GA was measured with the modified version of the Norudia GA assay using Cobas c702 (Roche Diagnostics International, Rotkreuz, Switzerland). The Norudia GA assay is capable of measuring GA levels from both serum and plasma, requiring approximately 10 minutes per test. Precision was evaluated following CLSI EP05-A3 [
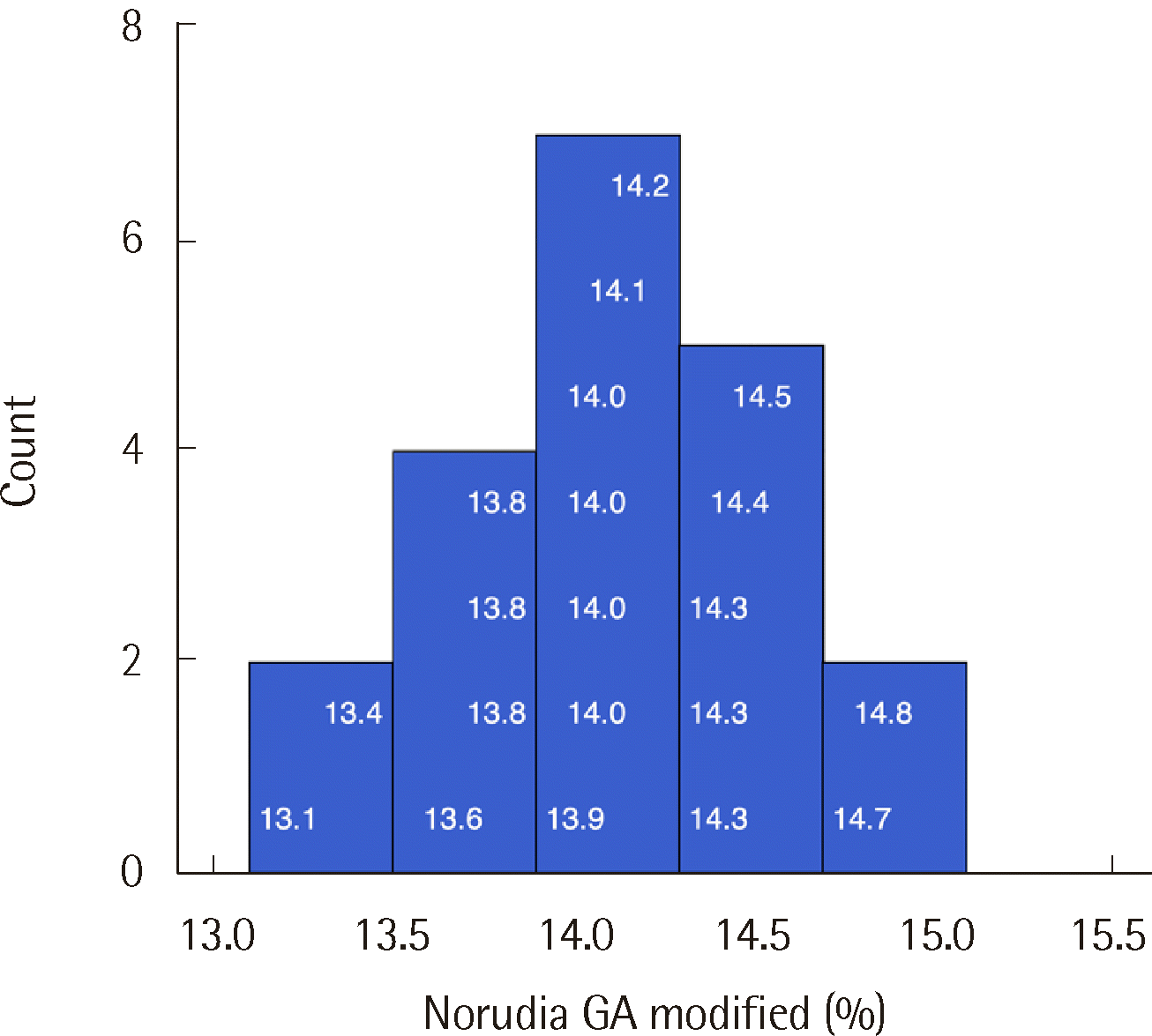
4]. Accordingly, two Norudia GA control materials (Lot 903RCT, Sekisui Medical Co., Ltd.) with estimated GA levels of 13.2% and 31.0% and one pooled serum sample with an estimated level of 20.0% were used. The samples were measured in duplicate, twice daily, for 30 days to assess repeatability and within-laboratory precision. The goal for imprecision was 2.6% or less, which is the allowed imprecision in the Westgard database [
