This article has been corrected. See "Inhibitory effect of temozolomide on apoptosis induction of cinnamaldehyde in human glioblastoma multiforme T98G cell line" in Volume 57 on page 332.
Abstract
Glioblastoma is the most common primary malignant brain tumor in adults. Temozolomide (TMZ) is an FDA-approved drug used to treat this type of cancer. Cinnamaldehyde (CIN) is a derivative of cinnamon extract and makes up 99% of it. The aim of this study was to investigate the in vitro combined effect of CIN and TMZ on human glioblastoma multiforme T98G cell line viability. In this study, we used 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl-tertazolium bromide (MTT) method to evaluate the extent of IC50, acridine orange, Giemsa and Hoechst staining to evaluate the manner of apoptosis and the Western blotting method to examine the expression change of apoptotic proteins. Our results show that TMZ has an inhibitory effect on CIN when both used in combination at concentrations of 300 and 100 μM (P<0.05) and has a cytotoxic effect when used alone at the same concentrations (P<0.05). The western blotting result showed that TMZ at concentrations of 2,000 and 1,000 μM significantly increased Bax expression and decreased Bcl2 expression (P<0.05), indicating that TMZ induced apoptosis through the mitochondrial pathway. However, CIN had no effect on Bax and Bcl2 expressions, thus causing apoptosis from another pathway. Also, the Bax:Bcl2 expression ratio at concentrations combined was lower than that for TMZ 1,000 μM and higher than that for CIN 150 and 100 μM (P<0.05), which confirms the inhibitory effect of TMZ on CIN. From the present study, we conclude that TMZ in combination with CIN has an inhibitory effect on increasing the cytotoxicity rate.
Glioblastoma multiforme (GBM), is the most common, aggressive, and deadly form of brain tumor, accounting for approximately 15% of all primary brain and central nervous system (CNS) tumors and 50% of all malignant primary brain and CNS tumors [1, 2]. The 5-year survival rate for GBM is limited to 5% [3]. Standard treatment includes the most reliable surgical resection followed by radiotherapy plus simultaneous and adjuvant chemotherapy with an alkylating agent such as temozolomide (TMZ) [4, 5].
TMZ has a lipophilic nature, which allows it to penetrate the blood-brain barrier and be administered orally [6]. TMZ as a DNA alkylating agent induces cell cycle arrest in phase G2/M and eventually initiates apoptosis [7]. TMZ causes cytotoxicity by adding methyl groups to N7 and O6 sites on guanines and the O3 site on adenines in genomic DNA [6]. However, the development of TMZ resistance in the treatment of GBM has adverse effects and leads to a poor prognosis [8, 9]. O6-methyl-guanine DNA methyl transferase (MGMT) is reported to act by removing alkyl groups from DNA and converting them into an internal cysteine residue [10]. Preclinical and clinical studies have concluded that MGMT is responsible for reducing TMZ-mediated GBM cell mortality [11, 12]. The studies indicated that several cell lines have been identified as TMZ sensitive or TMZ resistant, including T98G, which is one of the TMZ resistant GBM cell lines [6].
Cancer treatment has always been one of the most complex processes in medical science. Scientists have always sought treatment with better efficacy and fewer side effects. Because a number of GBM cell lines such as the T98G cell line are resistant to TMZ, we decided to evaluate the cell viability by combining herbal medicines such as cinnamaldehyde (CIN).
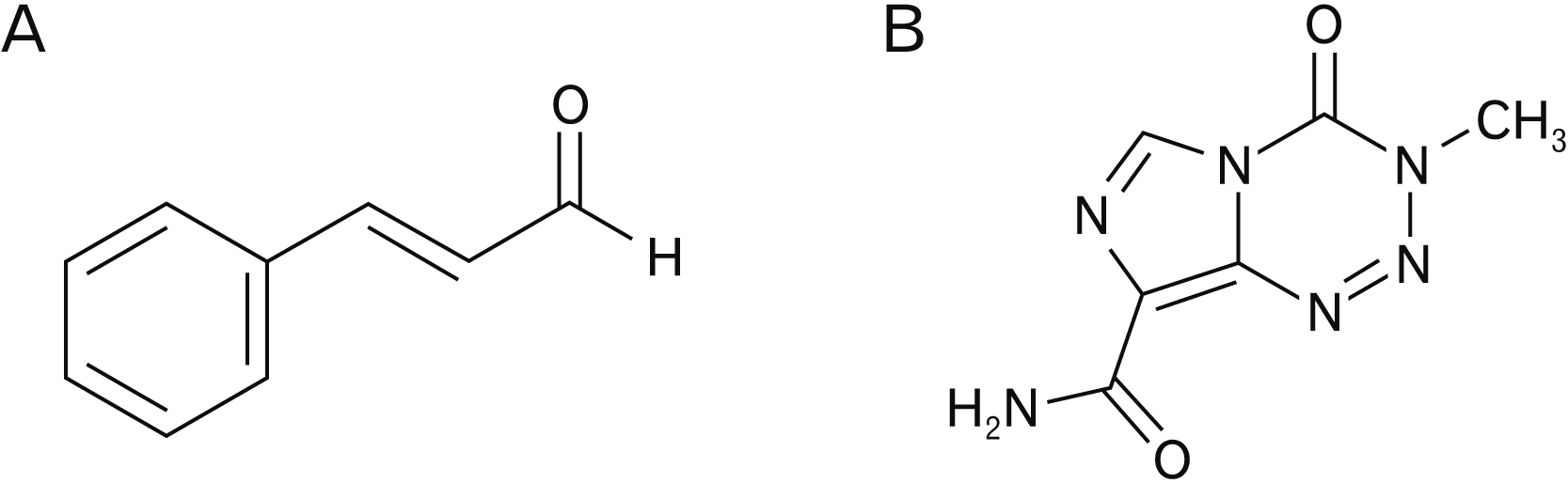
Trans-cinnamaldehyde (TCA) (Fig. 1A), also known as CIN, is an active compound derived from the stem bark of the cinnamon tree. Overall, about 250 species of cinnamon have been identified from trees spread all around the world [13, 14]. CIN is widely used for adding flavor in foods such as beverages, ice cream, sweets, and chewing gum. A number of biological activities have been reported for CIN such as peripheral vasodilatory, antitumor, antifungal, cytotoxic, and mutagenic/antimutagenic activities [15-17]. Recent documented data showed TCA could induce the apoptosis of several human tumor cell lines, including human promyelocytic leukemia HL-60 cells [18, 19]. Recent studies showed that CIN induces apoptosis in a p53-dependent manner via activation of p53 and CD95 (APO-1) signaling pathways in human hepatic HepG2 cells. Furthermore, the p53 transcriptional inhibitor, pifithrin-α, diminished the effect of CIN-induced apoptosis via upregulation of the anti-apoptotic factor Bcl-XL and inhibition of the pro-apoptotic factor Bax, suggesting that p53 transcriptional activity is essential for CIN induced apoptosis, at least in a HepG2 cellular model [20-24]. However, the mechanism of how CIN induces apoptosis in cancer cells is presently unclear.
In the present study, our aim was to evaluate the effect of CIN and TMZ alone and in combination on the induction of apoptosis and autophagy in the human GBM T98G cell line. The cell viability, the morphology of apoptotic cell, and DNA fragmentation were investigated as well as Bax and Bcl2 protein expression.
Dulbecco’s modified Eagle’s medium (DMEM), dimethyl sulfoxide (DMSO), 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl-tertazolium bromide (MTT), penicillin-streptomycin, trypsin-ethylene diamine tetra acetic acid (EDTA), and fetal bovine serum (FBS) were obtained from Bio Idea (IRI). CIN and TMZ were obtained from Sigma-Aldrich. Phosphate buffer saline (PBS) was obtained from Sigma-Aldrich. Acridine orange dye was obtained from Merck. Giemsa dye solution and Hoechst 33342 were obtained from Invitrogen. Anti-Bax, anti-Bcl2, anti-β-actin antibody, and goat anti-mouse horseradish peroxidase antibody were obtained from RF-Chem Company.
The human GBM T98G cell line was obtained from the Pasteur Institute. Cells were grown in DMEM medium supplemented with 10% FBS and 1% penicillin and streptomycin. They were maintained at 37°C in a humidified atmosphere of 5% CO2.
The CIN stock solution was prepared in DMSO at a concentration of 500 mM and was stored at –20°C until use. The TMZ stock solution was prepared in DMSO at a concentration of 1 M and was stored at –20°C until use. The final concentration of DMSO in this study did not exceed 0.2%.
MTT assay was used to determine cell viability in this study. Briefly, the cells (1×104) were seeded in each well containing 100 μl of the DMEM medium supplemented with 10% FBS in a 96-well plate. After 24 hours, CIN at increasing concentrations from 0 to 1,000 μM and TMZ at increasing concentrations from 0 to 2,000 μM were added, and the solvent group was incubated with 0.2% DMSO. Also, to evaluate the combined effect of these two drugs, constant concentrations of CIN (300, 150, and 100 μM) were combined with increasing concentrations of TMZ (0–2,000 μM) and added to the culture medium. After 48 hours, 10 µl of MTT (5 mg/ml stock solution) was added in each well (0.05 mg/well), and the plates were incubated for an additional 4 hours. The medium was then discarded and the formazan blue, which formed in the cells, was dissolved with 100 µl DMSO/well. The optical density was measured at 490 nm. Cell viability rate was calculated using the following formula:
(ODExperiment/ODControl) ×100.
This test was performed three times for each drug and also to evaluate their combined effect.
