Abstract
Heavy reliance on glucose metabolism and a reduced capacity to use ketone bodies makes glioblastoma (GBM) a promising candidate for ketone-based therapies. Ketogenic diet (KD) is well-known for its promising effects in controlling tumor growth in GBM. Moreover, synthetic ketone ester (KE) has demonstrated to increase blood ketone levels and enhance animal survival in a metastatic VM-M3 murine tumor model. Here, we compared the efficacy of a KE-supplemented Atkins-type diet (ATD-KE) to a classic KD in controlling tumor progression and enhancing survival in a clinically relevant orthotopic patient-derived xenograft GBM model. Our findings demonstrate that ATD-KE preserves body weight (percent change from the baseline; 112±2.99 vs. 116.9±2.52 and 104.8±3.67), decreases blood glucose (80.55±0.86 vs. 118.6±9.51 and 52.35±3.89 mg/dl), and increases ketone bodies in blood (1.15±0.03 mM vs. 0.55±0.04 and 2.66±0.21 mM) and brain tumor tissue (3.35±0.30 mM vs. 2.04±0.3 and 4.25±0.25 mM) comparable to the KD (results presented for ATD-KE vs. standard diet [STD] and KD, respectively). Importantly, the ATD-KE treatment significantly enhanced survival compared to the STD and was indistinguishable from the KD (47 days in STD vs. 56 days in KD and ATD-KE), suggesting that a nutritionally balanced low carbohydrate ATD combined with KE may be as effective as the KD alone in reducing brain tumor progression. Overall, these data support the rationale for clinical testing of KE-supplemented low-carb diet as an adjunct treatment for brain tumor patients.
Glioblastoma (GBM) is the most common and deadliest malignant brain tumor in adults with 5-year survival of less than 5% despite aggressive therapies [1, 2]. GBM tumor has a high rate of glucose metabolism mainly through aerobic glycolysis, a phenomenon known as the Warburg effect [3]. While this metabolic feature is primarily adopted by fast dividing GBM cells to generate ATP and build tumor biomass, slow-dividing cancer stem cells can still use glucose via oxidative phosphorylation and in scarcity of glucose they can process other available nutrients such as glutamine and fatty acids [4-7]. Although this metabolic heterogeneity and adaptability contribute to poor therapy outcomes [8-10], the extreme glucose dependency of GBM tumor for optimizing cellular proliferation and biomass production posit a weakness that could be exploited for developing effective treatments against GBM.
Dietary interventions, in particular diets with high fat, low carbohydrate and adequate protein such as the ketogenic diets (KDs) have been shown to effectively diminish tumor growth and enhance overall survival in animal studies [11-14] and show promising results in human clinical studies of brain tumors [15]. Although KD holds promise as a safe and effective therapeutic option with low toxicity for brain cancer patients, stringency of the diet can impact compliance and nutritional sufficiency. We have previously demonstrated that a nutritionally balanced high fat/low carbohydrate diet, similar to the KD, can reduce tumor progression and enhance survival in an orthotopic xenograft model [12]. However, while this diet is less restrictive than the classic KD it still involves significant changes to a patient’s usual diet. Mechanistically, two of the primary physiological changes associated with KD are a reduction in glucose and an increase in ketone bodies such as β-hydroxybutyrate (β-HB), acetoacetate (AcAc). These physiological changes can be mimicked by providing ketone esters (KEs) in the diet. We have previously demonstrated that KE can reduce glucose levels, increase ketone bodies, and improve survival in a metastatic cancer model [13], utilizing animals that were on a high-carbohydrate standard diet (STD). In this study, we posited that the combination of KE (1,3-butanediol AcAc diester) with an Atkins-type diet (ATD), characterized by lower carbohydrate content (16% vs. 60%) and higher levels of protein (28% vs. 18%) and fat (56% vs. 6.2%) compared to a STD, would yield more favorable outcomes in terms of blood glucose and ketone levels. Concurrently, we aimed to formulate a more nutritionally balanced diet that would help maintain body weight when compared to the classic KD (8.6% protein, 75.1% fat, and 3.2% carbohydrates).
Our results demonstrate that ATD-KE not only preserves body weight but also elevates blood ketone levels to the same extent as STD-KE. Furthermore, it significantly reduces blood glucose levels compared to STD-KE. Notably, our dietary intervention in tumor-bearing animals using a clinically relevant, patient-derived orthotopic xenograft model of GBM, with STD, ATD-KE, and KD, has revealed that ATD-KE not only maintains body weight but also lowers blood glucose, increases blood and brain ketone levels, and negatively impacts tumor progression, resulting in enhanced animal survival—comparable to the effects of a KD. In summary, these findings provide support for the rationale to conduct clinical trials investigating the potential of KE-supplemented low-carb diets as adjunct treatments for brain tumor patients.
