Abstract
The exocrine part of the pancreas has a duct system called the pancreatic ductal system (PDS). Its mechanism of development is complex, and any reorganization during early embryogenesis can give rise to anatomical variants. The aim of this study is to collect, classify, and analyze published evidence on the importance of anatomical variants of the PDS, addressing gaps in our understanding of such variations. The MEDLINE, Web of Science, Embase, and Google Scholar databases were searched to identify publications relevant to this review. R studio with meta-package was used for data extraction, risk of bias estimation, and statistical analysis. A total of 64 studies out of 1,778 proved suitable for this review and metanalysis. The meta-analysis computed the prevalence of normal variants of the PDS (92% of 10,514 subjects). Type 3 variants and “descending” subtypes of the main pancreatic duct (MPD) predominated in the pooled samples. The mean lengths of the MPD and accessory pancreatic duct (APD) were 16.53 cm and 3.36 cm, respectively. The mean diameters of the MPD at the head and the APD were 3.43 mm and 1.69 mm, respectively. The APD was present in only 41% of samples, and the long type predominated. The pancreatic ductal anatomy is highly variable, and the incorrect identification of variants may be challenging for surgeons during ductal anastomosis with gut, failure to which may often cause ductal obstruction or pseudocysts formation.
The pancreas is a retroperitoneal organ with endocrine and exocrine functions. The exocrine part of the gland drains into multiple lobular ducts which makes “herringbone pattern” by uniting with the pancreatic duct or accessory duct. The single pancreatic duct starts in the tail of the pancreas and usually ends at the major duodenal papilla, which is also known as the pancreatic duct of Wirsung or the main pancreatic duct (MPD). The accessory duct starts in the head of the pancreas and usually has a small caliber and communicates with MPD or its inferior branch at the neck of the gland and terminates at the minor duodenal papilla about 2 cm proximal to the major duodenal papilla. The accessory duct is also known as the pancreatic duct of Santorini or the accessory pancreatic duct (APD). These duct systems are called the pancreatic ductal system (PDS), which reflects variable embryological development of pancreatic buds along with their ducts. The pancreas develops by fusion of the ventral and dorsal endodermal pancreatic buds in the seventh week, and any divergence in this process can result in too many individual anatomical variants (Fig. 1) [1-5]. Anatomical variations of the PDS are commonly reported as incidental findings during imaging procedures during adulthood, but their prevalence is neither well documented nor well recognized [4]. The development of the PDS and its variations occur during embryogenesis when the pancreas begins to differentiate. Some examples of these variants include pancreas divisum, where there is no communication between the MPD and APD, and ansa pancreatica, which is a loop or S-shaped either in APD or in MPD. Except for pancreas divisum and ansa pancreatica, the effects of these variants on exocrine pancreatic function have not been extensively studied [6].
The methodology for studying the PDS has evolved over the years. Early methods used the injection of cadaveric specimens with delicate anatomical dissections and pancreatography. Duct visualization has evolved further during the last few decades, leading to endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) [7]. ERCP is still considered the gold standard for visualizing the ducts, but it has been replaced by non-invasive MRCP, which is safer and can also visualize the parenchyma. In addition, s-MRCP (secretin-stimulated MRCP), a newer technique that uses secretin to dilate the ducts temporarily, improves visualization and therefore the evaluation of duct morphology and the assessment of exocrine function [8]. Unfortunately, most studies on exocrine pancreatic function have provided little or no information about PDS variation, ductal morphology, and its effects [9].
The present study focuses on the frequency and clinical significance of PDS variants other than pancreas divisum and determines the pooled prevalence of different types of ductal variations in different populations and ethnic groups. The aim of this review is to collect, organize, and interpret published data on the relevance of anatomical variants of the PDS in order to preclude diagnostic errors, fill gaps in our knowledge about pancreatic duct variations, and identify areas where further research is needed.
We adopted the SPIDER model for this systematic review and metanalysis of the PDS [10].
· Sample: cadaveric or dissection, pancreatography, ERCP, and MRCP.
· Phenomenon of interest: pancreatic ductal variation related to configuration, course, termination, and dimensions.
· Design: cross-sectional, prospective or retrospective study.
· Evaluation: proportion of variants and mean dimension of ducts.
· Research: quantitative estimation.
PRISMA guidelines provided the basis for the search strategy, data extraction, and statistical analysis. In order to be included in the review, a study had to meet the following criteria:
Inclusion criteria
(1) Studies reporting data on anatomical variants of the PDS along with morphometric measurements of the ducts.
(2) Studies including classification of PDS variants into three categories: “normal variant PDS”, ansa pancreatica, and pancreas divisum or embryonic. Studies that provided further distinctions within the “normal PDS” category were also eligible.
Exclusion criteria
(1) Studies that only reported results for pancreas divisum and ansa pancreatica without data on other PDS variants.
(2) Systematic reviews, editorials, case reports, and case series.
(3) Studies reporting data associated with pancreatic disorders such as pseudocysts and tumors, which distorted the duct anatomy.
The MEDLINE, Web of Science, Embase, and Google Scholar databases were searched to identify publications relevant to this review. The search was limited to human subjects, but no time and language filters were applied. The included terms were “pancreas”, “pánkreas”, “duct”, “ductus” “pancreatic”, “pancreaticum” “ductal system”, “variations”, “variantes”, “variants”, “anatomic”, “anatomico”, and “anatomical”. The references listed in the included studies were also hand-searched.
