INTRODUCTION
Class III skeletal malocclusion is a common clinical dental abnormality. Maxillary protraction promotes maxillary growth by simulating suture growth around the maxilla. Conventional facemask therapy inevitably leads to dental side effects such as maxillary incisor protrusion, molar distalization, and clockwise rotation of the mandible.
1 Several researchers have attempted to use facemasks with temporary anchorage devices to eliminate side effects and obtain the maximum skeletal effect. Sar et al.,
2 Cevidanes et al.,
3 and Zhou et al.
4 have applied titanium plates for maxillary protraction to achieve favorable maxillary protraction outcomes. However, traditional titanium plate surgery is associated with trauma and the risk of tooth root damage. A three-dimensional (3D)-printed individual titanium plate was designed based on the surface morphology of the individual bone tissue, cortex bone thickness, and root distribution location. The titanium plate perfectly matched the cortical bone of the implant area and was positioned away from the root of the tooth.
5,6
The 3D finite element method can be employed to analyze the distribution pattern of stress and possible biomechanical changes after loading. However, limited finite element studies have been conducted on maxillary protraction using miniplates. The locations of the miniplates and directions of the orthopedic forces varied in each experiment. Despite these variations, no study has presented sufficient evidence to support this hypothesis. Additionally, the impact of miniplate height on maxillary protraction has not been previously reported. Therefore, individual titanium plates were designed in different areas based on root distribution and bone condition.