Female NOD/SCID animals of 8 weeks old were purchased from Charles River laboratories and maintained according to standard murine husbandry protocol. All producers were performed based on the NIH Guide for care and Use of Laboratory Animals and approved by the University of Florida Institutional Animal Care and Use Committee (protocol# 201701502).
STD rodent chow (2018 Teklad Global, 18% protein, 6.2% fat and 60% carbohydrate), ATD (TestDiet, 28% protein, 56% fat and 16% carbohydrate) and KD (BioServ, 8.6% protein, 75.1% fat, 3.2% carbohydrate) were readymade. KE (butane 1, 3-diyl bis[3-oxobutanoate]) was prepared from Disruptive Enterprises, manufactured based on the formula developed by D’Agostino et al. [17]. KE was supplemented to ATD and STD at 10% by volume and 1% stevia was added for palatability. Animals were continuously monitored on a daily basis with access to fresh food ad libitum.
To choose which KE-supplemented dietary formulation (STD/KE vs. ATD/KE) could decrease blood glucose and increase blood ketone levels comparable to KD, after three weeks of different dietary treatments, animals were fasted overnight to ensure rapid feeding compliance, and then their blood glucose and ketone were measured before return of food (0 hr) and at 1, 4, 8, and 12 hours after feeding with free access to food during measurements [13]. Blood was collected via tail snipping method and glucose and β-HB were measured using Keto-Mojo blood glucose and β-ketone strips. AcAc were measured using AcAc ELISA kit (BioVision, K650-100) per manufacturer’s instruction.
After tumor implantation, blood metabolites in tumor bearing animals were measured on a weekly basis 1 hour after providing the animals with freshly prepared food, following an overnight fast. The average level for each metabolite during the first five weeks, before showing any neurological symptoms, was compared between different treatment groups. Animal weight was also measured on a weekly basis. Brain tumor β-HB and AcAc was measured upon animal sacrifice on tumor tissue lysate using β-HB (BioVision, K632-100) and AcAc ELISA kits per manufacturer’s instruction.
Animal health was monitored daily after tumor implantation to ensure normal function. Animals were sacrificed humanely upon reaching endpoint criteria (>20% body weight loss, body conditioning score≤2, and neurologic deficits) and their survival time was recorded.
Kaplan-Meier method was used to estimate survival curves. To compare survival curves individual log-rank test was used for survival studies but a stratified Max-Combo test was used for combined survival data from two survival studies to account between-experiment variation due to possible laboratory day effects [18]. Pairwise comparisons in the combined experiments were considered a priori of interest. All calculations were performed using GraphPad Prism6 (GraphPad Software Inc.), SAS Version 9.4 (SAS Institute) or R Version 3.6.1 (R Foundation for Statistical Computing). Percent body weight change and average blood metabolites were compared across different groups using one-way analysis of variance (ANOVA) with appropriate post-hoc test. Level of statistical significance was set at P<0.05.
To find the best dietary combination that mimics the effects of KD in lowering blood glucose and increasing blood ketones while preserving body weight during the course of treatment, we initially fed intact animals a STD, ATD, KD, STD+20% KE and ATD+20% KE for one week. 1% Stevia was added to KE diets to increase palatability. Compared to STD and ATD, animals fed with KE supplemented diets lost weight considerably during the first week. For better compliance, we decreased KE to 10% that resulted in a stabilized animal weight over the course of study (Fig. 1A).
After three weeks of applying different dietary treatments, animals were fasted overnight to ensure rapid feeding compliance, and blood glucose and ketones were measured before return of food (0 hr) and at 1, 4, 8, and 12 hours after feeding with free access to food during measurements. A mean of these measures from 1, 4, 8, and 12 hours after feeding were compared between treatment groups. While the animals on KE supplemented diets had lower blood glucose (104.2±2.73 mg/dl in STD/KE, 80±0.86 mg/dl in ATD/KE) and higher β-HB (1.05±0.04 mM in STD/KE, 1.15±0.03 mM in ATD/KE) compared to the STD group (118.6±9.5 mg/dl and 0.55±0.04 mM, respectively), we found that the ATD/KE group had a significantly lower plasma glucose level than the STD/KE (Fig. 1B, C). At 12-hour time-point, blood AcAc was significantly higher in KD, ATD/KE, and STD/KE (2.13±0.42 mM, 1.41±0.19 mM, and 1.56±0.19 mM) compared to STD (0.52±0.06 mM, Fig. 1D). Accordingly, we chose to assess the effect of ATD/KE on overall survival in GBM tumor-bearing animals comparing to STD (negative control) and KD (positive control) dietary interventions.