Two reviewers were assigned to search independently for the data, any discrepancies regarding study inclusion being resolved by senior investigators. The data extracted from the studies included information such as the authors and publication year, the country where the study was conducted, the setting of the study, the number and type of subjects involved, and the number and type of PDS variants described. The numerous inconsistent classification systems for such variants are difficult to compare. Therefore, we adopted a literature-based classification system for anatomical variants of the PDS as follows:
1. Configuration variants, based on the drainage pattern of the PDS. The classification of the PDS was adopted from Bülow et al. [11] with slight modifications, including: normal variant (type 1–3), pancreas divisum or embryonic (type 4), and ansa pancreatica (type 5) (Fig. 2).
· Type 1: Bifid, dual duct configuration with MPD draining into the major papilla and APD opening into the minor papilla (Fig. 2).
· Type 2: Bifid configuration with MPD opening into the minor papilla and APD reaching the major papilla with or without patency (reverse of type 1) (Fig. 2).
· Type 3: MPD draining into the major papilla with or without communication with APD (Fig. 2).
· Type 4: Embryonic or pancreas divisum (subtype, “complete”; subtype, “absent APD”; subtype, “incomplete”; subtype “reverse divisum”) (Fig. 2).
· Type 5: Ansa pancreatica: loop or S- or L-shaped communication between MPD and APD with or without patency (Fig. 2).
2. Variants of MPD: MPD are classified into five subtypes on the basis of course or shape: descending, vertical or ascending, sigmoid, loop, and horizontal or transverse (Fig. 3) [12].
3. Classification of the APD: In 2004, Kamisawa et al. [13] proposed a classification system for APDs based on their length relative to the MPD. This system is used to help identify and diagnose variations in PDS anatomy.
· The Long-type APD: a continuation of the main duct of the dorsal primordium (Fig. 4).
· The Short-type APD: is formed by the continuation of the long inferior branch, with an obliterated connection with the main duct of the dorsal primordium (Fig. 4).
Variants of pancreaticobiliary junction (PBJ) arrangement: This classification is based on variations in the arrangement of the junction between the pancreas and the biliary system.
The risk of bias was estimated using the Newcastle–Ottawa score for observational studies [14]. R studio with meta package was used for statistical analyses. The effect size, i.e., pooled weighted proportion and pooled weight mean, were computed using the generalized linear mixed (GLM) method and inverse variance (IV) method, respectively. Heterogeneity was examined using Higgins i2 statistics, tau-square and Cochrane Q. If the heterogeneity was more than 50%, effect size computed with a random effect model was adopted in place of the fixed-effect model.
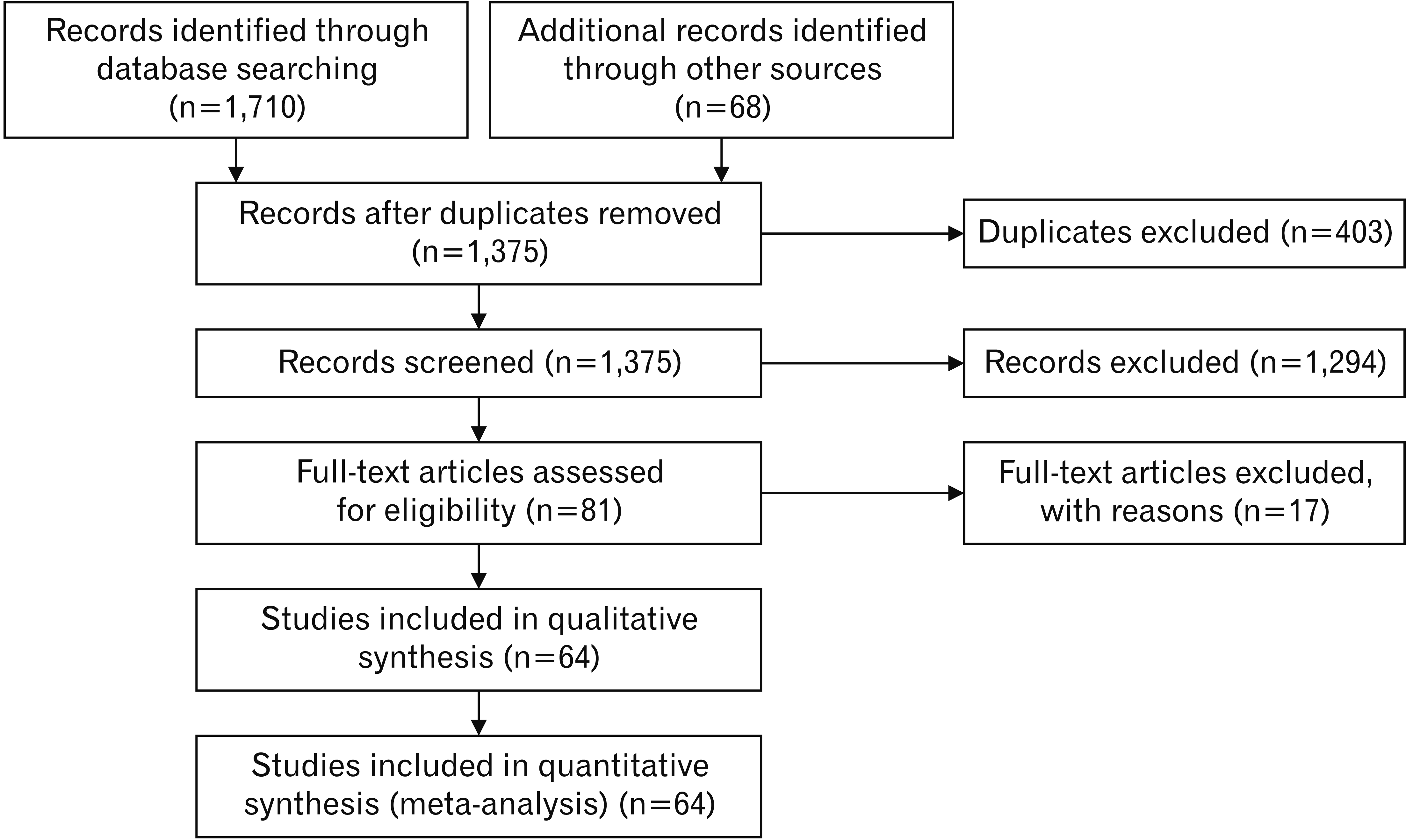
The search strategy yielded 1,778 citations, of which 403 were duplicates. After these duplicates were removed, abstracts were screened, and 1,294 citations were excluded because they did not report outcomes of interest. The full texts of eighty-one citations were evaluated by further screening, and 17 were excluded during the data extraction phase because outcome data were missing. Therefore, only 64 citations were suitable for qualitative and quantitative analysis (Fig. 5 and Table 1) [1-4, 9, 11, 15-69]. Thirteen reported configuration variations of the PDS (types 1–5), and 12 concerned variations in its course (descending, sigmoid, vertical, horizontal, and loop). The citations were subgrouped into MPD and APD.
A. Configuration variants of PDS: the prevalence of the normal form of the PDS was 92% (0.87–0.95). The prevalence of types 1–3 PDS variants were 27% (0.18–0.37), 1% (0.00–0.04), and 50% (0.39–0.62), respectively. Type 3 was the most common variant. The prevalences of pancreatic divisum and ansa were 6% (0.05–0.08) and 2% (0.00–0.07) (Table 2). The most common subtypes of pancreas divisum were “complete,” followed by “incomplete” and “absent APD.” The distribution of “reverse divisum” was not reported in the original reports.
B. MPD (Table 3):
I. Course variants of MPD: the most common course of the MPD was the “descending” type (40%, 0.19–0.65), followed by the “sigmoid” type (22%, 0.17–0.27) and the “vertical” type (10%, 0.05–0.21) (Table 3).
II. Termination of MPD: the MPD terminated at the major duodenal papilla in 81% (0.48–0.95) and at the minor duodenal papilla in 19% (0.05–0.52).
III. Dimensions of MPD: the mean length of the MPD was 16.53 cm (15.67–17.39 cm). The pooled estimates of MPD diameter were 3.43 mm (3.21–3.65 mm) at the head, 2.43 mm (2.27–2.59 mm) at the body, and 1.62 mm (1.41–1.81 mm) at the tail of the pancreas (Table 3 and Fig. 6A).
C. APD (Table 2):
I. Course variants: the prevalence of the APD was 41% (0.31–0.52) in 1,286 samples. The subtypes of the APD were studied in 467 samples. The long subtypes were the most common (52%, 0.37–0.67), followed by the short subtype (21%, 0.10–0.39). Both the embryonic and Ansa subtypes of the APD were 8% (Tables 2-4).
II. Termination of APD: the APD terminated at the major duodenal papilla in 18% (0.07–0.39) and at the minor duodenal papilla in 80% (0.58–0.95).
III. Dimensions of APD: the length of the APD was 3.36 cm (2.62–4.09), and its diameter was 1.69 mm (1.60–1.77) (Table 2 and Fig. 6B).
D. There was significant heterogeneity in almost every parameter owing to sample variations; subgroup analysis based on ethnicities and modalities of the investigation could not reduce this heterogeneity. There was publication bias in some parameters, corrected by trim-fill analysis.
Based on population normal variant of PDS was observed to be most prevalent in the Asians (0.96) while the embryonic variant was mostly seen in South Americans (0.11), and the ansa pancreatica was reported to be prevalent in North Americans (0.17). The Europeans were observed to possess the longest (18.05 cm) as well as the widest MPD (at head [3.88 mm] and body [2.49 mm]), whereas the diameter of the same at tail was widest for the South Americans (2.34 mm). Among the observed populations, only the South Americans (0.62) and Europeans (0.5) had APD prevalent in over half of their sample size (Table 4).