Autophagy is the process of sequestrating cytoplasmic proteins into the lytic component and is characterized by the formation and promotion of acidic vesicular organelles (AVOs). In acridine orange-stained cells, the cytoplasm and nucleolus fluoresce bright green and dim red, whereas acidic compartments fluoresce bright red. To detect the development of AVOs, we treated tumor cells as described above and then performed vital staining with acridine orange [20, 21]. Briefly, the treated tumor cells (104 cells/well in 96-well plates) were stained with acridine orange (1 µg/ml) for 15 minutes [22]. Samples were then examined under a fluorescence microscope (Olympus) and photographed at ×20 magnifications under a red filter. The staining was repeated three times, and in each repetition, at least five fields were photographed.
In this study, Hoechst staining was used to detect nuclear fragmentation, so that fragmented nuclei and DNA are seen in bright blue and normal nuclei are seen in dark blue. T98G cells were fixed with cool methanol for 15 minutes after treatment at room temperature and washed once with PBS. Cells were incubated with Hoechst 33342 stock solution (1:100 in PBS) for 20 minutes at room temperature and then washed with PBS. Apoptotic cells were identified by the condensation and fragmentation of their nuclei. Samples were examined under a fluorescence microscope and photographed at ×20 magnifications under a blue filter. The staining was repeated three times, and in each repetition, at least five fields were photographed [22].
Apoptosis morphology by Giemsa staining encompasses small, shrinkage, and pyknotic nuclei, membrane blebbing, and formation of apoptotic bodies. After treatment, cells were fixed by cool methanol for 15 minutes at room temperature and washed once with PBS. Cells were incubated with Giemsa staining stock solution (1:10 in PBS) for 30 minutes at room temperature and then washed with PBS until the excess dye came out. Samples were then examined under a light microscope (Olympus) and photographed at ×20 magnifications. The staining was repeated three times, and at each repetition, at least five fields were photographed [23].
After the treatments described above, cells were harvested and washed with PBS, and then resuspended in a RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM sodium chloride, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS], ddH2O and protease inhibitor [Roche] added fresh). After incubation on ice for 30 minutes, the lysates were centrifuged at 12,000 g for 20 minutes, the supernatants were collected, and protein concentrations were determined by Nano drop (Thermo Fisher Scientific). Equal amounts of proteins were separated by 12% SDS-polyacrylamide gel electrophoresis, which were subsequently transferred onto a polyvinylidene fluoride membrane (Merck) using a semi-dry method. After transfer, the membrane was blocked with 5% non-fat skimmed milk in Tris-buffered saline with Tween-20 (TBST) containing 0.05% Tween-20 for 1 hour and subsequently incubated overnight with the following antibodies: anti-β-actin 1:500, anti-Bcl2 1:1,000, and anti-Bax 1:300 (RF-Chem). After incubation with primary antibody, the membranes were washed three times for 10 minutes with TBST, then incubated for 90 minutes with a 1:5,000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgG (RF-Chem), and then washed four times for 10 minutes with TBST. Finally, protein bands appeared on the radiological film done using an excellent chemiluminescent substrate (ECL) kit, and then densitometered using the Image J software.
All quantitative data are represented as mean±SD. The normal distribution of data was measured using the Shapiro–Wilk test. IC50 (half maximal inhibitory concentration) was analyzed and calculated in the GraphPad Prism v.8 software (GraphPad). All other quantitative variables were analyzed using one-way ANOVA and Tukey’s post-hoc test. Differences were considered statistically significant at P< 0.05.
In this study, an MTT test was used to obtain IC50 of CIN and TMZ. The IC50 of CIN after 48 hours of cell treatment was estimated to be 211.96±14.82 μM (Fig. 2). Also, the cell viability rate for TMZ at a concentration of 2,000 μM was 55.94%.
In order to investigate the combined effect of CIN on TMZ, constant concentrations of CIN in combination with variable concentrations of TMZ were applied to glioblastoma cells for 48 hours. This test was performed three times with concentrations of 300, 150, and 100 μM of CIN in combination with concentrations of 25, 50, 100, 250, 500, 1,000, and 2,000 μM of TMZ. The reason for choosing these concentrations was the obtained IC50 of CIN, which we considered higher and lower concentrations. The average viability rate in each combined group (T+C) and single concentrations of TMZ and CIN were obtained. Then, in order to investigate the combined effect of two drugs, it was necessary to obtain the amount of cell cytotoxicity in each concentration. For this purpose, we subtract the cell viability rate in each concentration from 100 to obtain the cytotoxicity rate, and then we sum the cytotoxicity rate in a single TMZ concentration and a single CIN concentration together and compare the obtained number with the cytotoxicity rate in the same combined concentration. If the cytotoxicity rate in the combined group is greater than the sum of cytotoxicity in single concentrations, the effect of two drugs is synergistic, and if it is less, it is an inhibitory effect.
Combining CIN at a constant concentration of 300 μM with variable concentrations of TMZ (0, 25, 50, 100, 250, 500, 1,000, and 2,000 μM) (Fig. 3), we found that there was a significant difference at P≤0.0001 between the cytotoxicity induced by the concentrations of TMZ 2,000 μM (44.05%) and CIN 300 μM (75.35%) separately and the cytotoxicity induced at the combined concentration of T2000+C300 (98.30%), which indicates the inhibitory effect of the two drugs. Also, the cytotoxicity of total concentrations of TMZ 100 μM (0%) with CIN 300 μM (75.35%) was statistically significantly different from the cytotoxicity at the combined concentration of T100+C300 (66.22%) (P=0.01). Also, the total cytotoxicity at TMZ 50 μM (0%) and 25 μM (0%) with CIN 300 μM (75.35%) was significantly different at P=0.004 and P=0.001, respectively, from that of the combined concentrations (64.90% and 63.20%), which indicates the inhibitory effect of the two drugs on each other. Comparing the cell viability rate at single concentrations of CIN 300 μM and TMZ with that at combined concentrations, we found that the rate of cell viability in the combined group T2000+C300 (1.69%) was significantly different from the two groups TMZ2000 (55.94%) and CIN300 (24.64%) when considered individually at P≤0.0001. The same relationship exists between T1000+C300 (6.20%) and TMZ1000 (80.02%) and CIN300. Although the viability rate in this combined group is statistically significantly different from the viability rates at the same concentrations of TMZ and CIN when considered individually, according to Fig. 3A, it cannot be said that these two drugs have a synergistic effect, because the cytotoxicity rate in the combination group is less than the sum of cytotoxicity induced at the same concentrations considered individually. The viability rate of T500+C300 (20.50%) was significantly different from that of TMZ500 (100%) at P≤0.0001, but not significantly different from that of CIN300. The same relationship exists between T250+C300 (26.01%) and TMZ250 (100%) and CIN300 considered individually. There is a significant difference in cell viability between T100+C300 (33.77%) and TMZ100 (100%) at P≤0.0001 and CIN300 at P=0.02, which indicates the inhibitory effect of TMZ on CIN. There is a significant difference in cell viability between T50+CIN300 (35.09%) and TMZ50 (100%) at P≤0.0001 and CIN300 at P=0.008, which indicates the inhibitory effect of TMZ on CIN. There was a significant difference in cell viability between T25+CIN300 (36.79%) and TMZ 250 (100%) at P≤0.0001 and CIN300 at P=0.002, which confirms the inhibitory effect of TMZ on CIN.