To acclimate the animals to the new diet before tumor implantation, we randomly assigned animals of the same age and weight into different dietary interventions one week before tumor implantation, and their weight was measured before tumor implantation (baseline) and reassessed on a weekly basis. While KD and ATD/KE groups remained relatively stable, the STD group gradually gained weight during the course of their survival (Fig. 2A). On average, blood glucose and β-HB in ATD/KE (83.6±1.3 mg/dl, 1.38±0.07 mM) were significantly different to both STD (148±6.32 mg/dl, 0.67±0.08 mM) and KD (57.5±6.32 mg/dl, 2.95±0.21 mM) groups during the survival period (Fig. 2B, C).
Log-rank test of Kaplan–Meier survival curve found both the KD and ATD/KE diets significantly increased survival (median survival of 56 days in KD and ATD/KE vs. 47 days in STD, P=0.0003 and P=0.002 respectively, Fig. 3A).
Moreover, KD and ATD/KE significantly increased β-HB (4.25±0.25 mM, 3.35±0.30 mM) in freshly resected tumor tissue (Fig. 3B) comparing to the STD (2.04±0.3 mM). While both KD and ATD/KE demonstrated a trend of increased AcAc levels (Fig. 3C) in tumor tissue, the differences were not significant compared to the STD.
We found that a ATD-KE is comparable to the classic KD in controlling brain tumor growth and increasing overall survival. Our results indicate that while ATD-KE maintains body weight and elevates blood ketone levels to the same extent as STD-KE, it significantly reduces blood glucose levels in contrast to STD-KE. This finding aligns with our previous in vitro study, demonstrating that reduced glucose levels, in combination with ketone body supplementation, further reduces proliferation in several human GBM cell lines when compared to reduced glucose alone [12].
Therapeutic ketosis is typically achieved through the administration of high-fat KDs. However, the primary challenges associated with implementing KDs are compliance and low adherence to the dietary intervention necessary for optimal therapeutic results [19]. Some of the primary factors contributing to low adherence include the unappealing nature of ketogenic meals and the challenges associated with incorporating this dietary regimen into family life. Other significant drawbacks of the KD include malnourishment, sarcopenia, and cancer cachexia, which can result in weight loss, reduced quality of life, and poor clinical outcomes, especially in terminally ill patients. In order to resolve these problems, we hypothesized that supplementing a nutritionally balanced, relatively low glucose diet with an exogenous ketone precursor (butane 1, 3-diyl bis[3-oxobutanoate]) would reduce blood glucose, increase blood ketones and enhance animal survival in an orthotopic mouse model of human GBM. Our results showed ATD supplemented with 10% KE maintained animal weight and significantly increased median survival time from 47 days in animals fed with STD to 56 days, comparable to the effect of a high-fat KD [12]. These findings demonstrate that ketone supplementation together with reduction in the overall carbohydrate intake presents an efficacious strategy to fight brain tumors, while potentially increasing compliance to dietary intervention and enhancing nutritional sufficiency of the diet.
To assess the effect of KE supplemented STD vs. ATD on weight change, and blood glucose and ketone, we tested dietary interventions on non-tumor bearing animals over a period of five weeks. Poff et al. [13] showed that STD/KE (10%) could effectively reduce blood glucose, increase blood ketones and improve animal survival. Here, we increased KE to 20% in an attempt to further increase the overall blood ketone body concentration as it is directly correlated to the dose of the consumed KE [13]. With 20% KE, both STD/KE and ATD/KE groups lost weight even more than the KD group while the animals in STD and ATD groups gained weight. Weight loss is anticipated in KDs and has been reported by us and other groups [12, 20] but in this study weight loss in STD/KE and ATD/KE groups seems to be more related to the amount of KE supplemented to the animal food; notably, KE would increase malonyl-CoA, an anorexigenic metabolite known to decrease food intake [21] and also high amount of KE can make the food less palatable. To prevent further weight loss, we decreased the amount of KE to 10% which resulted in weight gain and stabilization over the course of the next few weeks. Comparing to the STD and ATD alone, we found that while STD/KE and ATD/KE could similarly increase blood β-HB and AcAc levels, the ATD/KE was more potent in decreasing blood glucose, a condition that greatly mimics our in vitro condition that blocks tumor cells proliferation [12]. While administering exogenous ketone precursors proved to be efficacious in increasing blood ketones, it is important to balance the amount of ketone to prevent a reduction in food intake which could result in an undesirable level of weight loss.