The foetal development of the human pancreas is intricate leading to a wide range of congenital pancreatic and ductal abnormalities. The literature presents with distinct reports on prevalence of the normal form and the variants of the PDS across the globe. The study of pancreatic variation is important in medicine, medical imaging, surgery, and evolutionary biology, to elucidate the anatomy, function, and evolution of the pancreas.
The present meta-analysis computed the prevalence of the normal form of the PDS, found in 92% of 10,514 subjects. Type 3 variants of the PDS and “descending” subtypes of the MPD predominated in the pooled samples. The MPD terminated at the major duodenal papilla in 80%, and a similar proportion of APDs terminated at minor duodenal papilla (81%). The mean lengths of the MPD and APD were 16.53 cm and 3.36 cm, respectively. The mean diameter of MPD at the head and that of the APD were 3.43 mm and 1.69 mm, respectively. The APD was present in only 41% of samples, and the long type predominated.
A systematic review by Dimitriou et al. [7] revealed normal pancreatic ducts in 94.3% of 7,792 subjects. A review of nine studies by Dugic et al. [8] showed 89.9% of normal MPDs in 3,234 subjects. In both reviews, the MPD most commonly drained into the major papilla with or without communication with the APD [8]. The present study findings are similar to those of the two systematic reviews with respect to prevalence of MPDs. Furthermore, the present study adds the data related to the comparison between the dimensions of the MPD and APD, which has no prior mention in the literature.
Pancreatic duct variations can have several clinical implications. The ductal anastomosis with gut usually performed for chronic pancreatitis, trauma, tumor and other causes of ductal obstruction. Adequate identification pancreatic duct and its long inferior branch often confused with accessory duct. For example, a long APD can make a pancreaticoduodenectomy (a surgical procedure to remove the head of the pancreas) without injuring the duct more challenging [13]. Variations in pancreatic ductal anatomy can lead to the formation of pancreatic pseudocysts, fluid-filled sacs formed within the pancreas [11, 13, 17, 70, 71]. Pancreas divisum and ansa pancreatica can cause ductal obstruction, leading to chronic pancreatitis and recurrent acute episodes. However, not all patients with those variations develop pancreatitis; other factors such as genetics, lifestyle, and environment can also be involved [9, 12, 72, 73].
In ERCP, variations in pancreatic ductal anatomy, particularly if there is a long APD, can make it difficult to identify the MPD and to locate any blockages or strictures. This can lead to diagnostic errors, such as mistaking a blockage in the APD for one in the MPD or failing to identify a blockage in the MPD. Variations in pancreatic ductal anatomy can also increase the risk of complications during endoscopic procedures such as ERCP. For example, a long APD entails the risk of the contrast medium accidentally entering the duct, causing inflammation and infection during ERCP [73]. PDS variations can make therapeutic interventions such as stent placement more difficult to perform during ERCP, leading to diagnostic errors. They can also make it more difficult to diagnose conditions such as chronic pancreatitis, as the changes in ductal anatomy can mimic those seen in this condition [74, 75]. Pancreatic duct length and width can influence the diagnosis and management of pancreatic pathologies, both clinically and surgically, and can be used as indicators of pancreatic inflammation or injury. For example, in acute pancreatitis, the pancreas is often enlarged and wider; in chronic pancreatitis, it can be shrunken and narrower. Pancreatic length and width can also affect the surgical management of pancreatic pathologies. For example, in pancreatic cancer, a longer pancreas can make it more difficult to perform a pancreaticoduodenectomy (removal of the head of the pancreas) because it increases the risk of injuring adjacent structures. Similarly, a wider pancreas can make a distal pancreatectomy (removal of the tail of the pancreas) more difficult [71, 76]. Overall, knowledge of pancreatic ductal anatomy variations is vital for diagnosing, treating, and managing pancreatic conditions.
There are some flaws in our study. First, data from several studies that used different categories to define the PDS in their subjects were evaluated together. Second, the studies were either prospective or retrospective, and their subjects came from various demographic groups. Our study is also limited by the variety of subjects we used: healthy volunteers, active patients, and cadavers. The key problem was the dearth of population studies with enough information to calculate the prevalence of each particular type of MPD and APD. Morphometric data appropriate for the subtypes of PDS were not available for further meta-analysis of estimates.
In conclusion, the meta-analysis showed a 92% prevalence of the normal PDS. The most common variant subtype was type 3, followed by type 1. The MPD was mostly of the “descending type” followed by the “sigmoid” and “vertical” types, while a long APD was most common. The pancreatic divisum and ansa pancreatica were noticeably prevalent. Most of the MPDs (81%) terminated at the major duodenal papilla and the APDs at the minor duodenal papilla. Variations in pancreatic ductal anatomy can lead to the formation of pancreatic pseudocysts and can affect the surgical management of conditions such as pancreatic cancer and chronic pancreatitis. Variants such as pancreas divisum and ansa pancreatica can cause ductal obstruction, which can lead to further complications depending upon other factors such as genetics, lifestyle, and environment. Many of these variants can hinder ERCP, potentially cause diagnostic errors, and make therapeutic interventions difficult to perform. Variations in ductal length and width can affect the diagnosis of pancreatic inflammation or injury, and the surgical management of pancreatic pathologies.
Understanding the anatomical variations of the pancreatic ducts is critical for helping general surgeons to perform pancreatic anastomoses safely and effectively. Such knowledge is essential for invasive gastroenterology and ERCP as diagnostic and therapeutic methods. Rare structural alterations of the pancreatic ducts, such as pancreas divisum, afflict less than 6%.
Notes
References
1. Anand BS, Vij JC, Mac HS, Chowdhury V, Kumar A. 1989; Effect of aging on the pancreatic ducts: a study based on endoscopic retrograde pancreatography. Gastrointest Endosc. 35:210–3. DOI: 10.1016/S0016-5107(89)72760-7. PMID: 2759399.