5]. Precision was considered acceptable if the coefficient of variation (CV) was less than the total allowable error (7.2%) listed in the Westgard database [
5]. Linearity was evaluated following CLSI EP06-A [
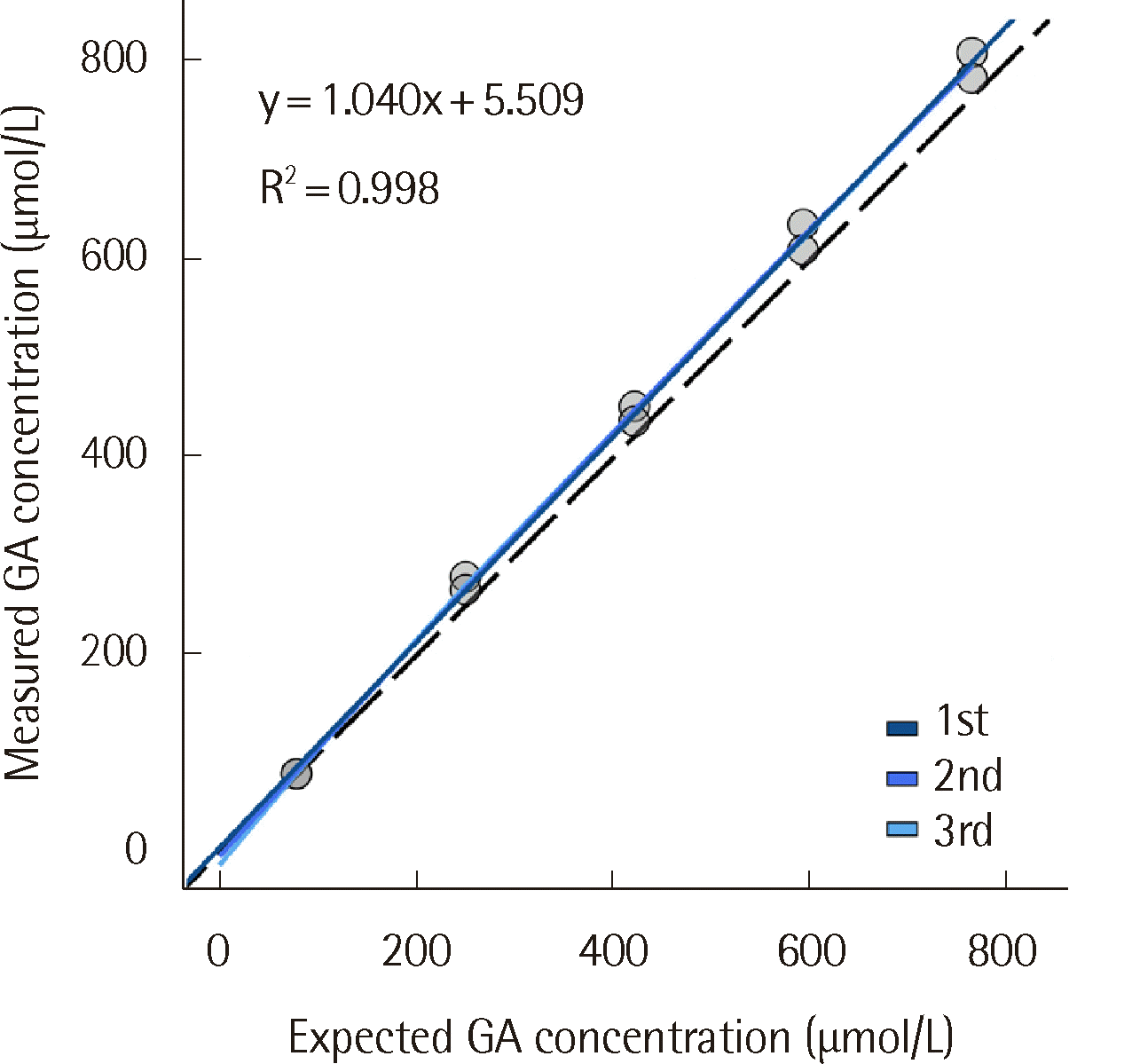
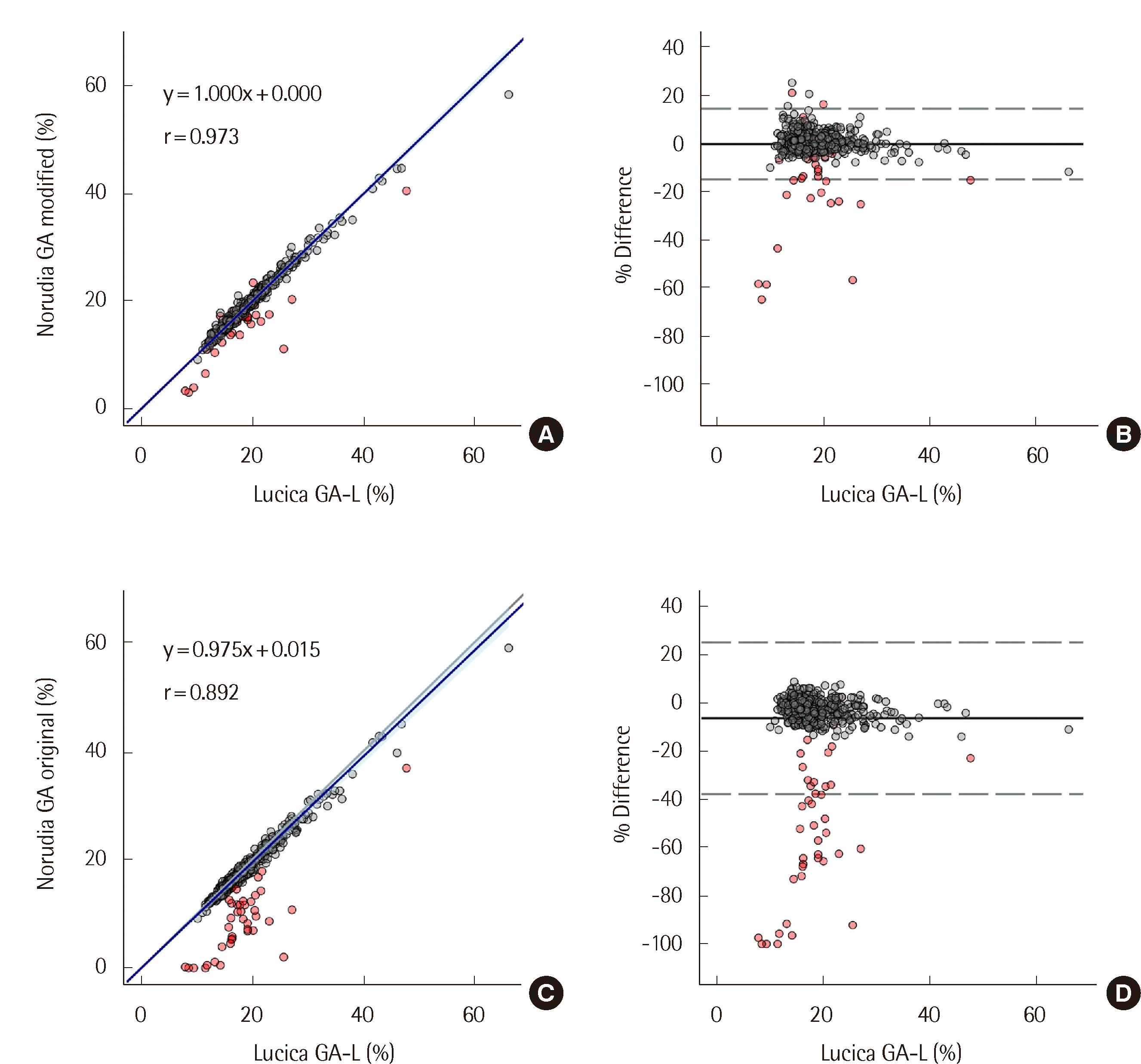
6]. To produce a high-concentration GA solution, pooled normal serum with a glucose level of 5 g/dL was created by adding D-(+)-glucose powder (Sigma-Aldrich, Saint Louis, MO, USA). The solution was incubated for three days at 37°C and then diluted to five concentration levels admixed with 0.85% saline. For method comparison, 539 serum samples, of which 39 were false negative samples, collected in a serum separating tube (Vacutainer SST II Tube 8.5 mL, #368972; Becton Dickinson, Sunnyvale, CA, USA) were used. The false negative samples were initially identified by their significant deviation from the corresponding patient’s