In the second experiment, T98G cells were treated at a combined concentration of TMZ and CIN 150 μM (Fig. 4). The results of this test showed that, in general, the cytotoxicity rate of the total concentrations of TMZ and CIN 150 μM is higher than the cytotoxicity rate in the combined concentrations of T+C150, but there is no statistically significant difference. There was a significant difference in cell viability rate in T2000+C150 (37.47%) compared to TMZ 2,000 μM (55.94%) and CIN 150 μM (72.68%) (P≤0.0001). The same relationship existed between T1000+C150 (48.79%) and TMZ 1,000 μM (80.02%) and CIN 150 μM. However, according to Fig. 4A, it cannot be said that these two drugs have a synergistic effect, because the cytotoxicity rate in these combined groups except T1000+C150 is less than the total cytotoxicity at the same individual concentrations. Although the cytotoxicity rate in the combined group of T1000+C150 is more than the cytotoxicity rate in the total concentrations of TMZ 1,000 μM and CIN 150 μM (Fig. 4A), this difference is not statistically significant. Thus, it cannot be said with certainty that these two drugs in combination have a synergistic effect. Cell viability rate was significantly different between the next combined groups from TMZ 500 μM (67.10%) to TMZ 25 μM (73.03%) and only TMZ (100%) (P≤0.0001) and cell viability in these combined groups was not significantly different from CIN 150 μM (72.68%).
In the third experiment, the cells were treated at a combined concentration of TMZ and CIN 100 μM (Fig. 5), and the results showed that the total cytotoxicity rate of individual concentrations of TMZ 2,000, 1,000, and 500 μM with CIN 100 μM was higher compared to the cytotoxicity at the same concentrations in combination, but there was no significant difference. The total cell cytotoxicity at individual concentrations of TMZ 250 μM with CIN 100 μM was significantly different from that at the same concentrations when combined at P≤0.0001, which indicates the inhibitory effect of TMZ on CIN. The same relationship was found when the total cell cytotoxicity at individual concentrations of TMZ 100 μM with CIN 100 μM was compared to that found at the same concentrations combined at P≤0.0001. Also, the total cytotoxicity at individual concentrations of TMZ 50 μM and 25 μM with CIN 100 μM was significantly different from that found at the same concentrations combined at P=0.002 and P=0.001, respectively, which confirms the inhibitory effect of TMZ on CIN. On the other hand, the cell viability rate in the T2000+C100 group (44.15%) was significantly different compared to that of TMZ 2,000 μM (55.94%) at P=0.001 and with CIN 100 μM (82.38%) at P≤0.0001. The viability rate in the T1000+C100 group (63.51%) was significantly different from that in TMZ 1,000 μM (80.02%) and CIN 100 μM at P≤0.0001. The viability rate in the T500+C100 group (86.60%) was significantly different from that in TMZ 500 μM (100%) at P≤0.0001 but not significantly different from that in CIN 100 μM. The cell viability in the two groups of T250+C100 (95.77%) and T100+C100 (98.42%) was not significantly different from that in TMZ 250 μM (100%) and TMZ 100 μM (100%), but was significantly different from that in CIN 100 μM at P≤0.0001. Also, the cell viability rate in the two groups of T50+C100 (92.18%) and T25+C100 (92.60%) was significantly different from that in TMZ 50 μM (100%) at P=0.004 and in TMZ 25 μM (100%) at P=0.008, and also from that in CIN100 at P≤0.0001. It should be noted that the difference in cell viability between the combined groups from TMZ 250 μM to TMZ 25 μM and CIN 100 μM confirms the inhibitory effect of TMZ on CIN.
In this staining, cells with autophagosomes (AVOs) were considered as autophagic cells and cells without autophagosomes were considered as normal cells. Bright red to orange spots in the cytoplasm of cells indicate the presence of AVOs and autophagy in them (Fig. 6A).
In Hoechst staining, bright blue nuclei were considered as apoptotic cells and dark blue nuclei were considered as normal cells. Fragmented nuclei and DNA fragments inside the nucleus appear bright blue, and apoptotic bodies can also be seen cumulatively from bright blue fragments between nuclei (Fig. 6C).
Cells with shrunken, pyknotic, and small nuclei with reduced cytoplasm, and in some cases with membrane blebbing as well as apoptotic bodies, were considered as apoptotic cells and cells without these characteristics were considered as normal cells (Fig. 6B). Finally, we observed that because in Giemsa staining only the morphology of the cell can be examined and the intracellular events of apoptosis are not visible and the cells are still in the early stages of apoptosis, we cannot see the characteristics of apoptosis well in this staining.
Western blot results showed that the amount of Bax expression in TMZ-treated cells (P≤0.0001) and in the T1000+C150 group (P=0.001) was significantly higher than that in the control and solvent groups (Fig. 7B), and also the amount of Bcl2 protein in TMZ-treated groups was significantly lower than that in the solvent and control groups (P=0.005) (Fig. 7C). The Bax:Bcl2 ratio in the groups treated with TMZ was significantly higher than the ratio in the control and solvent groups (P≤0.0001) (Fig. 7D). However, the amount of Bax and Bcl2 expression in the groups treated with CIN was not significantly different from that in the control and solvent groups (Fig. 7B, C). Also, the Bax:Bcl2 ratio in the groups treated with CIN was not significantly different from that in the control and solvent groups (Fig. 7D). However, the Bax:Bcl2 ratio in the T1000+C150 group was significantly higher than that in the control and solvent groups (P=0.001). Also, the Bax:Bcl2 ratio in T1000+C150 μM was significantly higher than that in CIN 150 μM (P=0.003). However, the Bax:Bcl2 ratio in the two combined groups was not higher than that in TMZ 1,000 μM (in fact, was significantly lower), which indicates the inhibitory effect of TMZ on CIN. The Bax:Bcl2 expression ratio of in the combined group T1000+C100 was slightly higher compared to that in CIN 100 μM, but there was no significant difference, and the same ratio in this combined group was much lower than that in TMZ 1,000 μM and had a significant difference at P≤0.0001, which confirms the inhibitory effect of TMZ on CIN (Fig. 7D).
Among primary brain tumors, GBM is the most common, malignant, and invasive brain tumor known to date. Our results show that [1] both CIN and TMZ induce apoptosis and autophagy in the T98G cell line; [2] TMZ in combination with CIN inhibits CIN and reduces its cytotoxicity; and [3] TMZ induces apoptosis via the mitochondrial pathway, while CIN has no effect on increasing Bax and decreasing Bcl2 expressions.
Initially, we needed a solvent to dissolve both drugs in the culture medium. For this purpose, we used DMSO, and during several tests, we found that the amount of DMSO more than 0.2% is toxic to the cell. On the other hand, TMZ did not have more than 1 M solubility in DMSO and the maximum concentration that could be used was 2,000 μM, and if we used the concentration higher than this amount, the amount of DMSO would exceed 0.2%.
The MTT method was used to obtain the IC50 of TMZ, and finally, we did not report the IC50 for TMZ because we could not increase the concentration of TMZ to more than 2,000 μM. Because according to the data obtained from Prism software, the IC50 value of TMZ was more than 2,000 μM and the cell viability rate at this concentration did not reach zero, we can only say that the cell viability rate at a concentration of 2,000 μM TMZ is 55.94% on average.
