Abstract
The Birt–Hogg–Dubé syndrome (BHDS) is a rare autosomal dominant disease caused by mutations in the FLCN gene and manifests as pulmonary cysts, pneumothoraces, skin fibrofolliculomas, and renal cancer. Currently, few cases of BHDS have been reported in the Korean population, with a prevalence of 5.67 per 10,000,000. Here, we reviewed the genetic and clinical characteristics of nine patients from five families who were confirmed to carry pathogenic FLCN variants using whole-exome sequencing and multiplex ligation-dependent probe amplification. Among these patients (mean age 49.2±18.2 years; 89% females), four pathogenic FLCN variants were identified, including c.1539-2A>G in two families, which had not been previously reported in Korean patients. Haplotype analysis suggested that these two families shared a common genetic background. We observed pulmonary cysts in 6/7 (86%) patients, fibrofolliculomas in 3/8 (38%), and renal cancer in 2/9 (22%), similar to those in previous Korean studies; however, pneumothorax was identified in 2/9 (22%) patients, indicating a markedly lower rate. Other cancers, such as breast cancer or thyroid carcinoma, were observed in 2/9 (22%) patients. Overall, our study describes the genetic and clinical heterogeneity among Korean patients with BHDS and may help better understand the comprehensive genetic and clinical profiles of BHDS in Korean patients.
초록
Birt-Hogg-Dubé 증후군(BHDS)은 FLCN 유전자의 돌연변이로 인해 발생하는 희귀 상염색체 우성 질환으로, 폐낭종, 기흉, 피부 섬유털집종 및 신장암 증상을 동반한다. 유병률은 10,000,000명당 5.67명이며, 현재 한국인 BHDS 환자로 보고된 예는 많지 않다. 이 논문에서는 WES (whole-exome sequencing) 및 MLPA (multiplex ligation-dependent probe amplification)를 사용하여 병원성 FLCN 변이가 있는 것으로 확인된 다섯 가족으로부터 9명의 BHDS 환자의 분자유전학적 및 임상적 특성을 검토하였다. 대상 환자군(평균 연령 49.2±18.2세, 여성 89%)에서 4개의 병원성 FLCN 유전자 변이가 확인되었는데, 이전에 한국인 BHDS 환자에서는 보고되지 않았던 c.1539-2A>G 변이가 두 가족에서 확인되었다. 일배체형 분석을 통해 해당 두 가족은 공통된 유전적 배경을 공유할 가능성이 있음을 알 수 있었다. 폐낭종은 6/7 (86%)의 환자에서 확인되었고, 섬유털집종은 3/8 (38%), 신장암은 2/9 (22%)의 환자에서 확인되어 이전의 국내 연구와 유사하였으나, 기흉은 2/9 (22%)의 환자에서 확인되어 이전 연구 결과보다 현저히 적었다. 유방암이나 갑상선암 등 악성종양도 2/9 (22%)의 환자에서 발견되었다. 본 연구는 한국인 BHDS 환자의 분자유전학적 및 임상적 다양성을 제시하여 한국인 BHDS 환자들의 특성에 대한 이해도를 높여줄 수 있다는 점에서 의의를 가진다.
Birt–Hogg–Dubé syndrome (BHDS) is a rare autosomal dominant disorder characterized by fibrofolliculomas, renal cancer, multiple pulmonary cysts, and spontaneous pneumothoraces [1]; however, its diagnosis requires satisfaction of one major or two minor diagnostic criteria as suggested by the European Birt-Hogg-Dubé consortium [1], and it predominantly results from mutations in the FLCN gene on chromosome 17p11.2, encoding folliculin, a tumor suppressor protein [2].
The estimated global prevalence of BHDS is 1.86 (95% confidence interval, 1.16–3.00) per million, with an unknown prevalence in Asia. BHDS can manifest at any age, typically at 40 [3]. Differences in incidence between the sexes remain debatable [4], as do genotype-phenotype correlations [3, 5]. BHDS exhibits phenotypic variability, with 80% of skin lesions [3, 6, 7], 80% of pulmonary cysts [3], 24–35% of pneumothorax [3, 8, 9], and 19–40% of renal cancer [3, 7, 10, 11] in Caucasian patients. Asian patients show slight differences, with fewer instances of skin lesions and renal cancer and more pulmonary manifestations, including pulmonary cysts [6, 8, 10, 12, 13]. Few BHDS cases have been reported in Korea, with clinical manifestations similar to those reported in previous Asian studies [5, 9]. This study aims to elucidate the clinical manifestations and genetic profiles of Korean patients with BHDS.
This study included nine patients from five families with pathogenic FLCN variants. Whole exome sequencing (WES) and multiple ligation-dependent probe amplification (MLPA) were performed on five probands, along with Sanger sequencing of the family members to confirm family-specific variants. The data included demographics, clinical traits, and test results from electronic medical records. Informed consent was obtained from the patients; the study adhered to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (H-2204-144-1319).
