Abstract
Objective
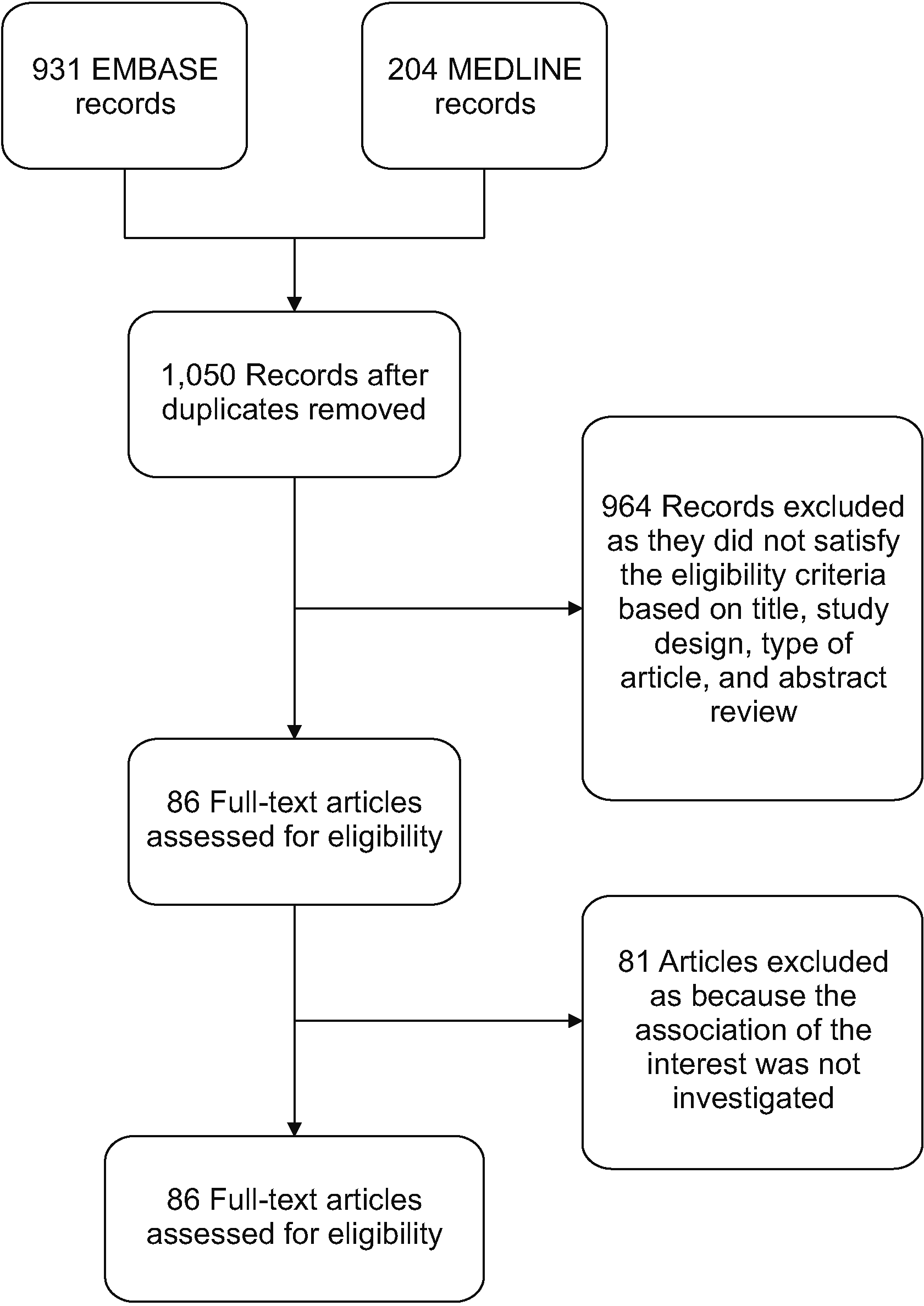
Methods
Results
Notes
AUTHOR CONTRIBUTIONS
P.W. and T.L. contributed equally to this work including the design and implementation. W.T., M.T., C.A., and T.N. performed the data collection and extraction. T.L. analyzed the data. M.T., C.A. and T.K. assessed the quality of the studies. P.W. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
REFERENCES
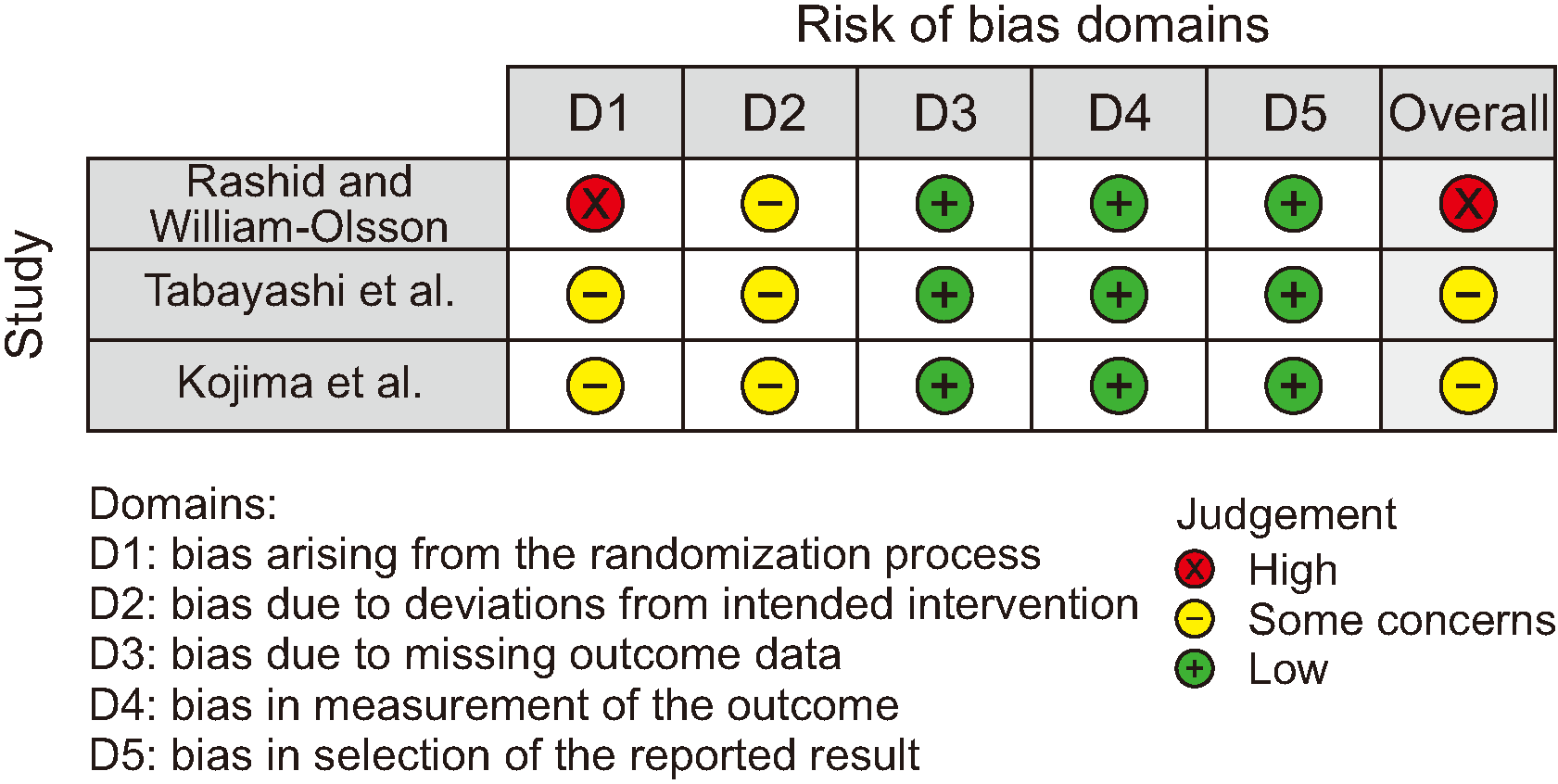
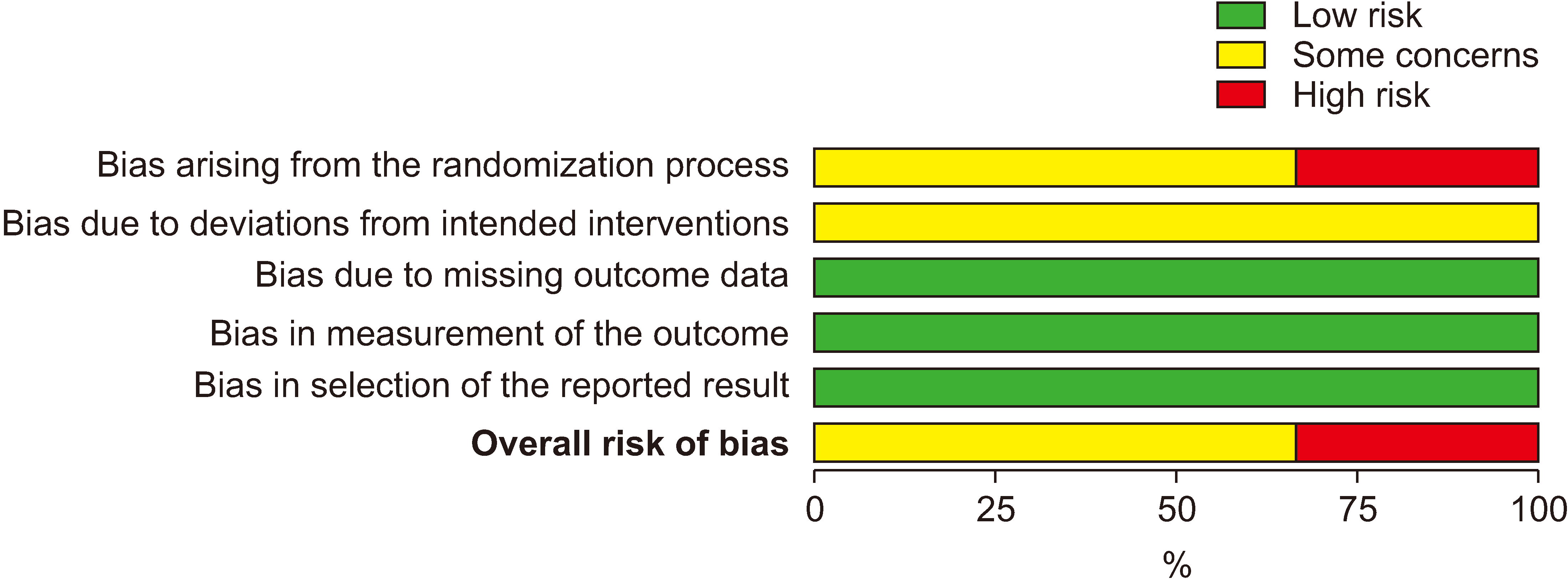
Figure 4
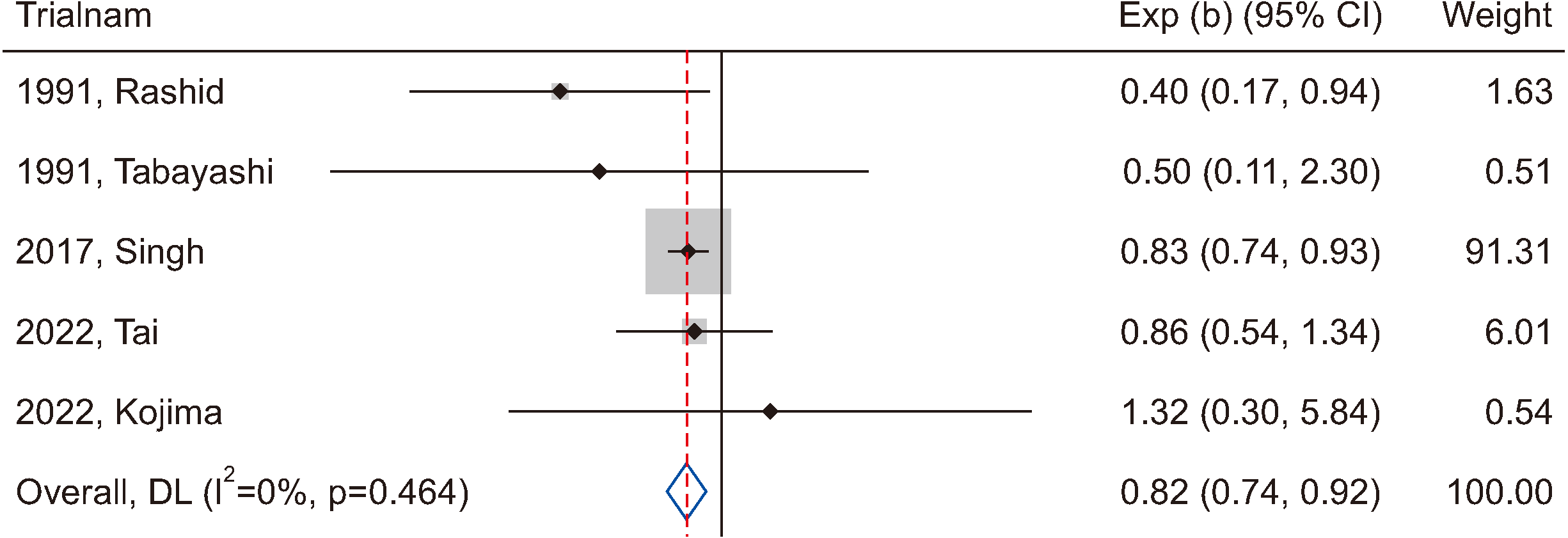
Figure 5
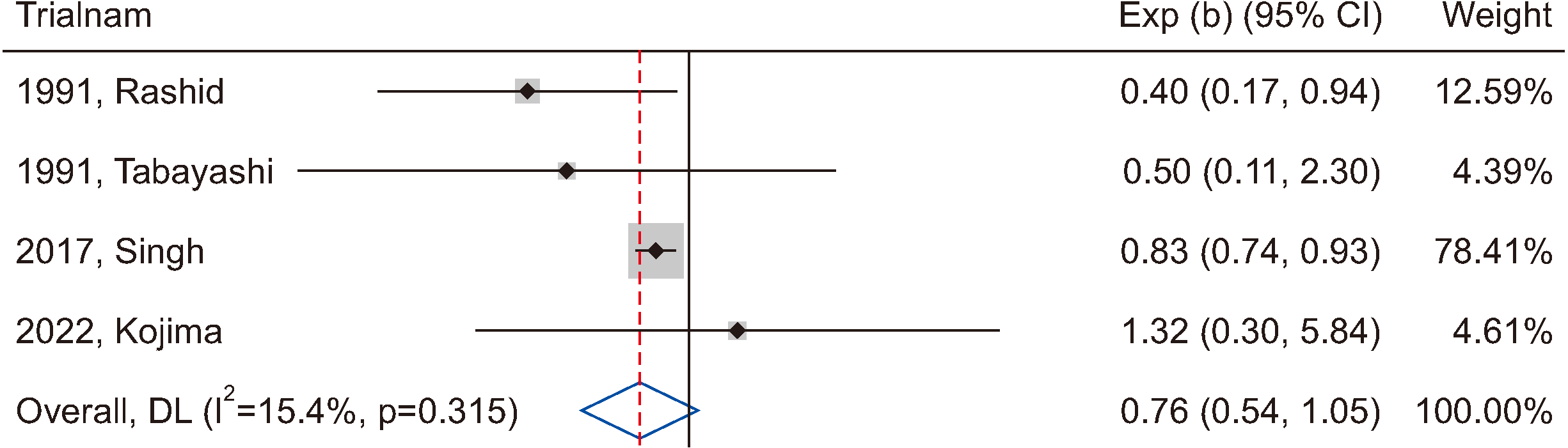
Figure 6
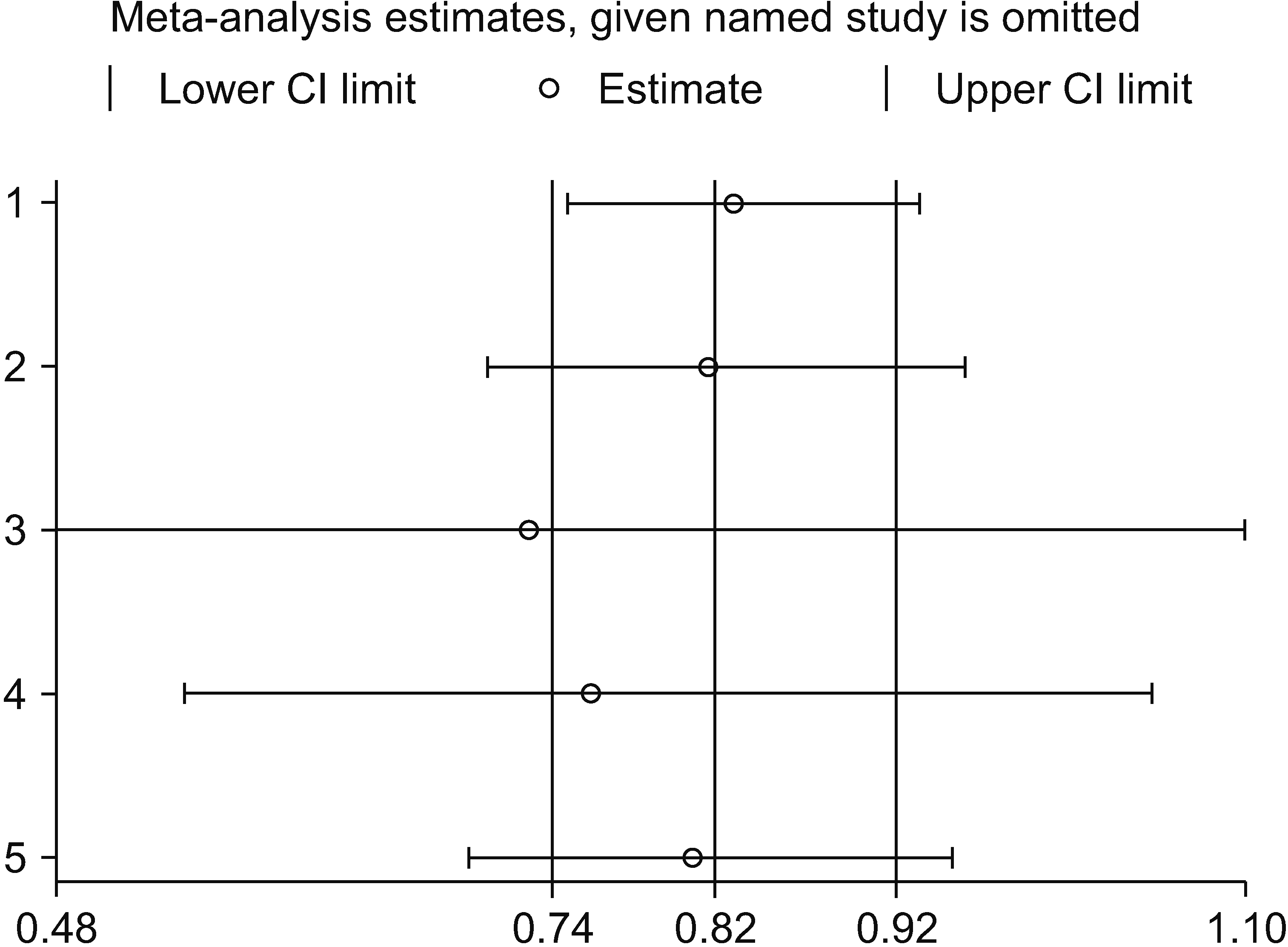
Table 1
| Rashid and William-Olsson [17] | Tabayashi et al.[12] | Singh and Yu [11] | Tai et al. [18] | Kojima et al. [16] | |