2. Arora A, Piplani M, Kapoor S, Bhatia B, Singh R, Verma P, Piplani S. 2011; A research study of santorini duct. J Clin Diagn Res. 5:1510–3.
3. Abdelkareem H, Ali R, Jibrini M, Nazzal Z, Maree M, Hamaida J, Demyati K. 2019; A study of the anatomic variations of the pancreatico-biliary system in Palestine: a national study. Int Surg J. 6:1020–8. DOI: 10.18203/2349-2902.isj20191066.

4. Covantsev S, Chicu C, Mazuruc N, Belic O. 2022; Pancreatic ductal anatomy: more than meets the eye. Surg Radiol Anat. 44:1231–8. DOI: 10.1007/s00276-022-03002-w. PMID: 35986117.

5. Renzulli M, Pagano N, Golfieri R. 2020; Pancreas divisum inversus. Clin Anat. 33:646–52. DOI: 10.1002/ca.23475. PMID: 31576611.

6. Jagielski M, Smoczyński M, Adrych K. 2018; The anatomical variations of pancreatic duct in the patients with pancreatic diseases. Eur J Transl Clin Med. 1:63–6. DOI: 10.31373/ejtcm/92069.

7. Dimitriou I, Katsourakis A, Nikolaidou E, Noussios G. 2018; The main anatomical variations of the pancreatic duct system: review of the literature and its importance in surgical practice. J Clin Med Res. 10:370–5. DOI: 10.14740/jocmr3344w. PMID: 29581798. PMCID: PMC5862083.

8. Dugic A, Nikolic S, Mühldorfer S, Bulajic M, Pozzi Mucelli R, Tsolakis AV, Löhr JM, Vujasinovic M. 2020; Clinical importance of main pancreatic duct variants and possible correlation with pancreatic diseases. Scand J Gastroenterol. 55:517–27. DOI: 10.1080/00365521.2020.1760345. PMID: 32393143.

9. Adibelli ZH, Adatepe M, Isayeva L, Esen OS, Yildirim M. 2017; Pancreas divisum: a risk factor for pancreaticobiliary tumors - an analysis of 1628 MR cholangiography examinations. Diagn Interv Imaging. 98:141–7. DOI: 10.1016/j.diii.2016.08.004. PMID: 27616039.
10. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. 2014; PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 14:579. DOI: 10.1186/s12913-014-0579-0. PMID: 25413154. PMCID: PMC4310146.

11. Bülow R, Simon P, Thiel R, Thamm P, Messner P, Lerch MM, Mayerle J, Völzke H, Hosten N, Kühn JP. 2014; Anatomic variants of the pancreatic duct and their clinical relevance: an MR-guided study in the general population. Eur Radiol. 24:3142–9. DOI: 10.1007/s00330-014-3359-7. PMID: 25120204.

12. Türkvatan A, Erden A, Türkoğlu MA, Yener Ö. 2013; Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J Radiol. 14:905–13. DOI: 10.3348/kjr.2013.14.6.905. PMID: 24265565. PMCID: PMC3835637.

13. Kamisawa T. 2004; Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 39:605–15. DOI: 10.1007/s00535-004-1390-1. PMID: 15293129.

14. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute;2011.
15. De Filippo M, Calabrese M, Quinto S, Rastelli A, Bertellini A, Martora R, Sverzellati N, Corradi D, Vitale M, Crialesi G, Sarli L, Roncoroni L, Garlaschi G, Zompatori M. 2008; Congenital anomalies and variations of the bile and pancreatic ducts: magnetic resonance cholangiopancreatography findings, epidemiology and clinical significance. Radiol Med. 113:841–59. DOI: 10.1007/s11547-008-0298-x. PMID: 18592141.

16. Bang S, Suh JH, Park BK, Park SW, Song SY, Chung JB. 2006; The relationship of anatomic variation of pancreatic ductal system and pancreaticobiliary diseases. Yonsei Med J. 47:243–8. DOI: 10.3349/ymj.2006.47.2.243. PMID: 16642555. PMCID: PMC2687635.

17. Kamisawa T, Takuma K, Tabata T, Egawa N. 2010; Clinical implications of accessory pancreatic duct. World J Gastroenterol. 16:4499–503. DOI: 10.3748/wjg.v16.i36.4499. PMID: 20857518. PMCID: PMC2945479.

18. Shahriah S, Nurunnabi ASM, Johora F, Siddiqua D, Ara S. 2014; Cadaveric study of the anatomical variations of the accessory pancreatic duct. J Bangladesh Soc Physiol. 9:83–8. DOI: 10.3329/jbsp.v9i2.22802.

19. Kim HJ, Kim MH, Lee SK, Seo DW, Kim YT, Lee DK, Song SY, Roe IH, Kim JH, Chung JB, Kim CD, Shim CS, Yoon YB, Yang US, Kang JK, Min YI. 2002; Normal structure, variations, and anomalies of the pancreaticobiliary ducts of Koreans: a nationwide cooperative prospective study. Gastrointest Endosc. 55:889–96. DOI: 10.1067/mge.2002.124635. PMID: 12024146.

20. Oracz G, Oralewska B, Pertkiewics J, Teisseyre M, Ryzko J, Socha J. 2006; Chronic pancreatitis associated with anatomic anomalies of pancreatic duct in children. J Pediatr Gastroenterol Nutr. 42:E58. DOI: 10.1002/j.1536-4801.2006.tb01738.x.