In tumor bearing animals, the KD group was showing a relatively stable weight during the course of treatment whereas the ATD-KE group not only maintained their weight but also showed a trend of weight gain that was evident until the fourth week after tumor implantation. This suggests that using KE at 10% by volume does not interfere with animal food consumption while still significantly reducing blood glucose and increasing blood ketone levels compared to the STD group. Starting from the fifth week after tumor implantation, the STD animals began to show neurological symptoms hence we did not include the blood glucose and ketone values in the average values to compare different groups as they possibly could be affected by the animal health status and food consumption rate. We evaluated animal survival in two different cohorts of animals showing significant increase in median survival in KD and ATD/KE groups compared to the STD animals, with no significant difference between KD and ATD/KE groups based on the log-rank test. We combined the survival data from the two experiments that were performed in identical situations but at different points in time (each with three treatment groups) and compared survival curves of the combined data using a stratified Max-Combo test, to account for between-experiment variation due to possible laboratory day effects. The stratified Max-Combo test incorporated stratified versions of the standard and weighted log-rank test components [18], and stratification by experiment. The Max-Combo test uses the distribution of the maximum test statistic obtained from considering the test statistics of the standard log-rank test and 3 weighted log-rank tests where the weights emphasize early, middle, and late differences between survival curves [22-24]. The Max-Combo test is robust against a wide variety of configurations of proportional and/or non-proportional hazards, whereas the standard log-rank test is most powerful under the proportional hazards assumption and loses power as survival curves deviate from proportional hazards [24]. ATD/KE significantly increased median survival comparable to KD (47 days in STD vs. 56 days in ATD-KE and KD groups, representing a 19% increase from STD group). Supporting this observation, while there was a trend of increased AcAc in tumor lysate in ATD/KE and KD groups compared to the STD, we also noticed a statistically significant increase of β-HB in the treatment groups. One possible explanation for this high level of ketone bodies in the tumor could be that the tumor was able to take in ketone bodies but not metabolize them efficiently and therefore it accumulated. Alternatively, the tumor was benefiting from the ketones and therefore taking in large amounts, or the tumor was producing ketones for some unknown reasons. Considering the survival data, it is evident that the state of ketosis is indeed impairing tumor growth. It is likely that due to the restricted availability of glucose in KD and ATD-KD treated animals, tumor cells may absorb significant quantities of ketone bodies from the bloodstream. Notably, impaired mitochondrial respiration [25] and the absence or low level of some ketone-metabolizing enzymes, such as succinyl CoA: 3-oxoacid CoA transferase (OXCT1), 3-hydroxybutyrate dehydrogenase 1 and 2 (BDH1 and BDH2), and acetyl-CoA acetyltransferase 1 (ACAT1) in GBM tumor cells [26], may prevent these ketone bodies from serving as an alternative energy source or providing essential metabolites required for tumor growth. Furthermore, it is possible that tumors have upregulated monocarboxylate transporters to expel excess lactate, which may explain the increased uptake of ketones as well [27]. Further studies are required to dissect out this phenomenon.
In conclusion, our results show that ketone diester supplemented low glucose ADT reduces overall tumor burden and increases survival to a similar extent as the high-fat KD but with better body weight preservation in a clinically relevant human GBM animal model. Therefore, we propose KE could be utilized in the management of GBM with less restriction on patient diet. Research is needed to explore the feasibility and efficacy of this KE supplemented less rigorous ATD in other cancers, particularly those in which loss of fat and muscle mass is associated with generally poorer outcomes.
Notes
References
1. Gallia GL, Brem S, Brem H. 2005; Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 3:721–8. DOI: 10.6004/jnccn.2005.0042. PMID: 16194460.