To explore the underlying mechanics, a finite element model of maxillary hypoplasia, including the suture, was established. The stress distribution characteristics under different traction directions and miniplate heights were analyzed to provide a theoretical basis for the clinical application of maxillary protraction using an individual titanium plate.
Go to :

DISCUSSION
In a comparative analysis of maxillary protraction using a titanium plate in the zygomatic alveolar crest and lateral nasal wall areas, Lee and Baek
8 observed greater stress values in the infrazygomatic crest. However, the titanium plate in the nasal sidewall area can reduce the counterclockwise rotation of the maxilla. Kim et al.
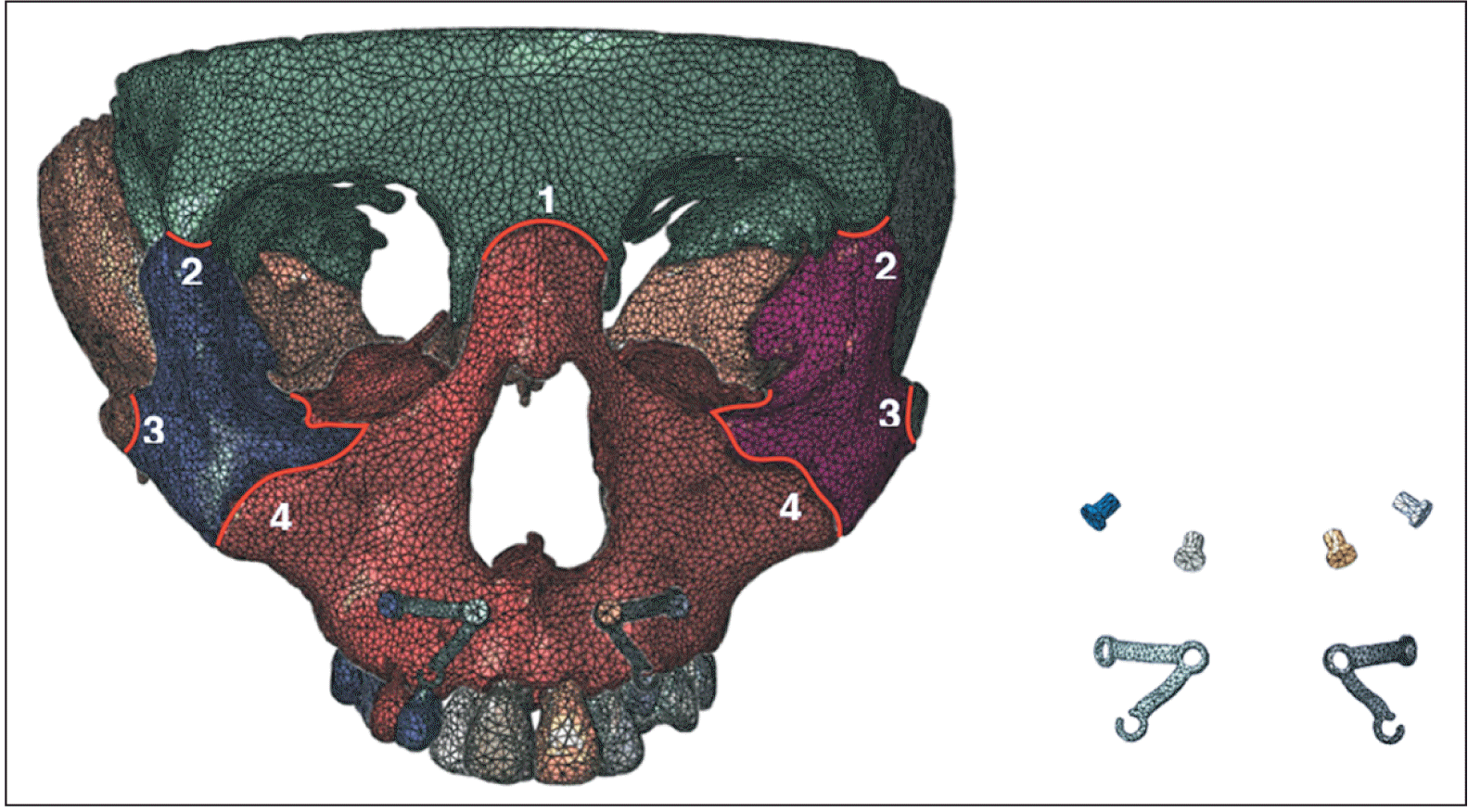
10 revealed that maxillary protraction with the palatal plate resulted in a broader stress distribution, increased forward displacement, and a greater tendency for counterclockwise rotation than the miniplate at the infrazygomatic crest area and conventional tooth-borne appliances. For patients with Class III skeletal malocclusion, counterclockwise rotation of the maxilla is not conducive to improving the facial pattern. Therefore, in this study, titanium plates were placed in the anterior teeth. In traditional titanium plate surgery, a titanium plate is implanted into the lateral edge of the pyriform aperture to prevent damage to the root and permanent germ.
