The term “ETosis” refers to the extracellular release of DNA, histones including citrullinated histone H3 (Cit-H3), proteins/enzymes such as myeloperoxidase (MPO), and other cytoplasmic components, with the formation of molecular webs called extracellular traps (ETs). The activation of this mechanism - initially studied in the context of programmed cell death - occurs upon recognition of a microbial pathogen (parasitic, bacterial, viral, fungal), followed by the release of ETs by inflammatory cells, particularly neutrophils (NETosis) and macrophages (METosis). The physiological action of ETs is to trap and eliminate the microbial agent, but it is now known that the persistence of ETosis is an autoimmune trigger, causing inflammation and cytotoxic tissue damage [1].
A 74-year-old man on immunosuppressive therapy for a liver transplant 14 years earlier presented with weight loss, dysthymia, seizures, and apyrexia. Contrast-enhanced computed tomography showed a hypodense brain lesion in the right frontal lobe with ring-enhancement, and magnetic resonance imaging confirmed a cortico-subcortical lesion of the right superior frontal gyrus. Neuroradiological differential diagnosis included inflammation/infection and post-transplant lymphoproliferative disorder: therefore, surgical excision was decided to have a histological answer.
Microscopy revealed areas of reactive gliosis with pseudocystic formations containing corpuscles compatible with an infectious etiology, particularly bradyzoites from a protozoan parasitic infection of Toxoplasma (Figure 1A). No concomitant fungal or alcohol-resistant bacterial infections were found; Epstein-Barr virus immunohistochemistry was also negative. Of note was a lymphocytic infiltrate organized in perivascular “caps” (Figure 1B), which on immunohistochemistry showed a T-cell profile (CD3+, with a predominance of CD4+) associated with widespread histiocytic infiltrates (Figure 1C). Molecular studies (polymerase chain