Peripheral blood from patients, stored in EDTA disodium salt tubes, was subjected to DNA extraction using a Gentra Puregene Blood kit (Qiagen, Hilden, Germany). DNA concentration and purity were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). After sonication of the DNA using a Covaris system (Covaris, Inc., Woburn, MA, USA), target enrichment was performed using SureSelect Human All Exon software (version 5; Agilent, Santa Clara, CA, USA). Paired-end sequencing after exome capture was performed using an Illumina HiSeq 2000 or 2500 platform (Illumina, San Diego, CA, USA). DNA sequence analysis was performed using Next-GENe software (v2.4.0.1) (SoftGenetics, State College, PA, USA), and the sequences were aligned with the GRCh37/hg19 human reference genome. NM_144997.5 served as the reference sequence for the FLCN gene. Benign variants were filtered using Genome Aggregation and Korean Reference Genome Databases. In silico prediction tools such as SIFT, Mutation Taster, and Poly-Phen2 were employed. The Human Gene Mutation and ClinVar databases were referenced. The pathogenicity of each variant was determined as per the American College of Medical Genetics and Association for Molecular Pathology (ACMG/AMP) guidelines [14] and the latest guidelines by the ClinGen Sequence Variant Interpretation Working Group [15-17]. Variants were visualized using ProteinPaint (https://proteinpaint.stjude.org).
SALSA MLPA Probemix P256-C1 FLCN (MRC-Holland, Amsterdam, The Netherlands) was used to screen for large deletions or duplications of the FLCN gene according to the manufacturer’s instructions. Deletions and duplications were indicated by normalized peak ratios <0.8 and >1.2, respectively. We selected four nearby SNPs within a 2.5 Mb range of FLCN, including two downstream (rs56926918, rs56153623) and two upstream SNPs (rs2072652, rs2075659). The FLCN haplotype was reconstructed using PHASE 2.1.1 with 56 unrelated normal controls and four patients.
Among the nine patients with BHDS, five were from unrelated families. At the time of diagnosis, the mean age of the nine patients was 49.2±18.2 years, and 8/9 (89%) patients were females (Table 1). In our study, we identified four different pathogenic FLCN variants in nine patients from five families (Table 2). Splice site variants were observed in 3/5 (60%) patients. A nonsense and a frameshift variant were observed in each of the 1/5 (20%) families. All four variants were classified as pathogenic according to the ACMG/AMP [14] and ClinGen guidelines [15-17]. These pathogenic variants were predominantly localized to the distal FLCN, spanning exons 11 and 14 and introns 11 and 13. Among the BHDS families meeting clinical diagnostic criteria [1] (3.I.2 and 4), all of them (100%) had pathogenic FLCN variants.
The FLCN c.1539-2A>G variant, previously unreported in Korean patients with BHDS, was discovered in two unrelated families (1 and 3.I.2). Nine haplotypes were reconstructed (Supplementary Table 1), and haplotype H1 was observed in both unrelated families with the FLCN c.1539-2A>G variant (Supplementary Table 2), suggesting a common genetic background between these two families. MLPA analysis showed no large deletions or insertions, including a mutational hotspot [11]. The genotype-phenotype correlation was not significant, consistent with previous findings [6].