2. Hau P, Baumgart U, Pfeifer K, Bock A, Jauch T, Dietrich J, Fabel K, Grauer O, Wismeth C, Klinkhammer-Schalke M, Allgäuer M, Schuierer G, Koch H, Schlaier J, Ulrich W, Brawanski A, Bogdahn U, Steinbrecher A. 2003; Salvage therapy in patients with glioblastoma: is there any benefit? Cancer. 98:2678–86. DOI: 10.1002/cncr.11845. PMID: 14669289.
3. Arismendi-Morillo GJ, Castellano-Ramirez AV. 2008; Ultrastructural mitochondrial pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies. J Electron Microsc (Tokyo). 57:33–9. DOI: 10.1093/jmicro/dfm038. PMID: 18230641.

4. Chinopoulos C, Seyfried TN. 2018; Mitochondrial substrate-level phosphorylation as energy source for glioblastoma: review and hypothesis. ASN Neuro. 10:1759091418818261. DOI: 10.1177/1759091418818261. PMID: 30909720. PMCID: PMC6311572.

5. Hoang-Minh LB, Siebzehnrubl FA, Yang C, Suzuki-Hatano S, Dajac K, Loche T, Andrews N, Schmoll Massari M, Patel J, Amin K, Vuong A, Jimenez-Pascual A, Kubilis P, Garrett TJ, Moneypenny C, Pacak CA, Huang J, Sayour EJ, Mitchell DA, Sarkisian MR, Reynolds BA, Deleyrolle LP. 2018; Infiltrative and drug-resistant slow-cycling cells support metabolic heterogeneity in glioblastoma. EMBO J. 37:e98772. DOI: 10.15252/embj.201798772. PMID: 30322894. PMCID: PMC6276884.