11 However, this approach often results in severe trauma. The shape of the individual titanium plate and distribution of the titanium screws can be designed based on the CBCT data. Under the premise of avoiding damage to the root, an individual titanium plate can be implanted at the lower edge of the pyriform aperture to decrease surgical trauma and postoperative reactions. In this study, the range of vertical movement of the titanium plate was selected from the lower edge of the pyriform aperture to 6 mm above the alveolar crest, covering the clinical application range.
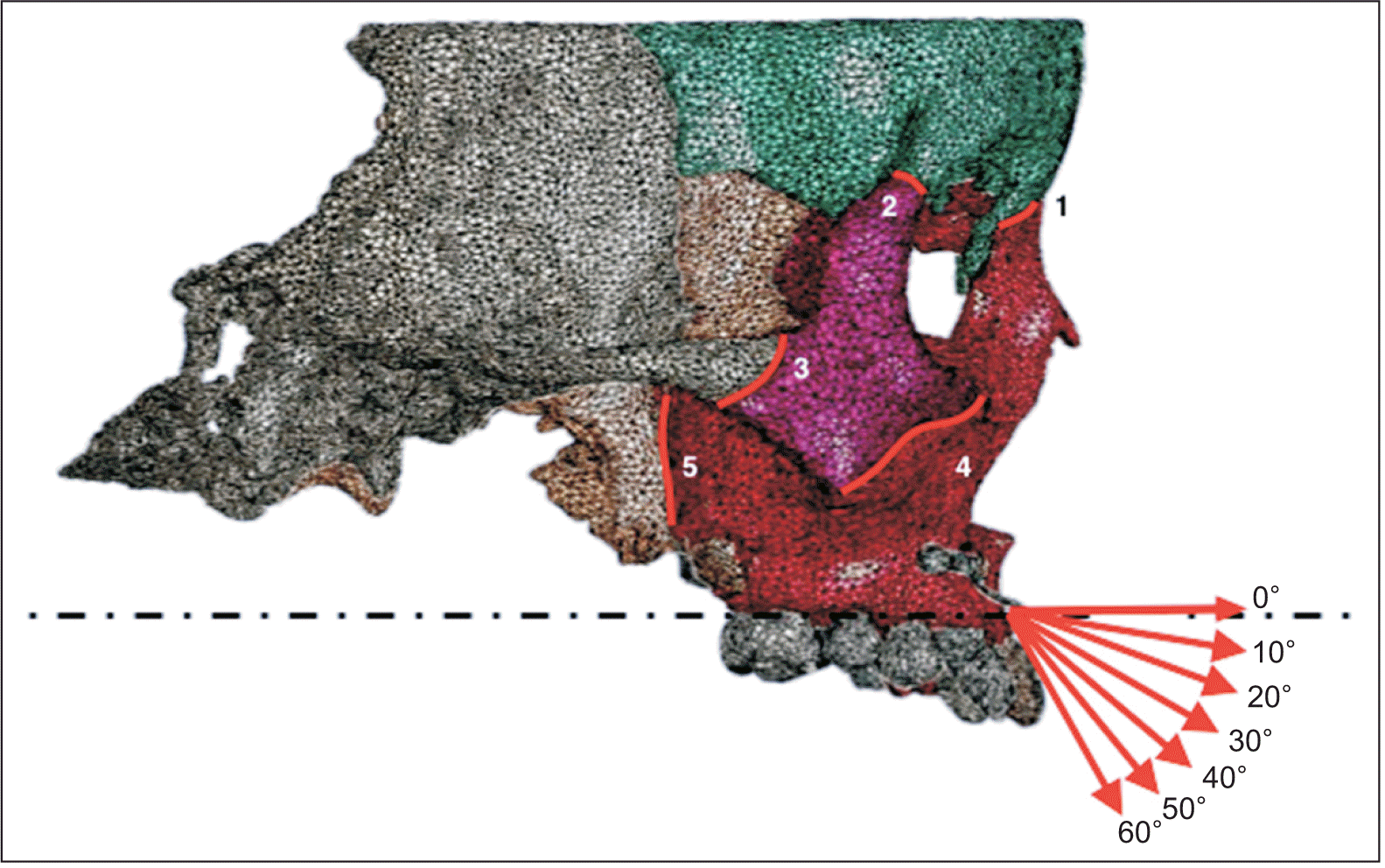
To determine the reference plane, previous studies, including the occlusal plane
10,12 and Frankfort horizontal plane,
13,14 were revisited. The occlusal plane, influenced by malocclusion severity and subject to significant variation among individuals, led us to choose the Frankfort horizontal plane as the reference.
Significant differences were observed in protraction force selection among prior studies. Lee and Baek,
8 Yan et al.,
15 and Zhang et al.
16 utilized a force of 500 g per side to simulate maxillary protraction in their finite element models, whereas Tanne et al.,
9 Miyasaka et al.,
17 and Gautam et al.
12 selected a force of 1,000 g. Zou and Lin
18 reported that the von Mises stresses, maximum principal stresses, and displacement of the cranial-maxillary complex increased with higher protraction forces. However, orthopedic forces > 500 g per side resulted in an evident counterclockwise rotational tendency and narrowing of the dental arch in the maxilla, as observed in both clinical practice and previous studies. Based on this information, we chose a protraction force of 500 g for this study.