21. Uomo G, Manes G, D'Anna L, Laccetti M, Di Gaeta S, Rabitti PG. 1995; Fusion and duplication variants of pancreatic duct system. Clinical and pancreatographic evaluation. Int J Pancreatol. 17:23–8. DOI: 10.1007/BF02788355. PMID: 8568331.
22. Prasanna LC, Rajagopal KV, Thomas HR, Bhat KM. 2015; Accessory pancreatic duct patterns and their clinical implications. J Clin Diagn Res. 9:AC05–7. DOI: 10.7860/JCDR/2015/11539.5660. PMID: 25954609. PMCID: PMC4413057.

23. Prasad M, Rout S, Putta T, Kurien RT, Chowdhury SD, Eapen A, Hepsy YS, Rabi S. 2019; Anatomical patterns of the pancreatic ductal system - a cadaveric and magnetic resonance cholangiopancreatography study. J Morphol Sci. 36:279–85. DOI: 10.1055/s-0039-1698371.

24. Koshariya M, Behram S, Singour JP, Tiwari S, Khare V. 2019; Anomalous anatomical variation in extrahepatic biliary tree and pancreas and its related vessels: a cadaveric study. Int Surg J. 6:3111–6. DOI: 10.18203/2349-2902.isj20193658.

25. Gonoi W, Akai H, Hagiwara K, Akahane M, Hayashi N, Maeda E, Yoshikawa T, Kiryu S, Tada M, Uno K, Okura N, Koike K, Ohtomo K. 2013; Santorinicele without pancreas divisum pathophysiology: initial clinical and radiographic investigations. BMC Gastroenterol. 13:62. DOI: 10.1186/1471-230X-13-62. PMID: 23570616. PMCID: PMC3637151.

26. Darnis E, Cronier P, Mercier P, Moreau P, Pillet J, Boyer J. 1984; A radio-anatomical study in vivo on pancreatic ducts. Anat Clin. 6:109–16. DOI: 10.1007/BF01773162. PMID: 6497998.
27. Schmitt F, Maignan A, Ploteau S, Hamel A, Lagier S, Blin Y, Rogez JM, Le Borgne J. 2010; New anatomical data on the drainage patterns of the uncinate process of the pancreas. Surg Radiol Anat. 32:777–81. DOI: 10.1007/s00276-010-0680-y. PMID: 20490492.

28. Smanio T. 1969; Proposed nomenclature and classification of the human pancreatic ducts and duodenal papillae. Study based on 200 post mortems. Int Surg. 52:125–41. PMID: 5793091.
29. Dawson W, Langman J. 1961; An anatomical-radiological study on the pancreatic duct pattern in man. Anat Rec. 139:59–68. DOI: 10.1002/ar.1091390109. PMID: 14025604.

30. Berman LG, Prior JT, Abramow SM, Ziegler DD. 1960; A study of the pancreatic duct system in man by the use of vinyl acetate casts of postmortem preparations. Surg Gynecol Obstet. 110:391–403. PMID: 13799593.
31. Ishaque I, Ilyas A, Khan O, Kazmi QH, Ladak AH, Rafay A. 2020; Role of magnetic resonance cholangiopancreatography (MRCP) in learning anatomical variations in pancreatic duct. J Pak Med Assoc. 70:472–6. DOI: 10.5455/JPMA.15767. PMID: 32207428.

32. Johansson K, Mustonen H, Seppänen H, Lehtimäki TE. 2022; Anatomical pancreatic variants in intraductal papillary mucinous neoplasm patients: a cross-sectional study. BMC Gastroenterol. 22:394. DOI: 10.1186/s12876-022-02465-w. PMID: 35989322. PMCID: PMC9394057.

33. Malathi K, Kishan Reddy C. 2019; The study of anatomy of main pancreatic duct and its variations. Perspect Med Res. 7:31–7.
34. Shu J, Zhang XM, Zeng N. 2006; Normal pancreatic duct: evaluation with MR cholangiopancreatography. Chin J Med Imaging Technol. 4:2004–6.
35. Kasugai T, Kuno N, Kobayashi S, Hattori K. 1972; Endoscopic pancreatocholangiography. I. The normal endoscopic pancreatocholangiogram. Gastroenterology. 63:217–26. DOI: 10.1016/S0016-5085(19)33306-2. PMID: 5048325.
36. Kreel L, Sandin B. 1973; Changes in pancreatic morphology associated with aging. Gut. 14:962–70. DOI: 10.1136/gut.14.12.962. PMID: 4785285. PMCID: PMC1412863.

37. Kang JK, Chung JB, Moon YM, Choi HJ. 1989; The normal endoscopic pancreatogram in Koreans. Korean J Intern Med. 4:74–9. DOI: 10.3904/kjim.1989.4.1.74. PMID: 2487408. PMCID: PMC4534962.

38. Vinay Kumar N, Lokanadham S. 2019; A study on incidence of accessory pancreatic duct and its clinical importance. Maedica (Bucur). 14:22–5. DOI: 10.26574/maedica.2019.14.1.22. PMID: 31123508. PMCID: PMC6511670.
39. Hayashi TY, Gonoi W, Yoshikawa T, Hayashi N, Ohtomo K. 2016; Ansa pancreatica as a predisposing factor for recurrent acute pancreatitis. World J Gastroenterol. 22:8940–8. DOI: 10.3748/wjg.v22.i40.8940. PMID: 27833385. PMCID: PMC5083799.

40. Govindraj N, Shabna C. 2017; Variations in the duct system of pancreas: a cadaveric study. Int J Anat Res. 5:4136–43. DOI: 10.16965/ijar.2017.269.

41. Liessi F, Manfredi R, Liessi G, Mucelli RP. Anatomical variants of the pancreatic ducts. ECR;2010. https://dx.doi.org/10.1594/ecr2010/C-0174. DOI: 10.1594/ecr2010/C-0174.
42. Varley PF, Rohrmann CA Jr, Silvis SE, Vennes JA. 1976; The normal endoscopic pancreatogram. Radiology. 118:295–300. DOI: 10.1148/118.2.295. PMID: 1250961.

43. Classen M, Hellwig H, Rösch W. 1973; Anatomy of the pancreatic duct a duodenoscopic-radiological study. Endoscopy. 5:14–7. DOI: 10.1055/s-0028-1098203.

44. Cotton P. 1974; The normal endoscopic pancreatogram. Endoscopy. 6:65–70. DOI: 10.1055/s-0028-1098599. PMID: 1250961.

45. Seifert E, Wagner HH, Otto P. 1973; Value and indication of endoscopic pancreato-cholangiography. Gastroenterol Jpn. 8:205–12. DOI: 10.1007/BF02779900.