reaction) performed on paraffin-embedded tissue finally showed polyclonality of both T and B lymphocytes, excluding the hypothesis of a lymphoproliferative disease. The diagnosis was therefore neurotoxoplasmosis associated with vasculitis of the small cerebral vessels (a fresh tissue fragment was also sent to microbiology, which later confirmed the presence of Toxoplasma gondii).
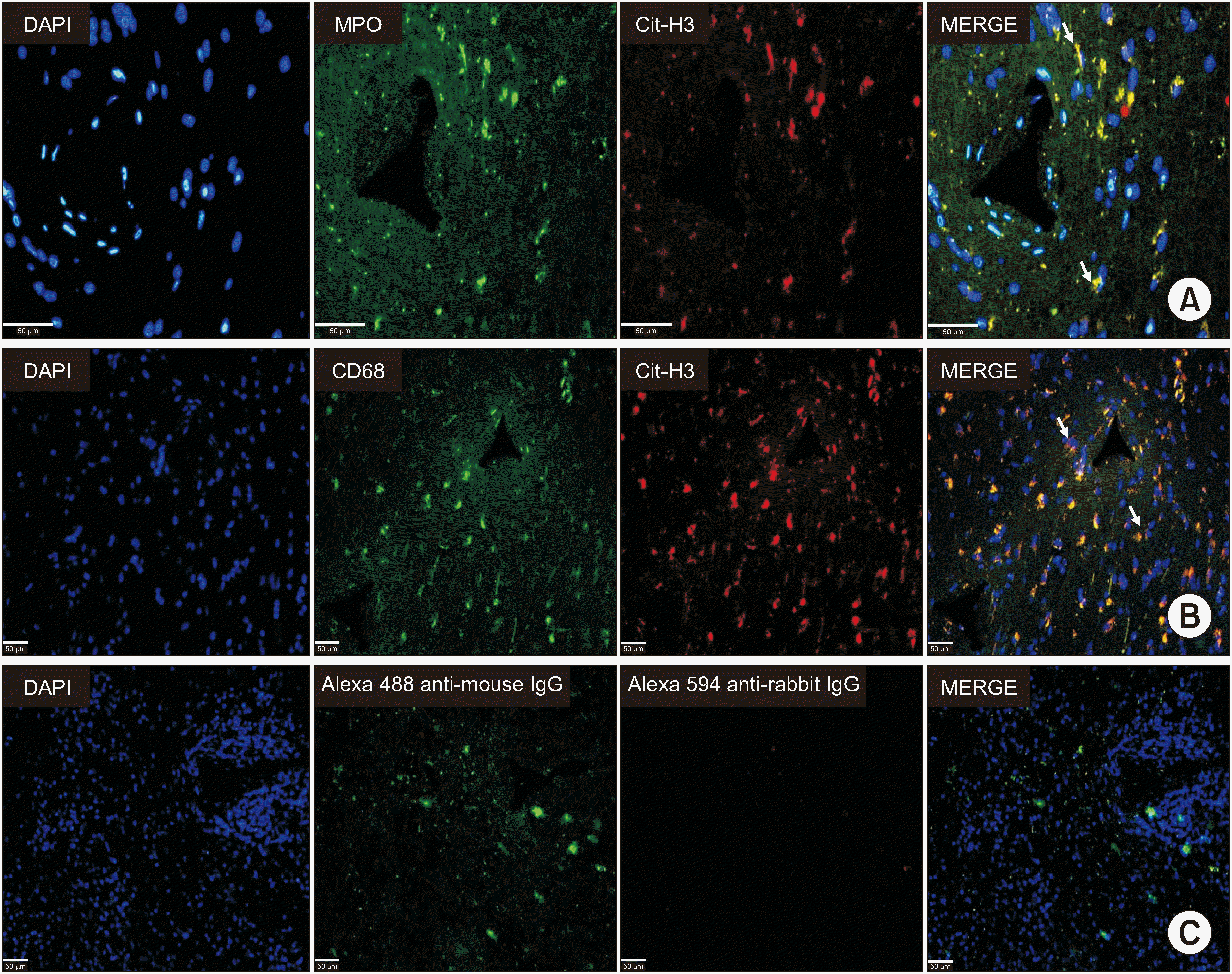
The most intriguing aspect was then investigated, namely the coexistence of lymphocytic vasculitis and macrophages in a subject pharmacologically immunosuppressed due to a previous liver transplantation: immunohistochemistry showed a striking positivity for MPO (Figure 1D), raising the hypothesis of vascular inflammation induced by ETosis. Immunofluorescence analysis was then performed and confirmed the presence of ETs positive for anti-Cit-H3 and anti-MPO antibodies in close topographical association with CD68+ macrophages (Figure 2), confirming that the vasculitis was associated with METosis.
There are literature data correlating ETosis with inflammation, including vasculitis [2], or with parasitic infection [3], as well as some reports describing the association between neurotoxoplasmosis and cerebral vasculitis [4]. However, to the best of our knowledge, there are no data to date that microscopically document the continuum of neurotoxoplasmosis-ETosis-brain vasculitis in humans.
Finally, a therapeutic consideration: although there are very rare reports of fatal neurotoxoplasmosis [5], cerebral vasculitis is certainly more dangerous for the patient’s life, and the case described makes one reflect on the “strength” of ETs, trumping that of post-transplant immunosuppressive drugs, supporting the scientific community’s growing attention to potential pharmacological targets to control the formation or release of ETs [3].
REFERENCES
1. Konsoula Z. 2019; Extracellular traps - NETosis and METosis. Mater Methods. 9:2714. DOI: 10.13070/mm.en.9.2714.

2. Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. 2009; Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 15:623–5. DOI: 10.1038/nm.1959. PMID: 19448636. PMCID: PMC2760083.

3. Koh CC, Gollob KJ, Dutra WO. 2023; Balancing the functions of DNA extracellular traps in intracellular parasite infections: implications for host defense, disease pathology and therapy. Cell Death Dis. 14:450. DOI: 10.1038/s41419-023-05994-8. PMID: 37474501. PMCID: PMC10359321.

4. Yang HX, Liu HR, Zhang FC. 2017; Pitfalls of PACNS: a rare case of central nervous system vasculitis associated with toxoplasmosis. J Rheumatol. 44:1290–1. DOI: 10.3899/jrheum.161472. PMID: 28765346.

5. Gaggero G, Campora M, Dose B, Taietti D, Vena A, Delfino E. 2022; Neuro-toxoplasmosis and fatal necrotizing cerebellitis. Autops Case Rep. 12:e2021363. DOI: 10.4322/acr.2021.363. PMID: 35252054. PMCID: PMC8893200.

Figure 1
Histology and immunohistochemistry. (A) Photomicrograph (hematoxylin and eosin [H&E], 400×) showing the presence of pseudocysts (arrows) with multiple microinclusions in the context of reactive gliosis, consistent with Toxoplasma bradyzoites. (B) Photomicrograph (H&E, 200×) showing (in the circle) the presence of perivascular lymphocytic “caps” configuring a small cerebral vessel vasculitis. (C) Immunohistochemistry for CD68-PGM1 (100×), a macrophage marker, showing (in the circle) many positive elements, especially around the small vessels. (D) Immunohistochemistry for myeloperoxidase (100×) showing (in the circle) widespread positivity overlapping with that of macrophages, suggesting the presence of METosis.
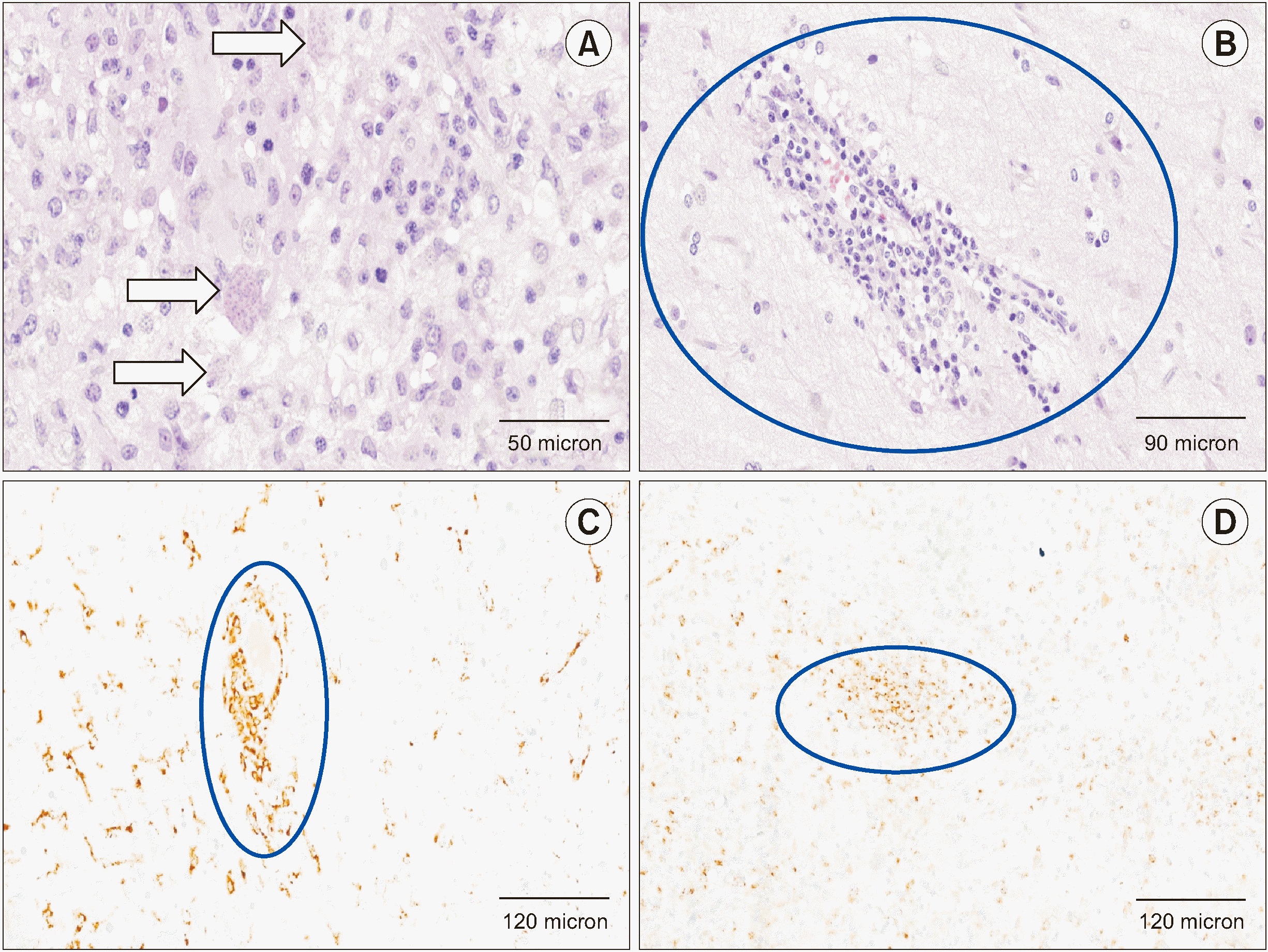
Figure 2
Immunofluorescence. (A) Representative images of METs. METs are identified as extracellular structures where MPO (green) and Cit-H3 (red) colocalize (shown by white arrows). Mets are stained in yellow. Cell nuclei are blue (images are acquired at 400× magnification. Scale bars=50 μm). (B) To identify METs and macrophages, tissue is immunostained with CD68 (green) and Cit-H3 (red). White arrows highlight colocalized areas that are stained yellow and orange (combination of red and green). Cell nuclei are stained in blue (DAPI) (magnification 200×, scale Bar=50 μm). (C) The images depict negative controls. Only Alexa 488 (green) and Alexa 594 (red) antibodies are used. Cell nuclei are stained in blue (magnification 20×, Bar=50 μm). METs: macrophages extracellular traps, MPO: myeloperoxidase, Cit-H3: citrullinated Histone H3, DAPI: 4’,6-Diamidino-2-Phenylindole.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download