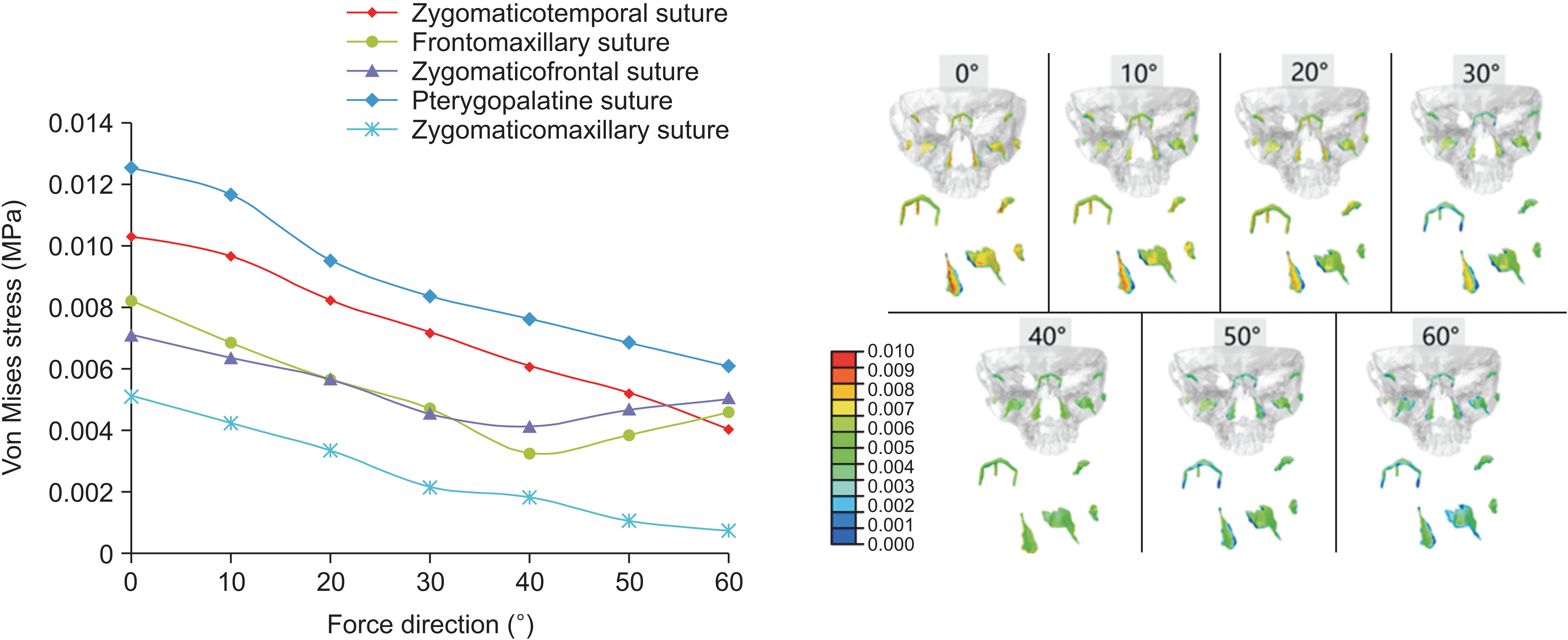
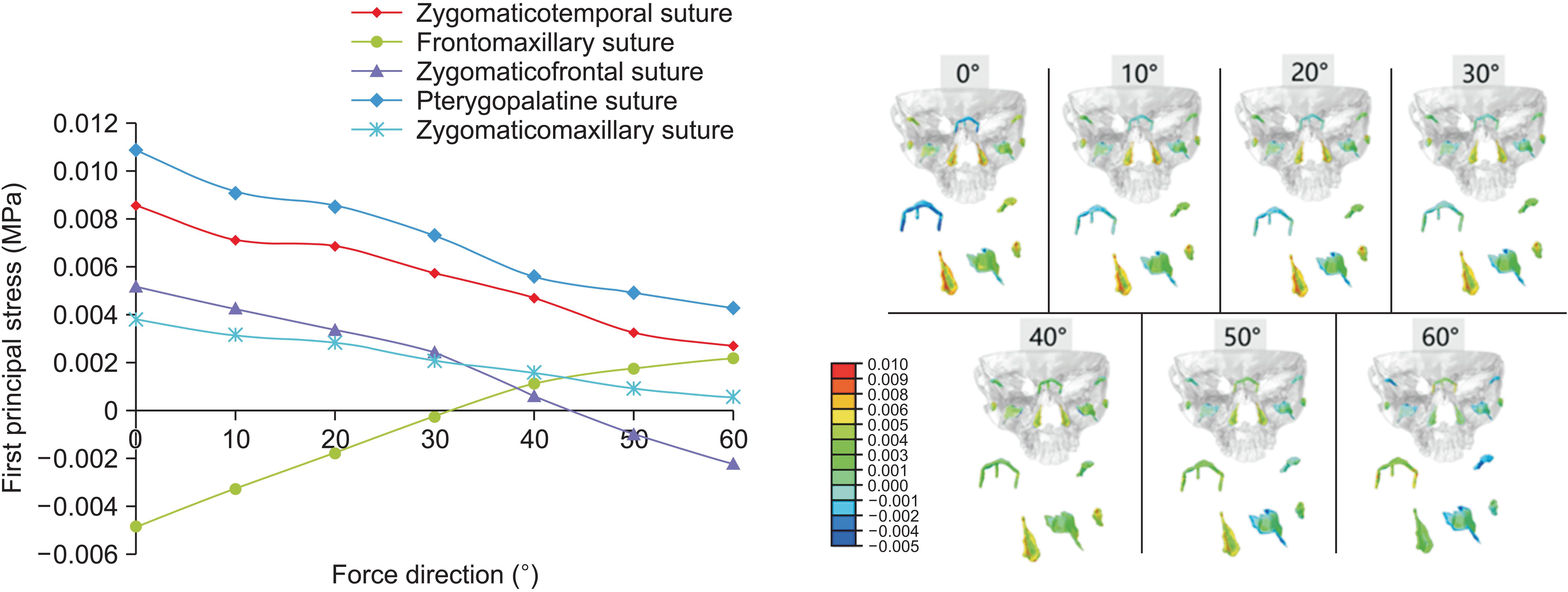
In the finite element analysis, each node was influenced by principal stresses in three directions, with the first principal stress having the largest absolute value among them. A negative value for the 1st principal stress indicates compressive stress in the sutures, whereas a positive value suggests tensile stress. Because the protraction force guides the growth direction of the maxilla, we focused on the first principal stress, representing the maximum traction force on the sutures used to analyze the growth direction of the maxilla. The frontomaxillary suture experienced compressive stress at a protraction angle of 0° to 30°, whereas the other sutures experienced tensile stress. Situated on the anterosuperior side of these sutures, the frontomaxillary suture showed a tendency for counterclockwise rotation in the maxilla. At a protraction angle of 40°, all sutures experienced tensile stress, indicating translational motion of the maxilla. Between a protraction angle of 50° and 60°, the zygomaticofrontal suture received compressive stress, whereas the other sutures received tensile stress. Positioned on the posterosuperior side of sutures, the zygomatic frontal suture indicated a tendency for clockwise rotation in the maxilla. The pterygopalatine, zygomaticotemporal, and zygomaticomaxillary sutures, distributed nearly longitudinally, generally experienced tensile stress under the horizontal component of maxillary protraction. For Class III patients with a deep overbite tendency, a protraction angle < 40° with respect to the Frankfort horizontal plane is recommended. Patients with a normal overbite may benefit from a protraction angle of approximately 40°, allowing translational movement of the maxilla along the translational direction. For Class III patients with an open bite tendency, an angle > 40° is suggested.
According to previous studies, Tanne et al.
9 and Miyasaka et al.
17 supported the idea that the optimal choice for maxillary protraction involves a forward and downward force angled at 30° with respect to the occlusal plane at the canines. The maxilla undergoes forward translation, and stress distribution is uniform. In this study, a titanium plate, chosen as the protraction device, was positioned at the lower edge of the pyriform aperture, causing its force point to move upward compared with that in the tooth anchorage, potentially influencing outcomes. Furthermore, prior studies did not construct a suture model and overlooked the significance of sutures in maxillary protraction. Yan et al.
15 suggested translational motion of the cranial-maxillary complex when the force direction is approximately 20° relative to the occlusal plane during bone anchorage protraction, with bone anchorage at the infrazygomatic crest, which is not consistent with our findings. Qu et al.