In a study conducted on TMZ resistance in human GBM cells, the author summarized a number of studies conducted on TMZ and the IC50 range for this drug in the T98G cell line was more than 250 μM to 1,585 µM is mentioned [6].
In another study that investigated the cytotoxic effect of TMZ on the T98G cell line, the IC50 of TMZ after 4 days of cell treatment by the MTT method was reported as 1,446 µM [25].
In another study that investigated the role of autophagy in TMZ-induced cytotoxicity in some glioma cell lines, the IC50 level in six cell lines (U373-MG, U-251MG, GB-1, U87-MG, and A172) was reported as less than 200 µM and on the other hand, the IC50 of TMZ in T98G cell line was reported as more than 1,000 μM due to the resistance of this line to TMZ. The method of calculating the viability rate in this study was cell counting, and the cells were treated with TMZ at concentrations of 5–1,000 µM for 72 hours [21].
In a 2020 study, Chen et al. [26] examined the effect of CIN and TMZ on the viability and expression of the chemokine receptor gene in the T98G cell line. MTT results showed that the cell viability rate in the combined group of TMZ 300+CIN 75 μM was significantly lower compared to that in TMZ 300 μM, but the difference was not statistically significant. Also, the combined group of TMZ300+CIN50 μM did not differ significantly in cell viability compared to that TMZ 300 and CIN 50 μM when considered individually. Western blot results in this study showed that CIN in response to TMZ reduced cell viability possibly by reducing the Cxcr4 expression [26].
In a study that examined the synergistic effect of TMZ and quercetin on the induction of apoptosis in the T98G cell line, it was found that TMZ in combination with quercetin had a synergistic effect and increased apoptosis within 24 to 48 hours in the T98G cell line [22].
In this study, for the molecular evaluation of apoptosis, we used the western blotting method to study changes in Bcl2 and Bax protein expression. We found that TMZ alone significantly increased the Bax:Bcl2 ratio in the treated groups compared with the control and solvent groups. However, in the CIN groups, this increase was not present, and in fact, Bax expression was not significantly different from that of Bcl2 in these groups compared to the control and solvent groups. Two reasons can be attributed to this result. First, as mentioned, 25 members of the Bcl2 protein family have been identified so far, and it is possible that CIN induces apoptosis through other members of this family except Bax and Bcl2. The second reason is that CIN may not induce apoptosis at all from the mitochondrial pathway; rather, it may cause apoptosis in the cell through the extrinsic pathway and activation of cell death receptors, which may explain the inhibitory effect of TMZ on CIN.
In this regard, a study by Wu et al. [27] concluded that CIN decreased Bcl-2 and Mcl-1 expression and increased Bax expression in PLC/PRF/5 cells (human hepatoma). It also activates caspase-8 and cleaved bid. In addition, they found that CIN activates and phosphorylates JNK, ERK, and P38 kinase in cells, which activates apoptosis via the mitogen-activated protein kinases (MAPK) pathway [27]. CIN decreases Bcl-XL and increases Bax in human hepatoma cells. In contrast, pifithrin-α and MAPK-specific inhibitors prevent the induction of apoptosis by CIN in this cell line by inhibiting the breakdown of poly (ADP-ribose) polymerase (PARP) as well as inhibiting the phosphorylation of the JNK, P38, and ERK proteins [28].
In another study, the same team found that CIN induced apoptosis by releasing cytochrome C and Smac/DIABLO and HtrA2/Omi from the mitochondria to the cytosol and activating caspase-3. CIN also induces apoptosis by upregulating Bax protein expression and downregulating anti-apoptotic proteins such as Bcl-2 and the apoptosis inhibitor protein family (X-linked inhibitor of apoptosis protein [XIAP], cellular inhibitor of apoptosis protein [cIAP-1, cIAP-2]). Also, pretreatment with vitamin E markedly prevented CIN-mediated apoptosis, which was associated with the modulation of XIAP, cIAP-1, cIAP-2, Bcl-2, and Bax protein activity [29]. In another study, researchers found that CIN decreased Bcl-XL expression and increased CD95, P53, and Bax expression, as well as the PARP cleavage in human hepatoma cells [19]. Another study reported that CIN increased Fas expression (CD95) and decreased mitochondrial membrane potential in K562 cancer cells [30].
As explained in previous studies, TMZ is an aldehyde agent that causes DNA alkylation and, in fact, DNA damage in tumor cells, followed by activation of the P53 protein, which eventually leads the cell to apoptosis [6]. According to the above studies, CIN also causes DNA damage and activation of P53 protein by causing oxidative stress, and also there is evidence to support negative interactions between different forms of cell death [31, 32]. We can speculate that the combination of CIN and TMZ first activates P53. However, due to the inhibition of CIN by TMZ, this protein is seized by TMZ and the pathway of cell death goes to the intrinsic pathway and P53 protein induces apoptosis by increasing Bax expression. However, in the absence of TMZ, CIN increases CD95 expression by activating the p53 protein and activates the extrinsic pathway of apoptosis.
Another study in 2020 on the antagonistic effects of drug compounds on cell death kinetics addressed the issue of differences in the relative onset time of cell death. Thus, the difference in the relative onset time of cell death in the two drugs combined produces an antagonistic effect, meaning that the rapid-acting drug suppresses the slower drug [33].
Now, according to these explanations, we can propose another hypothesis for the inhibitory effect of TMZ on CIN. Considering that TMZ is a DNA alkylating agent and CIN may induce cell death through oxidative stress, we can hypothesize that TMZ acts faster than CIN and uses P53 proteins to its advantage, and this difference in the relative onset of cell death inhibits CIN. Of course, it should be noted that more studies are needed to confirm these hypotheses.
In addition, there are many studies that show that TMZ induces apoptosis through the mitochondrial pathway. For example, one study showed that TMZ in combination with honokiol reduced cell viability compared to concentrations of TMZ and honokiol considered individually. The combination of these two drugs also increases Bax expression compared to their concentrations considered individually in glioma cells [34]. In one study, researchers reported that TMZ increased apoptosis as well as the Bax:Bcl2 ratio in the T98G cell line. However, dexamethasone in combination with TMZ reduces the induction of apoptosis and the Bax:Bcl2 ratio compared to a concentration of TMZ alone in this cell line [35].
In conclusion, from the results of the present study, we suggest that CIN and TMZ have an inhibitory effect on the cell viability rate of the GBM T98G cell line. These two drugs also induce apoptosis and autophagy in this cell line. TMZ also induces apoptosis through the mitochondrial pathway by increasing the Bax:Bcl2 ratio compared to the control group. But, CIN has no effect on the Bax:Bcl2 expression ratio and may induce apoptosis in GBM cells through other proteins involved in the induction of apoptosis or through an extrinsic pathway.
Notes
References
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. 2018; CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 20(suppl_4):iv1–86. Erratum in: Neuro Oncol 2021;23:508-22. DOI: 10.1093/neuonc/noy131. PMID: 30445539. PMCID: PMC6129949.

2. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. 2017; Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 18:3–9.
3. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. 2014; CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 16(Suppl 4):iv1–63. DOI: 10.1093/neuonc/nou223. PMID: 25304271. PMCID: PMC4193675.

4. Preusser M, de Ribaupierre S, Wöhrer A, Erridge SC, Hegi M, Weller M, Stupp R. 2011; Current concepts and management of glioblastoma. Ann Neurol. 70:9–21. DOI: 10.1002/ana.22425. PMID: 21786296.

5. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. 2014; High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25 Suppl 3:iii93–101. DOI: 10.1093/annonc/mdu050. PMID: 24782454.

6. Lee SY. 2016; Temozolomide resistance in glioblastoma multiforme. Genes Dis. 3:198–210. DOI: 10.1016/j.gendis.2016.04.007. PMID: 30258889. PMCID: PMC6150109.

7. Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. 2007; Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 67:11499–504. DOI: 10.1158/0008-5472.CAN-07-5312. PMID: 18089777.

8. Ramirez YP, Mladek AC, Phillips RM, Gynther M, Rautio J, Ross AH, Wheelhouse RT, Sakaria JN. 2015; Evaluation of novel imidazotetrazine analogues designed to overcome temozolomide resistance and glioblastoma regrowth. Mol Cancer Ther. 14:111–9. DOI: 10.1158/1535-7163.MCT-14-0113. PMID: 25351918. PMCID: PMC4297195.
9. Park I, Mukherjee J, Ito M, Chaumeil MM, Jalbert LE, Gaensler K, Ronen SM, Nelson SJ, Pieper RO. 2014; Changes in pyruvate metabolism detected by magnetic resonance imaging are linked to DNA damage and serve as a sensor of temozolomide response in glioblastoma cells. Cancer Res. 74:7115–24. DOI: 10.1158/0008-5472.CAN-14-0849. PMID: 25320009. PMCID: PMC4253720.

10. Smalley S, Chalmers AJ, Morley SJ. 2014; mTOR inhibition and levels of the DNA repair protein MGMT in T98G glioblastoma cells. Mol Cancer. 13:144. DOI: 10.1186/1476-4598-13-144. PMID: 24909675. PMCID: PMC4061125.

11. Cen L, Carlson BL, Pokorny JL, Mladek AC, Grogan PT, Schroeder MA, Decker PA, Anderson SK, Giannini C, Wu W, Ballman KV, Kitange GJ, Sarkaria JN. 2013; Efficacy of protracted temozolomide dosing is limited in MGMT unmethylated GBM xenograft models. Neuro Oncol. 15:735–46. DOI: 10.1093/neuonc/not010. PMID: 23479134. PMCID: PMC3661094.

12. Melguizo C, Prados J, González B, Ortiz R, Concha A, Alvarez PJ, Madeddu R, Perazzoli G, Oliver JA, López R, Rodríguez-Serrano F, Aránega A. 2012; MGMT promoter methylation status and MGMT and CD133 immunohistochemical expression as prognostic markers in glioblastoma patients treated with temozolomide plus radiotherapy. J Transl Med. 10:250. DOI: 10.1186/1479-5876-10-250. PMID: 23245659. PMCID: PMC3551841.

13. Sangal A. 2011; Role of cinnamon as beneficial antidiabetic food adjunct: a review. Adv Appl Sci Res. 2:440–50.
14. Vangalapati M, Sree Satya N, Surya Prakash DV, Avanigadda S. 2012; A review on pharmacological activities and clinical effects of cinnamon species. Res J Pharm Biol Chem Sci. 3:653–63.
15. Koh WS, Yoon SY, Kwon BM, Jeong TC, Nam KS, Han MY. 1998; Cinnamaldehyde inhibits lymphocyte proliferation and modulates T-cell differentiation. Int J Immunopharmacol. 20:643–60. DOI: 10.1016/S0192-0561(98)00064-2. PMID: 9848396.

16. Kwon BM, Lee SH, Choi SU, Park SH, Lee CO, Cho YK, Sung ND, Bok SH. 1998; Synthesis and in vitro cytotoxicity of cinnamaldehydes to human solid tumor cells. Arch Pharm Res. 21:147–52. DOI: 10.1007/BF02974019. PMID: 9875422.
17. Shaughnessy DT, Setzer RW, DeMarini DM. The antimutagenic effect of vanillin and cinnamaldehyde on spontaneous mutation in Salmonella TA104 is due to a reduction in mutations at GC but not AT sites. Mutat Res. 2001; 480-481:55–69. DOI: 10.1016/S0027-5107(01)00169-5. PMID: 11506799.

18. Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, Lee KT. 2003; Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 196:143–52. DOI: 10.1016/S0304-3835(03)00238-6. PMID: 12860272.

19. Ng LT, Wu SJ. 2011; Antiproliferative activity of cinnamomum cassia constituents and effects of pifithrin-alpha on their apoptotic signaling pathways in Hep G2 cells. Evid Based Complement Alternat Med. 2011:492148. DOI: 10.1093/ecam/nep220. PMID: 20038571. PMCID: PMC3135661.
20. Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. 2003; Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 63:2103–8.
21. Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. 2004; Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 11:448–57. DOI: 10.1038/sj.cdd.4401359. PMID: 14713959.

22. Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. 2013; Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 34:2367–78. DOI: 10.1007/s13277-013-0785-0. PMID: 23580181. PMCID: PMC3713258.