6. Mukherjee P, Augur ZM, Li M, Hill C, Greenwood B, Domin MA, Kondakci G, Narain NR, Kiebish MA, Bronson RT, Arismendi-Morillo G, Chinopoulos C, Seyfried TN. 2019; Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol. 2:200. DOI: 10.1038/s42003-019-0455-x. PMID: 31149644. PMCID: PMC6541653.

7. Vallejo FA, Shah SS, de Cordoba N, Walters WM, Prince J, Khatib Z, Komotar RJ, Vanni S, Graham RM. 2020; The contribution of ketone bodies to glycolytic inhibition for the treatment of adult and pediatric glioblastoma. J Neurooncol. 147:317–26. DOI: 10.1007/s11060-020-03431-w. PMID: 32096068.

8. Badr CE, Silver DJ, Siebzehnrubl FA, Deleyrolle LP. 2020; Metabolic heterogeneity and adaptability in brain tumors. Cell Mol Life Sci. 77:5101–19. DOI: 10.1007/s00018-020-03569-w. PMID: 32506168. PMCID: PMC8272080.

9. Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H, Olson S, Gabrielli B, Osborne G, Vescovi A, Reynolds BA. 2011; Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain. 134(Pt 5):1331–43. DOI: 10.1093/brain/awr081. PMID: 21515906. PMCID: PMC3097894.

10. Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH. 2013; Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals (Basel). 6:1475–506. DOI: 10.3390/ph6121475. PMID: 24287492. PMCID: PMC3873674.

11. Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. 2016; Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 16:310. DOI: 10.1186/s12885-016-2337-7. PMID: 27178315. PMCID: PMC4866042.

12. Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, Griffith BG, Skinner CL, Suslov O, Deleyrolle LP, Reynolds BA. 2016; A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Cancer Res. 22:2482–95. DOI: 10.1158/1078-0432.CCR-15-0916. PMID: 26631612.