14 conducted maxillary protraction using an implant anchorage positioned between the lateral incisors and canines. They observed that the maxilla moved forward and rotated upward when the protraction angle was < 30°. At an angle of 40°, the suture in the nasion did not experience tensile or compressive stress, indicating translational motion of the maxilla. The maxilla moves forward and rotated downward when the angle exceeded 50°. The protraction loading position in this study was similar to that in Qu et al.,
14 with both studies using the Frankfort horizontal plane as the reference plane. Therefore, our study results closely resemble those reported by Qu et al.
14 This divergence might be attributed to the larger stress area of maxillary protraction with the titanium plate than the implant screws. Previous studies exhibited variations in maxillary protraction outcomes due to differences in finite element models, anchorage position, reference plane selections, and analysis indexes. In clinical applications, the direction of maxillary protraction should be determined based on the anchorage location and the vertical facial type of the patient.
In a 3D solid structure, the stress distribution is intricate and cannot be captured by a single directional stress value. The von Mises stress is often employed for heterogeneous objects with complex mechanical properties, including three principal stresses in directions equivalent to the same axis. The von Mises stresses of the pterygopalatine, zygomaticotemporal, and zygomaticomaxillary sutures decreased with an increasing protraction angle. The highest stress was observed when the force direction was approximately 0° with respect to the Frankfort horizontal plane. These results are consistent with those obtained by Yan et al.
15 This consistency is attributed to the rough longitudinal distribution of the sutures. As the protraction angle increases, the component of the forward force gradually decreases, indicating that the protraction value should be appropriately increased with an increase in the protraction angle. The von Mises stresses on the frontomaxillary and zygomaticofrontal sutures initially decrease and then increase in accordance with the change in the 1st principal stress. In other words, the stress on the zygomaticomaxillary suture changes from negative to positive, whereas that on the zygomaticofrontal suture shows the opposite result.
Stress magnitude is influenced by several factors. First, stress magnitude is associated with the distribution of the sutures. The results showed higher stresses on the pterygopalatine and zygomaticotemporal sutures due to their vertical distribution and exposure to a greater forward component force. Secondly, stress is defined as the external force per unit area, correlating with the suture area. Kambara