23. Pasi F, Persico MG, Buroni FE, Aprile C, Hodolic M, Corbella F, Nano R, Facoetti A, Lodola L. 2017; Uptake of 18F-FET and 18F-FCH in human glioblastoma T98G cell line after irradiation with photons or carbon ions. Contrast Media Mol Imaging. 2017:6491674. DOI: 10.1155/2017/6491674. PMID: 29097931. PMCID: PMC5612615.
24. Niknezhad F, Sayad-Fathi S, Karimzadeh A, Ghorbani-Anarkooli M, Yousefbeyk F, Nasiri E. 2019; Improvement in histology, enzymatic activity, and redox state of the liver following administration of Cinnamomum zeylanicum bark oil in rats with established hepatotoxicity. Anat Cell Biol. 52:302–11. DOI: 10.5115/acb.18.180. PMID: 31598360. PMCID: PMC6773892.

25. Figul M, Söling A, Dong HJ, Chou TC, Rainov NG. 2003; Combined effects of temozolomide and the ribonucleotide reductase inhibitors didox and trimidox in malignant brain tumor cells. Cancer Chemother Pharmacol. 52:41–6. DOI: 10.1007/s00280-003-0611-2. PMID: 12690517.

26. Chen JC, Hsieh PS, Chen SM, Hwang JH. 2020; Effects of cinnamaldehyde on the viability and expression of chemokine receptor genes in temozolomide-treated glioma cells. In Vivo. 34:595–9. DOI: 10.21873/invivo.11812. PMID: 32111758. PMCID: PMC7157882.

27. Wu SJ, Ng LT, Lin CC. 2005; Cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells through activation of the proapoptotic Bcl-2 family proteins and MAPK pathway. Life Sci. 77:938–51. DOI: 10.1016/j.lfs.2005.02.005. PMID: 15964311.

28. Wu SJ, Ng LT. 2007; MAPK inhibitors and pifithrin-alpha block cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells. Food Chem Toxicol. 45:2446–53. DOI: 10.1016/j.fct.2007.05.032. PMID: 17673346.

29. Wu SJ, Ng LT, Lin CC. 2004; Effects of vitamin E on the cinnamaldehyde-induced apoptotic mechanism in human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 31:770–6. DOI: 10.1111/j.1440-1681.2004.04091.x. PMID: 15566391.

30. Zhang JH, Liu LQ, He YL, Kong WJ, Huang SA. 2010; Cytotoxic effect of trans-cinnamaldehyde on human leukemia K562 cells. Acta Pharmacol Sin. 31:861–6. DOI: 10.1038/aps.2010.76. PMID: 20581850. PMCID: PMC4007726.

31. Grootjans S, Hassannia B, Delrue I, Goossens V, Wiernicki B, Dondelinger Y, Bertrand MJ, Krysko DV, Vuylsteke M, Vandenabeele P, Vanden Berghe T. 2016; A real-time fluorometric method for the simultaneous detection of cell death type and rate. Nat Protoc. 11:1444–54. DOI: 10.1038/nprot.2016.085. PMID: 27414760.

32. Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. 2008; Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 135:1311–23. DOI: 10.1016/j.cell.2008.10.044. PMID: 19109899. PMCID: PMC2621059.

33. Richards R, Schwartz HR, Honeywell ME, Stewart MS, Cruz-Gordillo P, Joyce AJ, Landry BD, Lee MJ. 2020; Drug antagonism and single-agent dominance result from differences in death kinetics. Nat Chem Biol. 16:791–800. DOI: 10.1038/s41589-020-0510-4. PMID: 32251407. PMCID: PMC7311243.

34. Chio CC, Tai YT, Mohanraj M, Liu SH, Yang ST, Chen RM. 2018; Honokiol enhances temozolomide-induced apoptotic insults to malignant glioma cells via an intrinsic mitochondrion-dependent pathway. Phytomedicine. 49:41–51. DOI: 10.1016/j.phymed.2018.06.012. PMID: 30217261.

35. Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL. 2005; Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia. 50:160–7. DOI: 10.1002/glia.20168. PMID: 15685605.