previous GA result. To confirm false negativity, these samples were retested using the Lucica GA-L assay (Asahi Kasei Pharma Corporation, Tokyo, Japan). Negative interference was defined as a % difference between the GA results of the Norudia GA and Lucica GA-L assays of
<−14.4%(Norudia GA−Lucica GA-LLucica GA-L<−0.144). The % differences were calculated relative to the results of the Lucica GA-L assay, rather than the mean values. Considering the total allowable error for GA (7.2%) suggested by Ricos et al. [
7], the cutoff for negative interference was set as twice the total allowable error (14.4%), assuming that the error in the compared assays in the opposite direction from the ground truth would be the maximum allowable error. During the comparison study, three assays were utilized: 1) Lucica GA-L, 2) Original Norudia GA, and 3) Modified Norudia GA. The Lucica GA-L assay was used as the reference in the comparison study of both the original and modified versions of the Norudia GA assay since the Lucica GA-L assay showed results consistent with the glycemic status of each patient. Furthermore, the Diabetes Mellitus Indices Committee of the Japanese Society of Clinical Chemistry reported that the Lucica GA-L assay correlates well with the isotope dilution–tandem mass spectrometry reference method in both the reference material (JCCRM611) and patient sera [
8]. The Passing-Bablok regression and Bland-Altman plots were used to compare the methods according to CLSI EP09-A3 [
9]. To avoid a biased positive correlation, the % differences in the Bland-Altman plots were plotted against the results of the Lucica GA-L assay, not the mean values [
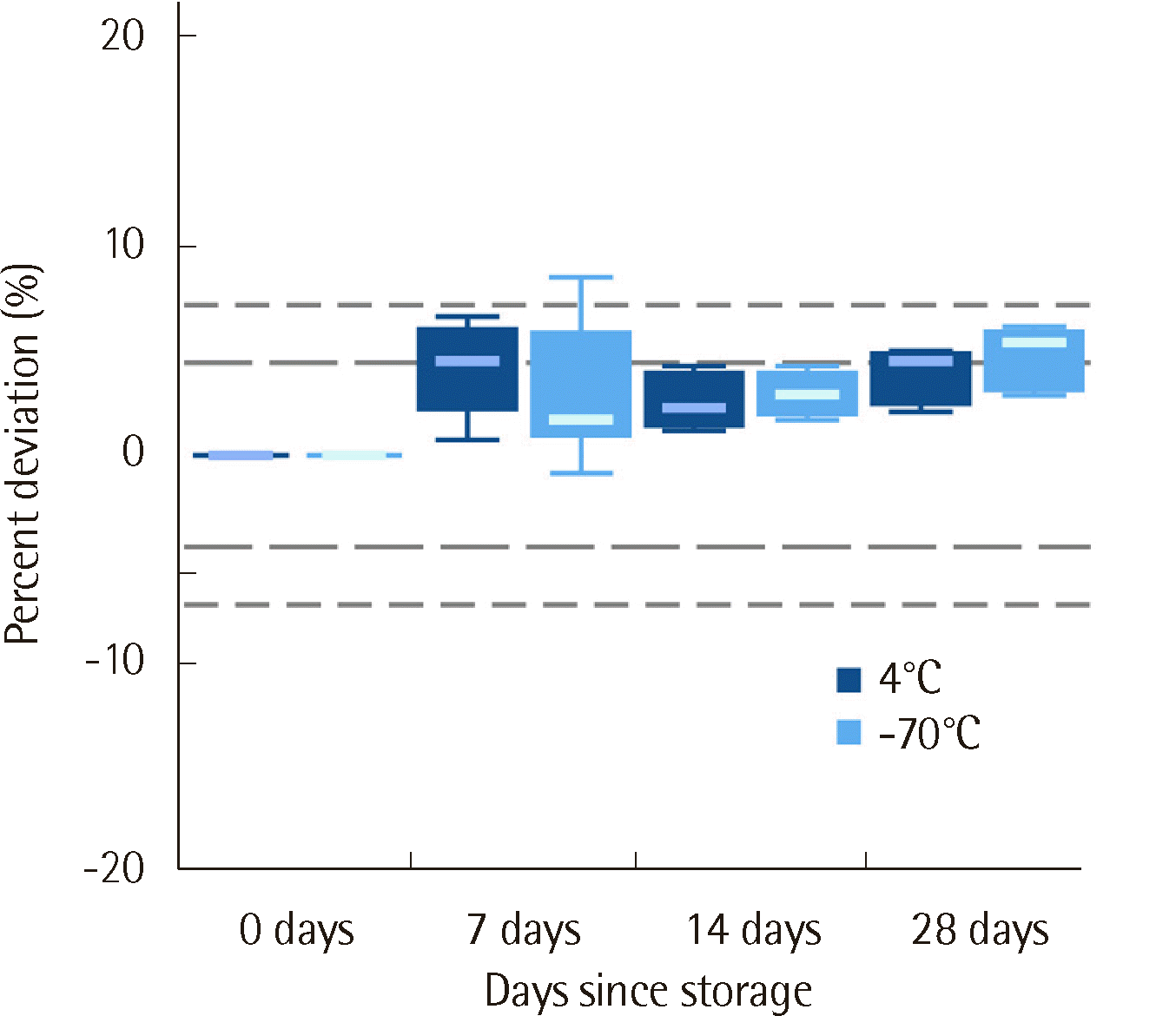
10]. Sample stability at 4°C and -70°C was evaluated using seven specimens, including one false negative specimen. The initial values and those after 7, 14, and 28 days of storage were evaluated. To verify the current GA reference interval of our institute, samples from 20 healthy controls were used following CLSI EP28-A3C [
11]. The healthy control group was selected from individuals who visited our institute for a routine medical check-up and had normal fasting glucose levels. All statistical analyses were conducted using Statistics 1.9.0, Polynomials 3.2.2, and GLM 1.8.3 on Julia 1.9. All plots were created using Gadfly 1.3.4 on Julia 1.9. The Institutional Review Board of Samsung Medical Center, Seoul, Korea, approved the study (IRB No. 2020-08-081-006) and waived the need for informed consent.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download