19 observed increased activity in the reconstruction of the zygomaticotemporal suture during maxillary protraction. The narrow shape of the suture led to stress concentration. Furthermore, stress is influenced by the distance from the protraction point and buffer during the force-transfer process. Therefore, stress magnitudes are the outcome of these combined factors.
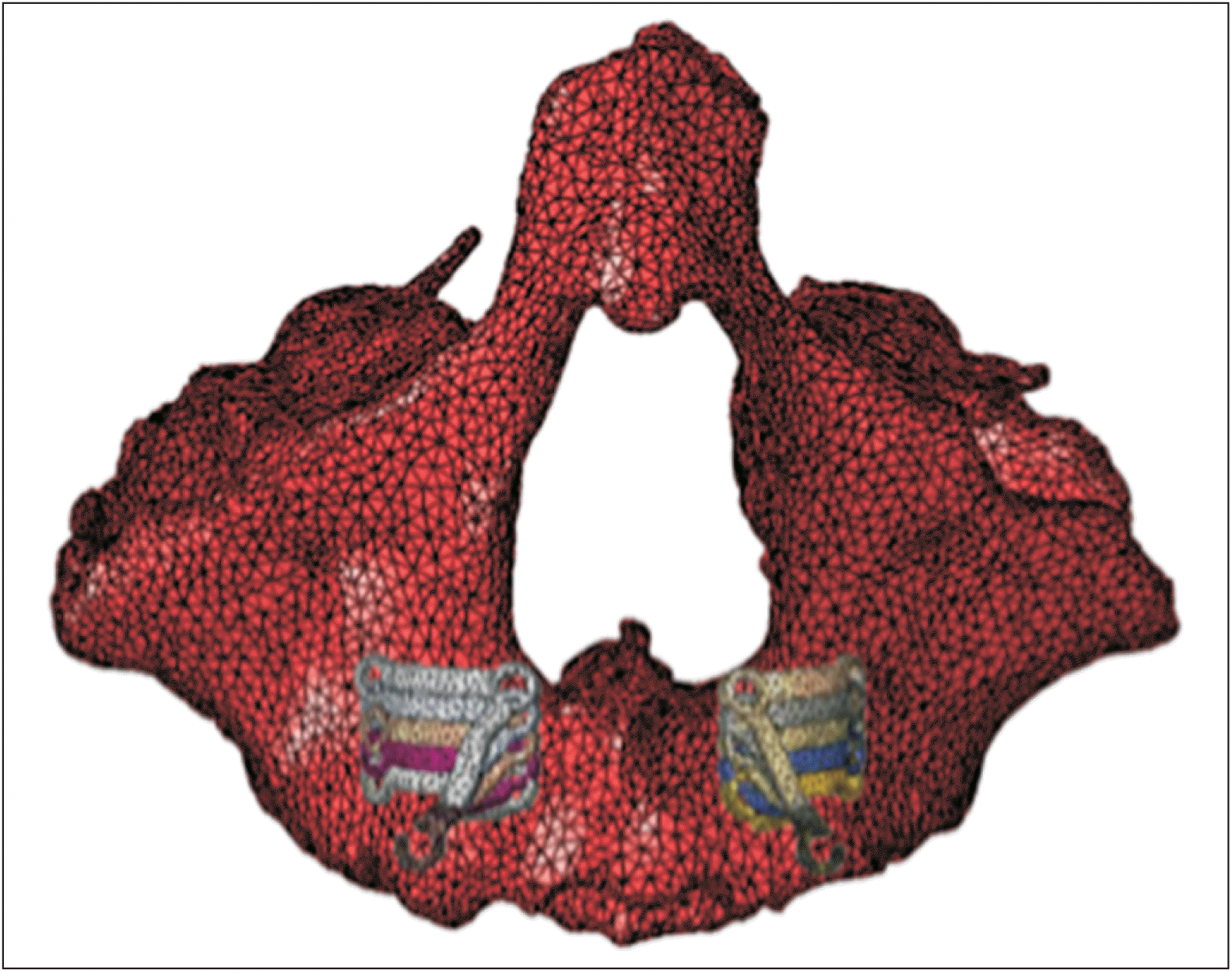
Individual titanium plates were custom-designed based on bone thickness and root distribution, offering a range of options for implant height. The position of the traction hook in the mouth remained fixed, whereas the titanium plate was adjusted upward by 2 and 4 mm and downward by 2 and 4 mm. This study included various miniplate heights to encompass a broad spectrum of clinical scenarios. The analysis of stress distribution trends aimed to provide guidance for determining the optimal position of the titanium plate.
When the titanium plate moved downward, the von Mises stress of each suture decreased, albeit at varying amplitudes. This suggests an improvement in protraction efficiency with an increase in the titanium plate position. In clinical practice, when designing a titanium plate in a lower position, the force applied should be appropriately increased. The decrease in stress may be attributed to the increased distance between the application point and the sutures. Although this study designed the titanium plate with different heights, the position of the hooks remained constant. Therefore, factors such as hook length and angle variation could potentially contribute to these observed outcomes.
Throughout the movement of the titanium plate, the 1st principal stresses consistently exhibited positive values. This indicates that all sutures experienced tensile stress, resulting in translational motion of the maxilla. The findings suggest that the height of the titanium plate had an insignificant effect on maxillary rotation within the designed range. Therefore, in clinical applications, the choice of titanium plate position can be made based on considerations such as root distribution and bone mass.
This study had several limitations. The accuracy of the model was constrained by the thickness of the image layer. Additionally, the assumption of homogeneous, continuous, and isotropic bone tissue in the analysis deviates from the actual complex structure. Although finite element analysis offers insights for clinical applications, the influence of soft tissues such as muscles and ligaments on growth could lead to variations between experimental outcomes and real-world effects. The shape of an individual titanium plate can be customized based on various requirements, offering more choices for positioning. Further research is necessary to enhance guidance for the design of titanium plates.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download