Fig. 2
Cell viability and IC50. 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl-tertazolium bromide (MTT) assay show the viability of T98G cells after exposure to increasing concentrations of cinnamaldehyde for 48 hours. Analysis of MTT assay results using the GraphPad Prism v.8 software (GraphPad). In these graphs, the x-axis shows the logarithm of the cinnamaldehyde concentration and the y-axis shows the cell viability rate in percentage. The mean of IC50 for cinnamaldehyde was estimated at 211.96±14.82 μM.
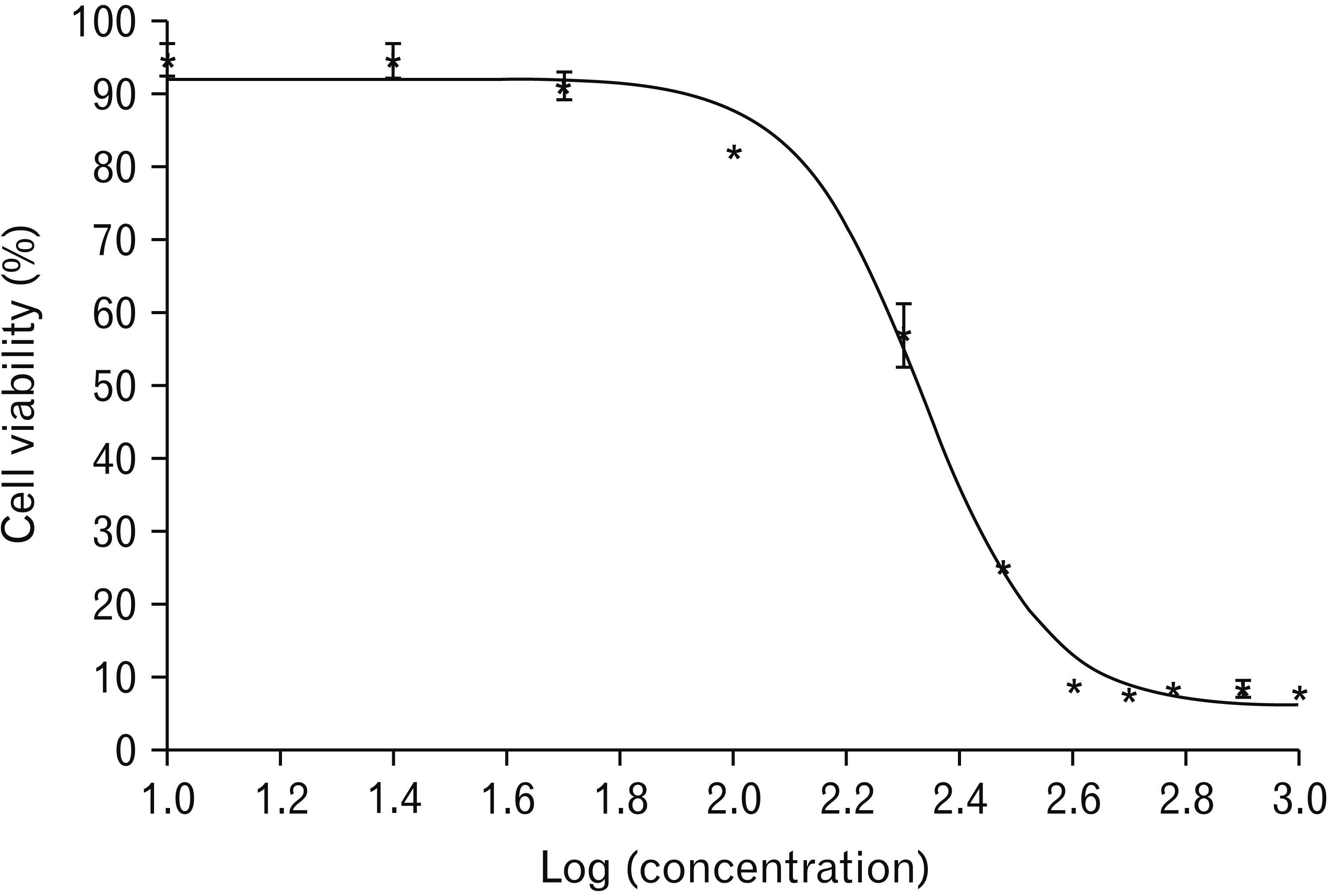
Fig. 3
(A) Comparison of cell cytotoxicity rate (mean±SD) in the combined groups of temozolomide (TMZ) and cinnamaldehyde (CIN) 300 μM (T+C300) with the sum of cytotoxicity at concentrations of TMZ and CIN 300 μM considered individually. ****P≤0.0001, ***P=0.004 and P=0.001 at concentrations of 50 and 25 μM, respectively. **P=0.01. (B) Comparison of cell viability rate (mean±SD) in the combined groups of TMZ with CIN 300 μM (T+C300) with that of the two groups of TMZ and CIN 300 μM. In this diagram, * shows the significance of the TMZ groups compared to the combined groups and # shows the significance of CIN 300 μM compared to the combined groups. ****P≤0.0001. ###P=0.008 and P=0.002, respectively, at concentrations of 50 and 25 μM. ##P=0.02, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
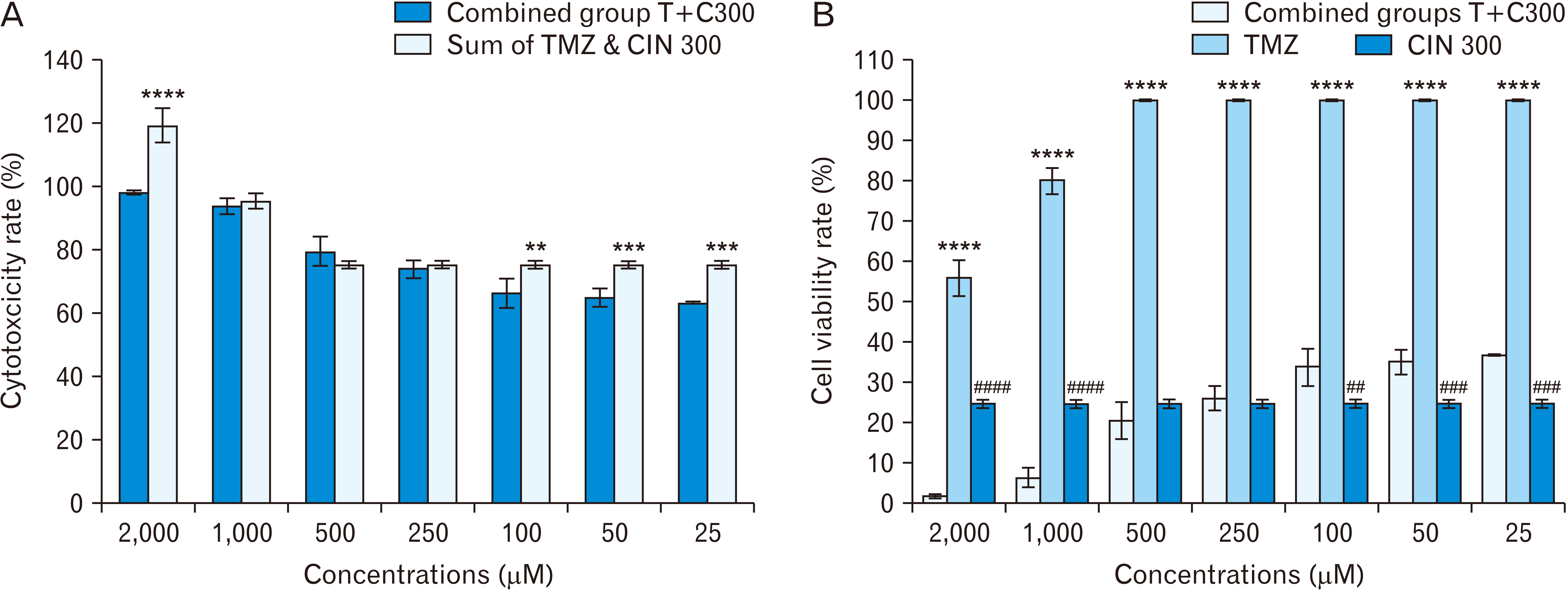
Fig. 4
(A) Comparison of cell cytotoxicity rate (mean±SD) in the combined groups of temozolomide (TMZ) and cinnamaldehyde (CIN) 150 μM (T+C150) with the sum of cytotoxicity at concentrations of TMZ and CIN 150 μM considered individually. (B) Comparison of cell viability rate (mean±SD) in the combined groups of TMZ and CIN 150 μM (T+C150) with that of the two groups of TMZ and CIN 150 μM. In this diagram, * shows the significance of the TMZ groups compared to the combined groups and # shows the significance of CIN 150 μM compared to the combined groups. ****P<0.0001 and ####P<0.0001.
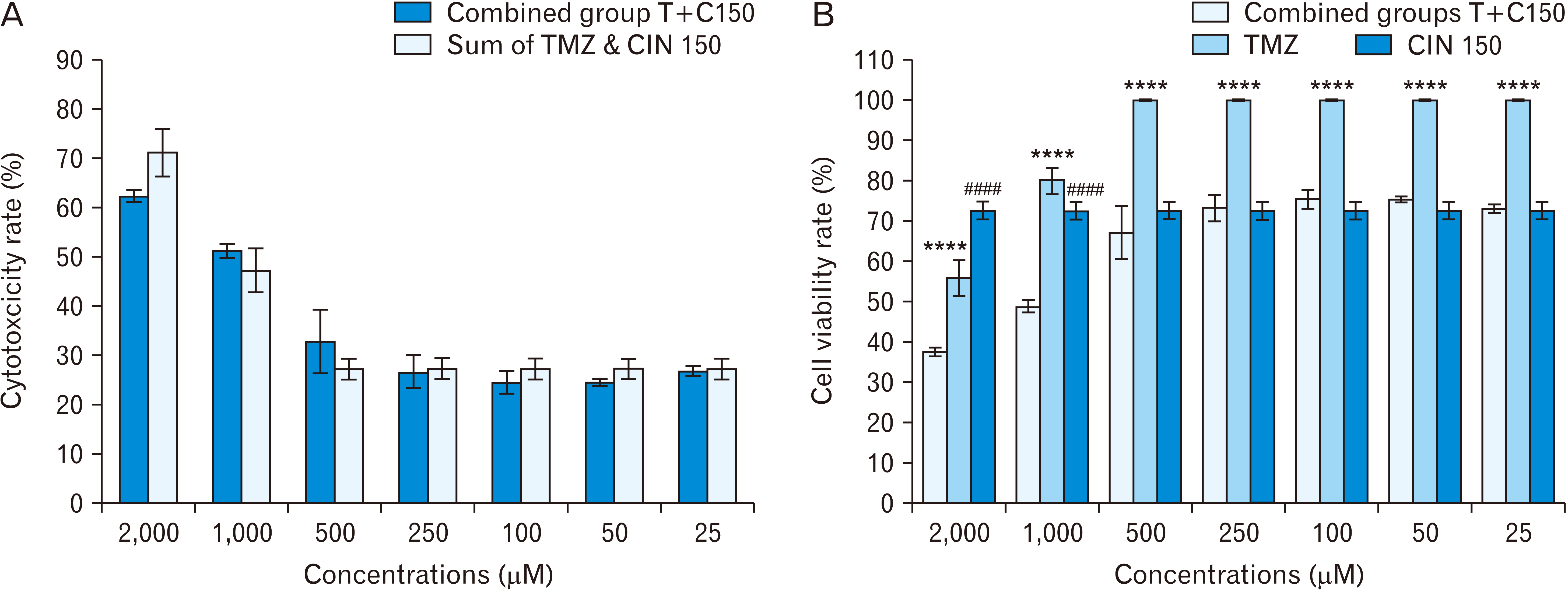
Fig. 5