46. Okuda K, Someya N, Goto A, Kunisaki T, Emura T, Yasumoto M, Shimokawa Y. 1973; Endoscopic pancreato-cholangiography. A preliminary report on technique and diagnostic significance. Am J Roentgenol Radium Ther Nucl Med. 117:437–45. DOI: 10.2214/ajr.117.2.437. PMID: 4346685.
47. Sivak MV Jr, Sullivan BH Jr. 1976; Endoscopic retrograde pancreatography: analysis of the normal pancreatogram. Am J Dig Dis. 21:263–9. DOI: 10.1007/BF01095900. PMID: 1266843.
48. Ara S, Shahriah S, Begum S. 2011; The length of main pancreatic duct in Bangladeshi cadaver at different age groups. Mymensingh Med J. 20:298–302. PMID: 21522104.
49. Kochhar R, Goenka MK, Nagi B, Aggarwal R, Singh K. 1996; Normal pancreatic duct morphology in a north Indian population. Trop Gastroenterol. 17:223–5. PMID: 9094863.
50. Sahni D, Jit I, Harjeet . 2001; Gross anatomy of the pancreatic ducts in north Indians. Trop Gastroenterol. 22:197–201. PMID: 11963324.
51. Fatima T. 2010; Anatomical variants of pancreatic duct system in human cadavers. J Fatima Jinnah Med Univ. 4:110–7.
52. Wilasrusmee C, Pongchairerks P. 1999; Pancreaticobiliary ductal anatomy in Thai people. J Hepatobiliary Pancreat Surg. 6:79–85. DOI: 10.1007/s005340050087. PMID: 10436241.

53. Pina L. 2016; Does the accessory pancreatic duct have any protective role in acute biliary pancreatitis? Anatomical findings from cadaver dissections. MOJ Anat Physiol. 2:180. DOI: 10.15406/mojap.2016.02.00071.
54. Hastier P, Buckley MJ, Dumas R, Kuhdorf H, Staccini P, Demarquay JF, Caroli-Bosc FX, Delmont JP. 1998; A study of the effect of age on pancreatic duct morphology. Gastrointest Endosc. 48:53–7. DOI: 10.1016/S0016-5107(98)70129-4. PMID: 9684665.

55. Akochi S, Ugwu A, Otuh I. 2018; Sonographic measurement of common bile duct diameter in apparently healthy adults in Abakaliki metropolis. Int J Sci Appl Res. 5:1–8.
56. Karak PK, Vashisht S, Tandon RK, Berry M. 1991; Normal endoscopic pancreatogram in an Indian referral hospital. Indian J Med Res. 94:426–9. PMID: 1774094.
57. Hadidi A. 1983; Pancreatic duct diameter: sonographic measurement in normal subjects. J Clin Ultrasound. 11:17–22. DOI: 10.1002/jcu.1870110105. PMID: 6403583.

58. Millbourn E. 1960; Calibre and appearance of the pancreatic ducts and relevant clinical problems. A roentgenographic and anatomical study. Acta Chir Scand. 118:286–303. PMID: 14422445.
59. Trapnell JE, Howard JM. 1966; Transduodenal pancreatography: an improved technique. Surgery. 60:1112–9. PMID: 5928106.
60. Hand BH. 1963; An anatomical study of the choledochoduodenal area. Br J Surg. 50:486–94. DOI: 10.1002/bjs.18005022303. PMID: 13952484.

61. Ogoshi K, Niwa M, Hara Y, Nebel OT. 1973; Endoscopic pancreatocholangiography in the evaluation of pancreatic and biliary disease. Gastroenterology. 64:210–6. DOI: 10.1016/S0016-5085(73)80031-9. PMID: 4686330.

62. Birnstingl M. 1959; A study of pancreatography. Br J Surg. 47:128–39. DOI: 10.1002/bjs.18004720203. PMID: 13800918.

63. MacCarty RL, Stephens DH, Brown AL Jr, Carlson HC. 1975; Retrograde pancreatography in autopsy specimens. Am J Roentgenol Radium Ther Nucl Med. 123:359–66. DOI: 10.2214/ajr.123.2.359. PMID: 1115312.

64. Toda J, Ueno E, Takada Y, Okawa T. 1998; Demonstration of normal bile duct and pancreatic duct with MR cholangiopancreatography. Nihon Rinsho. 56:2830–5. Japanese. PMID: 9847605.
65. Aubé C, Hentati N, Tanguy JY, Fournier HD, Papon X, Lebigot J, Mercier P. 2003; Radio-anatomic study of the pancreatic duct by MR cholangiopancreatography. Surg Radiol Anat. 25:64–9. DOI: 10.1007/s00276-002-0082-x. PMID: 12647024.

66. Mchonde GJ, Gesase AP. 2014; Termination pattern of the main and accessory pancreatic ducts among Tanzanians. Anat J Afr. 3:223–7.
67. Jirasiritham J, Wilasrusmee C, Poprom N, Larbcharoensub N. 2016; Pancreaticobiliary ductal anatomy in the normal population. Asian Pac J Cancer Prev. 17:4363–5. DOI: 10.1016/j.pan.2017.07.034. PMID: 27797245.
68. Gosavi V, Gaikwad P. 1980; The duct system of the human pancreatic gland. J Anat Soc India. 29:142–6.
69. Cremer M, Toussaint J, Hermanus A, Deltenre M, De Tceuf J, Engelholm L. 1976; Primary chronic pancreatitis. A classification based on endoscopic pancreatography. Acta Gastroenter Belg. 39:522–46. In French. PMID: 1023728.
70. Malathi K, Chitra R. 2022; The study of anatomy of accessory pancreatic duct and its variations. Eur J Mol Clin Med. 9:6270–9.
71. Nealon WH, Walser E. 2002; Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg. 235:751–8. DOI: 10.1097/00000658-200206000-00001. PMID: 12035030. PMCID: PMC1422503.

72. Covantev S. 2018; Pancreas divisum: a reemerging risk factor for pancreatic diseases. Rom J Intern Med. 56:233–42. DOI: 10.2478/rjim-2018-0022. PMID: 30521477.

73. de Jong DM, Stassen PM, Poley JW, Fockens P, Timmer R, Voermans RP, Verdonk RC, Bruno MJ, de Jonge PJF. 2021; Clinical outcome of endoscopic therapy in patients with symptomatic pancreas divisum: a Dutch cohort study. Endosc Int Open. 9:E1164–70. DOI: 10.1055/a-1460-7899. PMID: 34222643. PMCID: PMC8216775.

74. Testoni PA. 2007; Endoscopic pancreatic duct stent placement for inflammatory pancreatic diseases. World J Gastroenterol. 13:5971–8. DOI: 10.3748/wjg.v13.45.5971. PMID: 18023085. PMCID: PMC4250876.