Bilateral multiple lung cysts were observed in 6/7 (86%) patients (mean age 52.2±12.0) from five unrelated families (Supplementary Fig. 1). Pneumothorax occurred in 2/9 (22%) patients, with one experiencing recurrent episodes starting five years before diagnosis. Pulmonary function tests were conducted in 4/9 unrelated patients, showing mild restrictive patterns in 1/4 (25%) and mildly decreased diffusion capacity in 2/3 (67%) (Supplementary Table 3). Further, 3/8 (38%) patients from the same family had multiple neck fibrofolliculomas, raising concerns regarding selection bias and necessitating histological confirmation. Renal cancer was observed in 2/9 (22%) patients, one with bilateral chromophobe renal cell carcinoma and the other with multilocular cystic renal neoplasm of low malignant potential. Renal cysts were observed in 4/5 (80%) patients. Malignant tumors, including breast cancer and papillary thyroid carcinoma, were observed in 2/9 (22%) unrelated patients. Benign tumors were observed in 6/9 (67%) patients and arterial hypertension in 3/9 (33%). Family history included pneumothorax in 3/9 (33%) and lung cancer in 1/9 (11%) patients, and five patients from one family had a family history of pathogenic FLCN variants. In family 3 with the c.1539-2A>G variant, the proband showed all pulmonary, renal, and skin manifestations. In contrast, the other family members showed varying clinical patterns. As BHDS is typically described in the 40s [3], patient 3.III.3 may not fully present the disease symptoms (Fig. 1).
This study investigated nine Korean patients with BHDS, with a mean diagnosis age of 49.2±18.2 years, consistent with previous Korean studies [5, 9]. The rates of pulmonary cysts (86%), renal cancer (22%), and skin lesions (38%) were similar to those reported in earlier studies on Korean patients with BHDS [9, 18]. However, the rate of pneumothorax (22%) was lower [5, 9, 18]. This pattern was comparable to that reported in previous studies on Asian patients [8, 13]. Compared to Caucasians, Korean patients with BHDS exhibited similar rates for pulmonary cysts [3], pneumothorax [3, 7, 8], and renal cancer [3, 7, 10, 11]; however, they had lower rates for skin lesions [3, 6, 7]. Recurrent pneumothorax, typically higher (75–80%) in Caucasians [4] and Koreans [9], was 50% in this study, emphasizing the need for larger-scale research due to the small population. The discrepant skin lesion rates in Asian patients [9, 12, 13] may be related to low biopsy rates [13], necessitating further research. The common renal cancers in Korean patients with BHDS are chromophobe renal cell carcinoma and hybrid oncocytic/chromophobe tumors [9]. Patients with BHDS have a sevenfold higher risk of renal cancer [3] and a 19.2% incidence of renal cysts [10]. Further, 4/5 (80%) of unrelated patients in this study had renal cysts, necessitating continuous monitoring. Breast cancer or papillary thyroid carcinoma was observed in 2/9 (22%) unrelated patients, underlining the importance of early screening.
Our study identified FLCN variants mainly in the exon and intron regions of FLCN, predominantly including splice site variants (60%), among nine patients from five families. This warrants further investigation into genotype-phenotype correlations. Similar to previous research [3, 7], a high variant detection rate of 100% was observed in clinical BHDS families that met the set criteria [1]; however, larger studies are needed for validation due to the limited number of included cases.
In conclusion, our study describes the genetic and clinical heterogeneity of BHDS in Korean patients and contributes to a better understanding of their comprehensive profiles.
Acknowledgments
This research was supported by the Genetic Diagnosis Program for Rare Diseases of the Korean Disease Control and Prevention Agency.
REFERENCES
1. Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, et al. 2009; Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 10:1199–206. DOI: 10.1016/S1470-2045(09)70188-3. PMID: 19959076.

