4] |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download