(A) Comparison of cell cytotoxicity rate (mean±SD) in the combined groups of temozolomide (TMZ) and cinnamaldehyde (CIN) 100 μM (T+C100) with the sum of cytotoxicity at concentrations of TMZ and CIN 100 μM considered individually. ****P≤0.0001 and ***at concentrations of TMZ 50 μM and 25 μM at P=0.002 and P=0.001, respectively. (B) Comparison of cell viability rate (mean±SD) in the combined groups of TMZ with CIN 100 μM (T+C100) with that in the two groups of TMZ and CIN 100 μM. In this diagram, * shows the significance of the TMZ groups compared to that of the combined groups and # shows the significance of CIN 100 μM compared to that of the combined groups. ****P≤0.0001 and ***at concentrations of TMZ 2,000, 50, and 25 μM at P=0.001, P=0.004, and P=0.008, respectively. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Fig. 6
Acridine orange, Giemsa, and Hoechst staining; the scale bar is 100 μm. (A) Acridine orange staining to identify acidic vesicular organelles under the red filter. (B) Giemsa staining to evaluate the morphology of apoptotic cells. The arrow indicates apoptotic cells. In the group T1000+C150, the star represents the pyknotic cell and a number of apoptotic bodies around it. (C) Hoechst staining to investigate nuclear fragmentation in apoptotic cells. The arrow indicates apoptotic bodies.
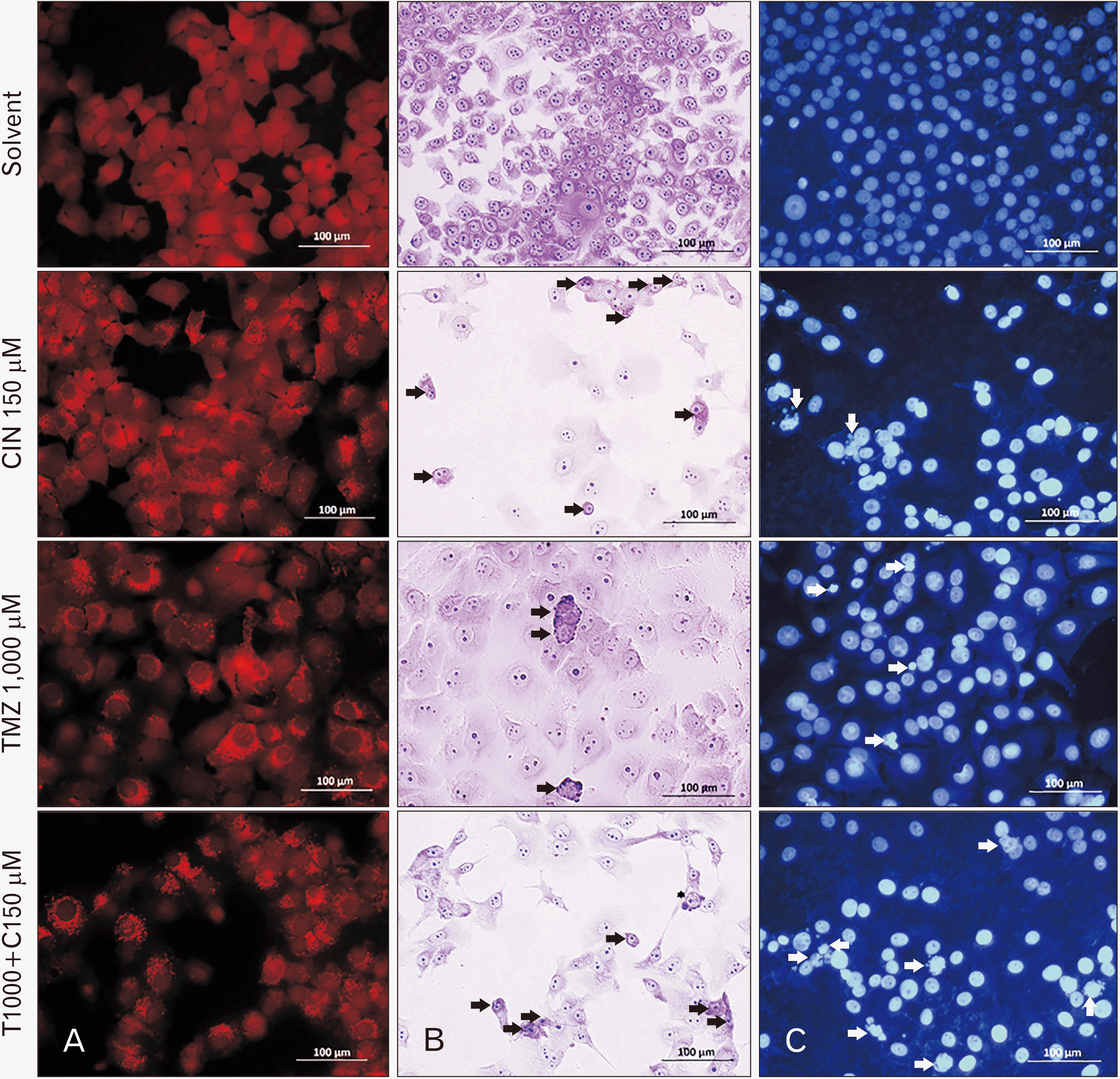
Fig. 7
Altered Bax and Bcl2 expression and Bax:Bcl2 expression ratio in TG98 cell line in different groups. (A) Bax expression (21 kDa) in different groups. Bcl2 expression (26 kDa) in different groups. β-Actin was used as a loading control (45 kDa). (B, C) Densitometric comparison of the average expression of Bax and Bcl2. (B) ANOVA, tukey-hoc test. ****P≤0.0001, ***P=0.001, **P=0.01. Data are represented as mean±SD, n=4. (C) ANOVA, tukey-hoc test. ****P≤0.0001, ***P=0.005, **P=0.01. Data are represented as mean±SD, n=4. (D) Bax:Bcl2 expression ratio in treatment groups. ANOVA, tukey-hoc test. ****P≤0.0001, ***P=0.001, **P=0.003. Data are represented as mean±SD, n=4.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download