2. Schmidt LS, Linehan WM. 2018; FLCN: the causative gene for Birt-Hogg-Dubé syndrome. Gene. 640:28–42. DOI: 10.1016/j.gene.2017.09.044. PMID: 28970150. PMCID: PMC5682220.
3. Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. 2005; Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 76:1023–33. DOI: 10.1086/430842. PMID: 15852235. PMCID: PMC1196440.
4. Gupta N, Sunwoo BY, Kotloff RM. 2016; Birt-Hogg-Dubé syndrome. Clin Chest Med. 37:475–86. DOI: 10.1016/j.ccm.2016.04.010. PMID: 27514594.

5. Park HJ, Park CH, Lee SE, Lee GD, Byun MK, Lee S, et al. 2017; Birt-Hogg-Dube syndrome prospectively detected by review of chest computed tomography scans. PLoS One. 12:e0170713. DOI: 10.1371/journal.pone.0170713. PMID: 28151982. PMCID: PMC5289479. PMID: 07a4e6c80ea3453981d94ad19f2e2b23.

6. Daccord C, Good JM, Morren MA, Bonny O, Hohl D, Lazor R. 2020; Birt-Hogg-Dubé syndrome. Eur Respir Rev. 29:200042. DOI: 10.1183/16000617.0042-2020. PMID: 32943413. PMCID: PMC9489184. PMID: 929b284661a947cfa3284f374cd99188.

7. Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, et al. 2008; BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 45:321–31. DOI: 10.1136/jmg.2007.054304. PMID: 18234728. PMCID: PMC2564862.
8. Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, et al. 2010; Clinical and genetic spectrum of Birt-Hogg-Dubé syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 47:281–7. DOI: 10.1136/jmg.2009.070565. PMID: 20413710. PMCID: PMC2981024.
9. Lee JH, Jeon MJ, Song JS, Chae EJ, Choi JH, Kim GH, et al. 2019; Birt-Hogg-Dubé syndrome in Korean: clinicoradiologic features and long term follow-up. Korean J Intern Med. 34:830–40. DOI: 10.3904/kjim.2018.119. PMID: 30360018. PMCID: PMC6610189. PMID: 22d1e3d1f8e447dea5ed898a8a71501a.

10. Furuya M, Yao M, Tanaka R, Nagashima Y, Kuroda N, Hasumi H, et al. 2016; Genetic, epidemiologic and clinicopathologic studies of Japanese Asian patients with Birt-Hogg-Dubé syndrome. Clin Genet. 90:403–12. DOI: 10.1111/cge.12807. PMID: 27220747.

11. Sattler EC, Reithmair M, Steinlein OK. 2018; Kidney cancer characteristics and genotype-phenotype-correlations in Birt-Hogg-Dubé syndrome. PLoS One. 13:e0209504. DOI: 10.1371/journal.pone.0209504. PMID: 30586397. PMCID: PMC6306193. PMID: f015baf639964d6d98d90c600438a555.

12. Chang HC, Sung CW, Chou PC. 2020; Comment on "Birt-Hogg-Dubé syndrome in Korean: clinicoradiologic features and long term follow-up". Korean J Intern Med. 35:474–5. DOI: 10.3904/kjim.2019.326. PMID: 31992019. PMCID: PMC7061016. PMID: b8f8c7e7f8034283be59c5056449f74b.

13. Lee JH, Jeon MJ, Song JS, Chae EJ, Choi JH, Kim GH, et al. 2020; Response to comment on "Birt-Hogg-Dubé syndrome in Korean: clinicoradiologic features and long term follow-up". Korean J Intern Med. 35:476–7. DOI: 10.3904/kjim.2019.430. PMID: 31992018. PMCID: PMC7060991. PMID: 88109721affe41f09d0299ebca0457eb.

14. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17:405–24. DOI: 10.1038/gim.2015.30. PMID: 25741868. PMCID: PMC4544753.

15. ClinGen. Sequence Variant Interpretation general recommendations for using ACMG/AMP criteria. https://clinicalgenome.org/workinggroups/sequence-variant-interpretation/. Last accessed on Sep 2023.
16. Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, et al. 2018; Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 39:1517–24. DOI: 10.1002/humu.23626. PMID: 30192042. PMCID: PMC6185798.

17. Harrison SM, Biesecker LG, Rehm HL. 2019; Overview of specifications to the ACMG/AMP variant interpretation guidelines. Curr Protoc Hum Genet. 103:e93. DOI: 10.1002/cphg.93. PMID: 31479589. PMCID: PMC6885382.

18. Park HJ, Choi YJ, Park CH, Kim TH, Lee SS, Moon DH, et al. 2023; Outstanding characteristics of Birt-Hogg-Dube syndrome in Korea. Diagnostics (Basel). 13:2047. DOI: 10.3390/diagnostics13122047. PMID: 37370942. PMCID: PMC10296880. PMID: 24b4fd5f5d694bea9f46372aeadbd8a3.

19. Johannesma PC, van de Beek I, van der Wel JW, Paul MA, Houweling AC, Jonker MA, et al. 2016; Risk of spontaneous pneumothorax due to air travel and diving in patients with Birt-Hogg-Dubé syndrome. Springerplus. 5:1506. DOI: 10.1186/s40064-016-3009-4. PMID: 27652079. PMCID: PMC5014776.