13. Poff AM, Ari C, Arnold P, Seyfried TN, D'Agostino DP. 2014; Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 135:1711–20. DOI: 10.1002/ijc.28809. PMID: 24615175. PMCID: PMC4235292.

14. Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. 2010; The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond). 7:74. DOI: 10.1186/1743-7075-7-74. PMID: 20831808. PMCID: PMC2949862.

15. Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kämmerer U, Coy JF, Weller M, Steinbach JP. 2014; ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 44:1843–52. Erratum in: Int J Oncol 2014;45: 2605. DOI: 10.3892/ijo.2014.2382. PMID: 24728273. PMCID: PMC4063533.

16. Rahman M, Azari H, Deleyrolle L, Millette S, Zeng H, Reynolds BA. 2013; Controlling tumor invasion: bevacizumab and BMP4 for glioblastoma. Future Oncol. 9:1389–96. DOI: 10.2217/fon.13.96. PMID: 23980685.

17. D'Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB. 2013; Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 304:R829–36. DOI: 10.1152/ajpregu.00506.2012. PMID: 23552496.
18. Akazawa K, Nakamura T, Palesch Y. 1997; Power of logrank test and Cox regression model in clinical trials with heterogeneous samples. Stat Med. 16:583–97. DOI: 10.1002/(SICI)1097-0258(19970315)16:5<583::AID-SIM433>3.0.CO;2-Z.

19. Ye F, Li XJ, Jiang WL, Sun HB, Liu J. 2015; Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol. 11:26–31. DOI: 10.3988/jcn.2015.11.1.26. PMID: 25628734. PMCID: PMC4302176.

20. Nakamura K, Tonouchi H, Sasayama A, Ashida K. 2018; A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients. 10:206. DOI: 10.3390/nu10020206. PMID: 29443873. PMCID: PMC5852782.

21. Kashiwaya Y, Pawlosky R, Markis W, King MT, Bergman C, Srivastava S, Murray A, Clarke K, Veech RL. 2010; A ketone ester diet increases brain malonyl-CoA and Uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar Rat. J Biol Chem. 285:25950–6. DOI: 10.1074/jbc.M110.138198. PMID: 20529850. PMCID: PMC2923987.

22. Karrison TG. 2016; Versatile tests for comparing survival curves based on weighted log-rank statistics. Stata J. 16:678–90. DOI: 10.1177/1536867X1601600308.

23. Knezevic A, Patil S. Combination weighted log-rank tests for survival analysis with non-proportional hazards. In : SAS Global Forum; 2020.
24. Lin RS, Lin J, Roychoudhury S, Anderson KM, Hu T, Huang B, Leon LF, Liao JJZ, Liu R, Luo X, Mukhopadhyay P, Qin R, Tatsuoka K, Wang X, Wang Y, Zhu J, Chen TT, Iacona R. 2020; Alternative analysis methods for time to event endpoints under nonproportional hazards: a comparative analysis. Stat Biopharm Res. 12:187–98. DOI: 10.1080/19466315.2019.1697738.

25. Seyfried TN, Arismendi-Morillo G, Mukherjee P, Chinopoulos C. 2020; On the origin of ATP synthesis in cancer. iScience. 23:101761. DOI: 10.1016/j.isci.2020.101761. PMID: 33251492. PMCID: PMC7677709.

26. Chang HT, Olson LK, Schwartz KA. 2013; Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 10:47. DOI: 10.1186/1743-7075-10-47. PMID: 23829383. PMCID: PMC3707813.