|---|---|---|---|---|---|
| Country | Sweden | Japan | USA | Taiwan | Japan |
| Study design | Prospective randomized study | Prospective double-blind randomized study | Retrospective cohort study | Retrospective cohort study | Prospective randomized study |
| Year of publication | 1991 | 1991 | 2017 | 2021 | 2022 |
| Patient/ Population | Patients who underwent elective CABG | Patients who underwent CABG or valve replacements or repairs | Patients without atrial fibrillation at baseline (at least 365 days) from the 5% random Medicare Claims data from 2006 to 2012 | Patients aged 20 years or older with a new diagnosis of in-hospital myocardial infarction by ICD-9-CM code: 410 and ICD-10-CM code: I21 and I22 between January 1, 2005, and December 31, 2016 | Patients with asymptomatic hyperuricemia from post hoc analysis of febuxostat for cerebral and cardiorenovascular events prevention study trial |
| Exposure | Patients who received allopurinol twice a day for 2 days preoperatively, 600 mg given as single dose on the morning of operation day and 300 mg given twice a day for 2 days postoperatively. | Patients who received 1,200 mg and 2,400 mg allopurinol preoperatively | Patients who received allopurinol as a new therapy, indicated by a filled allopurinol prescription, after a baseline period of at least 365 days | Patients who used XOIs (allopurinol or febuxostat) or uricosuric agents (benzbromarone, probenecid, or sulfinpyrazone), identified according to the Anatomical Therapeutic Chemical classification system and the corresponding drug codes in the NHI database. | Patients who received febuxostat |
| Total number of patients with ULT use | 45 | 60 | 5,754 | 963 | 537 |
| Comparators | Patients who did not receive allopurinol | Patients who did not receive allopurinol | Patients who did not receive allopurinol | Patients who did not receive ULT | Patients who did not receive febuxostat |
| Total number of comparators | 45 | 30 | 3,490 | 963 | 533 |
| Outcome | Atrial fibrillation, atrial flutter, sinus tachycardia, ventricular tachycardia, and blocks appearing more than 24 hours postoperatively | Atrial, supraventricular and ventricular arrhythmias | Incidient atrial fibrillation from 2006 to 2012 | Supraventricular tachycardia, ventricular tachycardia, atrial fibrillation, and atrial flutter | Atrial fibrillation (including paroxysmal atrial fibrillation) |
| Average age of participants at index date (yr), mean (SD) | |||||
| Patients with ULT | 61.9 (11) | 54.5 (12.4) | 78.4 (7.1) | 65.6 (13.3) | 75.4 (6.7) |
| Patients without ULT | 62.5 (9) | 51.8 (14.5) | 78.0 (7.3) | 65.5 (13.8) | 76 (6.5) |
| Percentage (%) of female | |||||
| Patients with ULT | 32.3 | 53.8 | 56.3 | 22.9 | 30.9 |
| Patients without ULT | 32.3 | 57.8 | 57.5 | 22.9 | 31 |
| Variables adjusted in multivariate analysis | None | None | Age, sex, race, Charlson–Romano comorbidity score and cardiac medications | Age, sex, other possible confounders and the use of major CV medications | Age, sex, and comorbidities |
| Newcastle-Ottawa score | Selection: 4 | Selection: 4 | |||
| Comparability: 1 | Comparability: 1 | ||||
| Outcome: 2 | Outcome: 2 |
CABG: coronary artery bypass grafting, ICD-9-CM: The International Classification of Disease, 9th Revision, Clinical Modification, ICD-10-CM: The International Classification of Disease, 10th Revision, Clinical Modification, XOIs: xanthine oxidase inhibitors, NHI: National Health Insurance, ULT: urate lowering therapy, SD: standard deviation, CV: cardiovascular.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download