20. Ray A, Chattopadhyay E, Singh R, Ghosh S, Bera A, Sarma M, et al. 2022; Genetic insight into Birt-Hogg-Dubé syndrome in Indian patients reveals novel mutations at FLCN. Orphanet J Rare Dis. 17:176. DOI: 10.1186/s13023-022-02326-5. PMID: 35477461. PMCID: PMC9044636. PMID: eb1b67e9eeff4ff9a549b0ee0f25978d.
Fig. 1
Pedigree of family 3 with Birt–Hogg–Dubé syndrome (BHDS). Numbers refer to the age (in years) at BHDS diagnosis. Arrow, proband; top left quadrant, FLCN variant proven; top right, lung cyst; bottom left, fibrofolliculoma, not proven by biopsy; bottom right, renal cancer. The Roman numerals indicate generations. Patient 3.II.2 shows thyroid cancer.
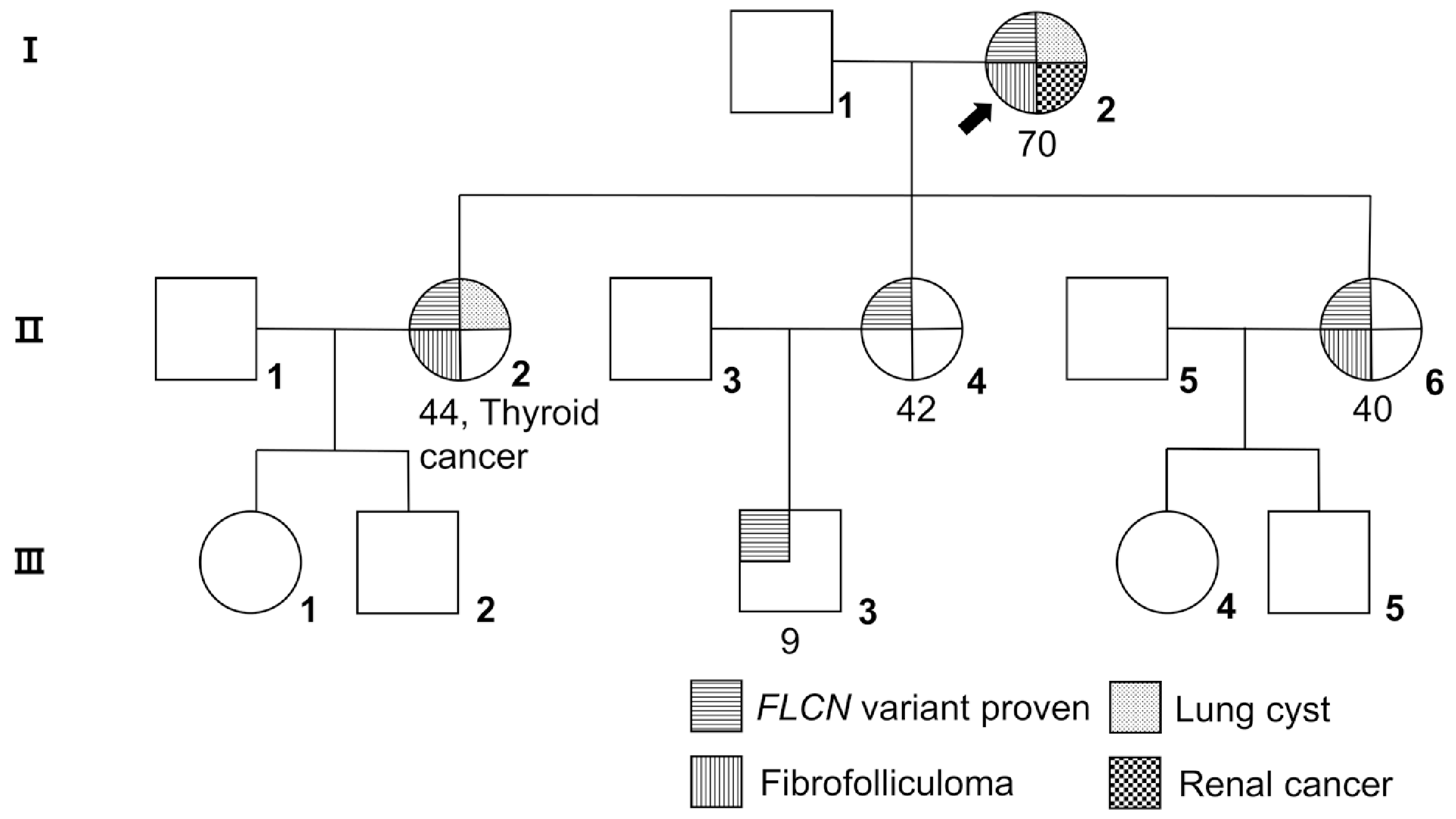
Table 1
Clinical characteristics of nine patients with Birt–Hogg–Dubé syndrome
| ID | Sex | Diagnosis, age | Lung | Skin (age at diagnosis) | Kidney | Other disease | Family history (number of family members) | Smoking | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pneumothorax (number of events, age at diagnosis) | Lung cysts (age at diagnosis) | Renal cancer (age at diagnosis) | Renal cysts (age at diagnosis) | Other cancers (age at diagnosis) | Benign tumors (age at diagnosis) | Other | ||||||
| 1 | F | 54.6 | No | Bilateral (52) | R/O Facial fibroma, NBx (55) | No | Simple cysts (53) (Bilateral, multiple, up to 1.0 cm) | Breast cancer (55) (Ductal carcinoma in situ) | Endometrial polyp (55) | Unruptured intracranial aneurysms | No | Never |
| 2 | F | 63.7 | Yes (1, 45) | Bilateral (62) | Bilateral facial rash, NBx (64) | No | Simple cysts (64) (Bilateral, multiple) | No | Esophageal tumor (61) | Arterial hypertension, Hyperlipidemia | Pneumothorax (5, father, mother, two younger brothers, a nephew), Lung cancer (1, father) | Never |
| 3.I.2 | F | 70.6 | No | Bilateral (70) | Multiple fibrofolliculomas on the neck, NBx (70) | MCRNLPM (71) (Right, single, 1.7 cm) | Simple cysts (67) (Bilateral, multiple, up to 5.2 cm) | No | Adrenal incidentaloma (67), Gall bladder adenomyomatosis (69), Sessile serrated adenoma in the colon (69) | DM, Arterial hypertension, Coronary artery disease, Glaucoma, Macular atrophy | BHDS (4, three daughters, a grandson) | Never |
| 3.II.2 | F | 44.3 | No | Bilateral (44) | Multiple fibrofolliculomas on the neck, NBx (44) | No | NT | Papillary thyroid carcinoma (44) (Left, single, up to 3.5 cm) | No | No | BHDS (4, mother, two younger sisters, a nephew) | NA |