27. Park SJ, Smith CP, Wilbur RR, Cain CP, Kallu SR, Valasapalli S, Sahoo A, Guda MR, Tsung AJ, Velpula KK. 2018; An overview of MCT1 and MCT4 in GBM: small molecule transporters with large implications. Am J Cancer Res. 8:1967–76.
Fig. 1
Weight change, blood glucose, and ketone levels in NOD/SCID mice treated with different dietary interventions. (A) Body weight was presented as a percent of weight at the start of dietary intervention. Animals in KD and KE supplemented diet groups lost weight in the first week. KD remained unchanged over the rest of the study period but STD+KE and ATD+KE animals reached a plateau after decreasing KE from 20% to 10% in their food. (B, C) Three weeks after putting animals on the diets, the average blood glucose and β-hydroxybutyrate (β-HB) levels were measured over a course of 12 hours. (D) Blood acetoacetate (AcAc) was also measured only at the 12-hour time-point. Animals on the KD and ATD+KE had significantly lower glucose levels compared to the STD (control diet). Although the highest levels of β-HB were observed in the KD treated animals, the β-HB levels in the ATD+KE and STD+KE animals were significantly higher than the STD alone group. Additionally, AcAc in KD and ATD+KE and STD+KE were significantly higher than the STD alone group (*P<0.05, **P<0.0021, ***P<0.0002, ****P<0.0001; one-way ANOVA). KD, ketogenic diet; STD, standard diet; ATD, Atkins-type diet; KE, ketone ester.
Fig. 2
Weight change, blood glucose, and ketone levels in brain tumor bearing NOD/SCID mice under STD, ATD+KE, and KD dietary interventions. (A) Body weight was presented as a percent of weight at the start of dietary intervention. Compared to STD, no statistically significant changes occurred in animals’ body weight. (B) Both the KD and ATD+KE had significantly lower glucose compared to the STD group, and also glucose level in the KD group was significantly lower than the ATD+KE. (C) β-hydroxybutyrate (β-HB) was significantly higher in the KD as compared to the STD and ATD+KE groups. Moreover, β-HB level in ATD+KE was also significantly elevated relative to the STD alone group (**P<0.0021, ****P<0.0001; one-way ANOVA). KD, ketogenic diet; STD, standard diet; ATD, Atkins-type diet; KE, ketone ester.
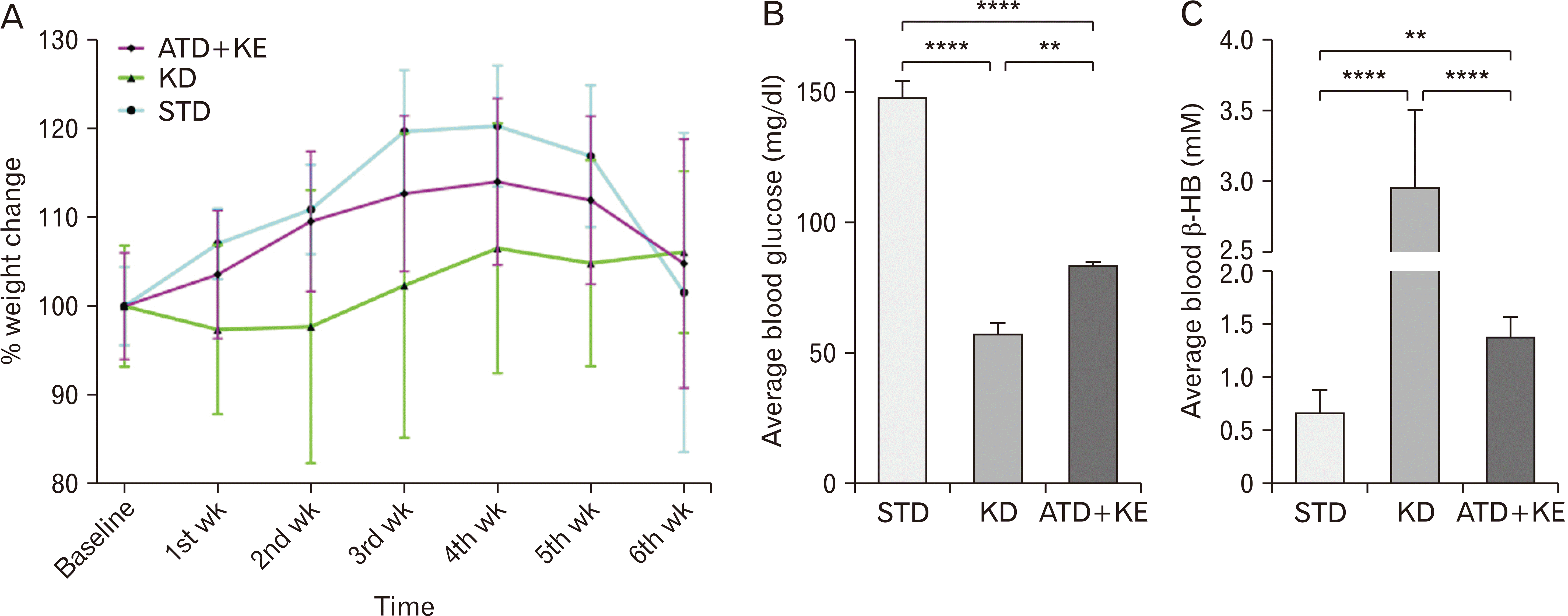
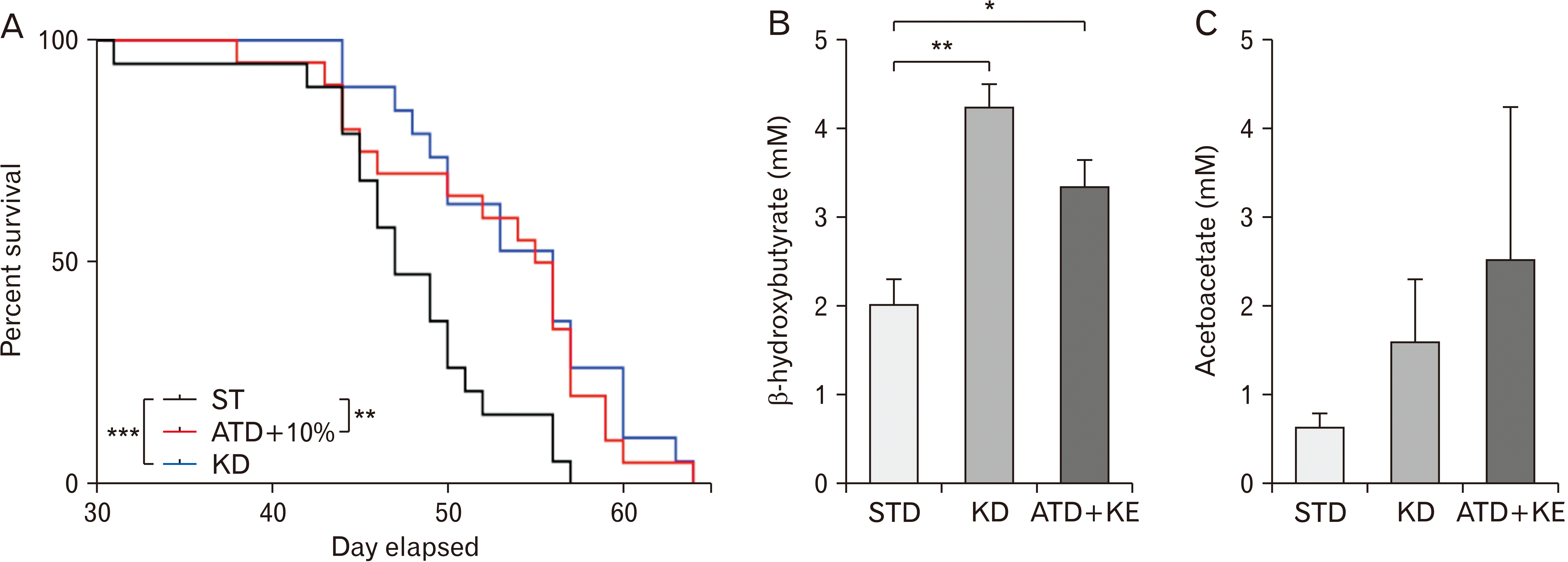
Fig. 3
(A) Kaplan–Meier survival curve with stratified Max-Combo test demonstrated a significant survival benefit of both KD (***P=0.0003) and ATD+KE (**P=0.002) dietary interventions compared to STD in L0 tumor bearing NOD/SCID mice. (B) Measurements of β-hydroxybutyrate (β-HB) from tumor tissue demonstrated a significant increase of intra-tumoral β-HB under both dietary interventions. (C) Intra-tumoral AcAc levels were elevated but were not significantly different when compared to controls (*P<0.05, **P<0.0021; one-way ANOVA). KD, ketogenic diet; STD, standard diet; ATD, Atkins-type diet; KE, ketone ester; AcAc, acetoacetate.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download