| 3.II.4 | F | 42.6 | No | NT | No | No | NT | No | Rectal hyperplastic polyps (42), Benign thyroid nodules (42) | Azotemia | BHDS (4, mother, an older sister, a younger sister, a son) | NA |
| 3.II.6 | F | 40.3 | No | No | Multiple fibrofolliculomas on the neck, NBx (40) | No | NT | No | Benign thyroid nodules (40) | No | BHDS (4, mother, two older sisters, a nephew) | NA |
| 3.III.3 | M | 9.0 | No | NT | NT | No | NT | No | No | No | BHDS (4, a grandmother, mother, two aunts) | Never |
| 4 | F | 57.1 | No | Bilateral (49) | No | ChRCC (49) (Bilateral, multiple, up to 4.0 cm) | No | No | Uterine myomas (49) | No | Pneumothorax (1, a daughter), Bronchiectasis (1, mother), Interstitial lung disease (1, mother), Breast tumor (1, a daughter) | Former smoker |
| 5 | F | 60.2 | Yes (4, 55–60) | Bilateral (58) | No | No | Simple cysts (58) (Bilateral, multiple) | No | No | Arterial hypertension | Pneumothorax (3, father, an older sister, a younger brother) | Never |
| Mean±SD | 49.2±18.2 | 55.4±6.1* | 52.2±12.0 | 51.3±16.3 | 60.0±15.6 | 60.5±6.2 | 49.5±7.8 | |||||
| All | 9 | 2/9 (22%) | 6/7 (86%) | 3/8 (38%) | 2/9 (22%) | 4/5 (80%) | 2/9 (22%) | 6/9 (67%) | 5/9 (56%) | 8/9 (89%) | 1/6 (17%) | |
Abbreviations: No, no lesion was detected; Yes, presence of lesions; NBx, cutaneous lesions were observed, but biopsy was not performed; MCRNLPM, multilocular cystic renal neoplasm of low malignant potential; DM, diabetes mellitus; NT, not tested; NA, not available; ChRCC, chromophobe renal cell carcinoma; SD, standard deviation.
Table 2
Characteristics of the four FLCN variants identified in this study on nine patients with Birt–Hogg–Dubé syndrome
| Family | ID | DNA variants | Protein alteration | Location | Variant type | Classification | Criteria [References] |
|---|---|---|---|---|---|---|---|
| 1 | 1 | c.1539-2A>G | p.? | Intron 13 | Splice site | Pathogenic | PVS1, PS4_supporting [19], PM2, PP1 |
| 2 | 2 | c.1215C>G | p.Tyr405* | Exon 11 | Nonsense | Pathogenic | PVS1, PS4_supporting [7], PM2 |
| 3 | 3.I.2 | c.1539-2A>G | p.? | Intron 13 | Splice site | Pathogenic | PVS1, PS4_supporting [19], PM2, PP1 |
| 3.II.2 | |||||||
| 3.II.4 | |||||||
| 3.II.6 | |||||||
| 3.III.3 | |||||||
| 4 | 4 | c.1557del | p.Phe519Leufs*18 | Exon 14 | Frameshift | Pathogenic | PVS1, PS4_supporting [9], PM2 |
| 5 | 5 | c.1300+1G>A | p.? | Intron 11 | Splice site | Pathogenic | PVS1, PS4_supporting [20], PM2 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download