75. Elbanna KY, Jang HJ, Kim TK. 2020; Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging. 11:58. DOI: 10.1186/s13244-020-00861-y. PMID: 32335790. PMCID: PMC7183518.

76. Nealon WH, Walser E. 2003; Duct drainage alone is sufficient in the operative management of pancreatic pseudocyst in patients with chronic pancreatitis. Ann Surg. 237:614–20. discussion 620–2. DOI: 10.1097/01.SLA.0000064360.14269.EF. PMID: 12724627. PMCID: PMC1514521.

Fig. 1
Development of pancreas. (A) the ventral (Ve) and dorsal (Do) buds of the pancreas develop opposite to each other at the junction of foregut and midgut. (B) The ventral bud undergoes clockwise rotation around the caudal part of the foregut. (C) The dorsal and ventral pancreatic ducts fuse in the region of the neck of pancreas. (D) The fused ventral pancreatic duct and the proximal part of dorsal pancreatic duct forms the main pancreatic duct, while the remaining distal portion of the dorsal pancreatic duct may persist as accessory pancreatic duct.
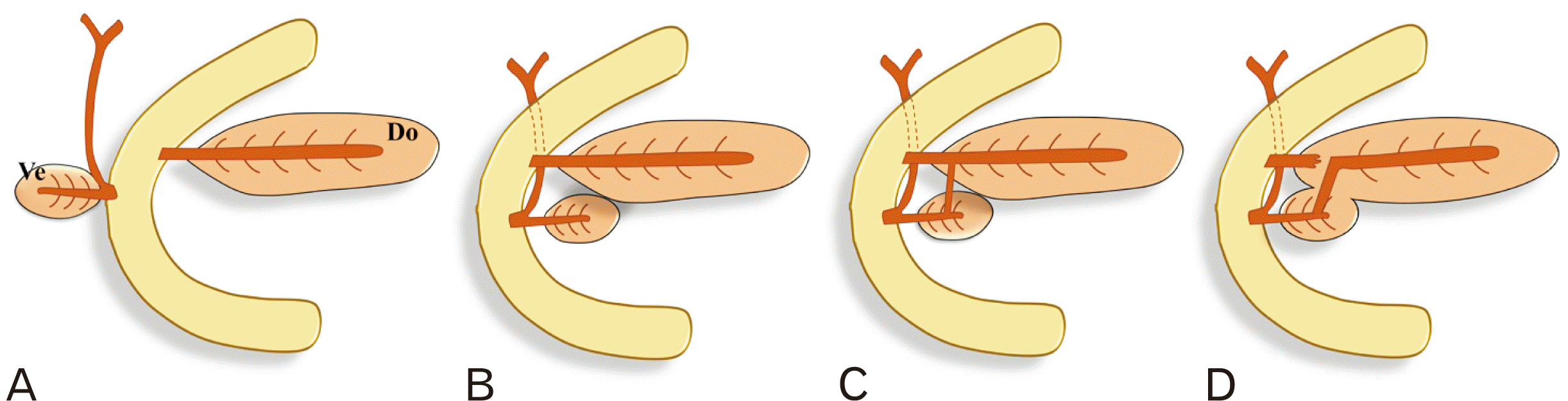
Fig. 2
Shows the configurational variants of the pancreatic ductal system (PDS). Types 1–3 shows normal pancreatic variants, and types 4, 5 shows variants of pancreas divisum. MP, major duodenal papilla; MiP, minor duodenal papilla.
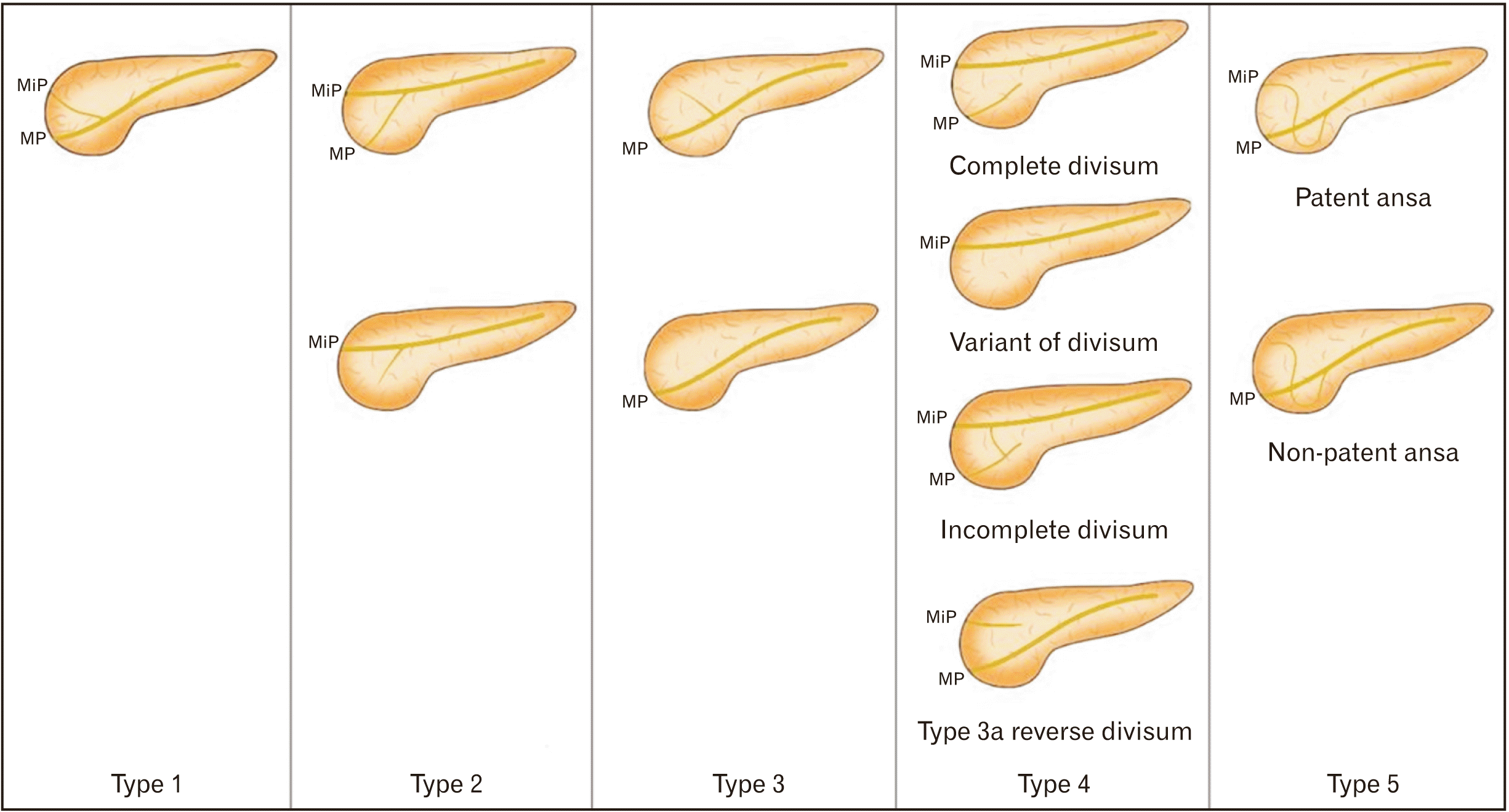
Fig. 3
Variants of the main pancreatic duct. Adapted from Türkvatan et al. Korean J Radiol 2013;14:905-13 [12].
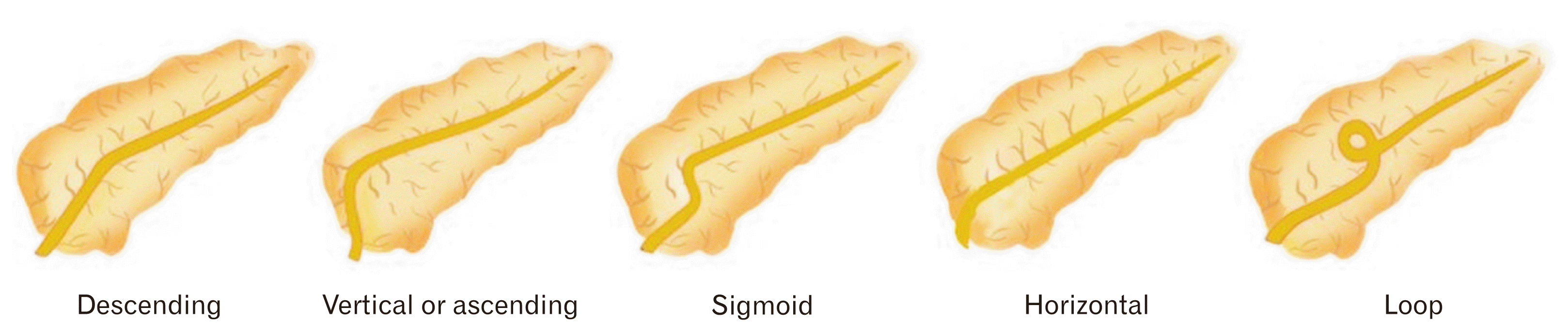
Fig. 4
Variants of the accessory pancreatic duct (A) long type, (B) short type. MP, major duodenal papilla; MiP, minor duodenal papilla. Adapted from Kamisawa et al. World J Gastroenterol 2010;16:4499-503 [17].
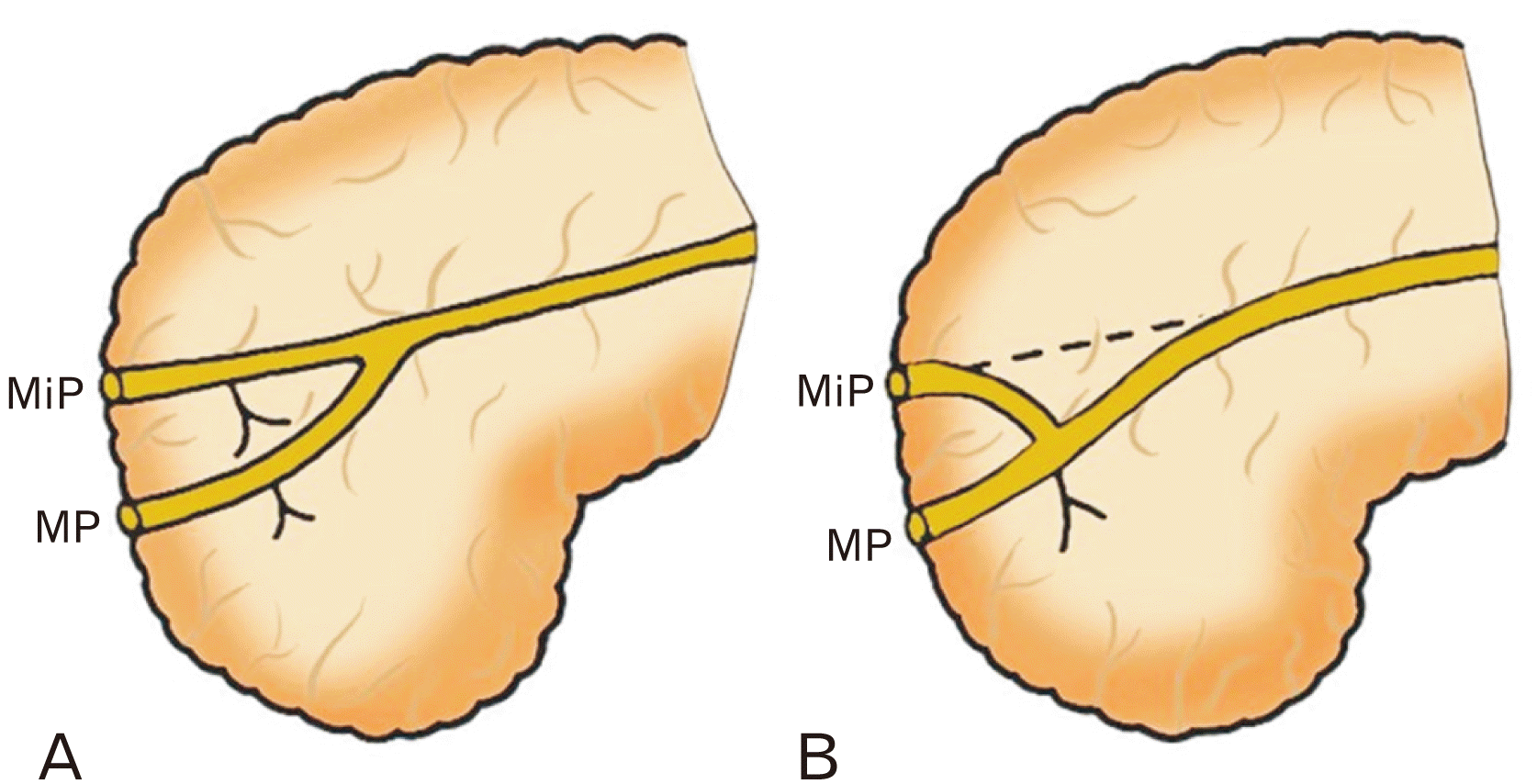
Fig. 6
Forest plot showing pooled estimate of the length of (A) the main pancreatic duct, and (B) the accessory pancreatic duct. SD, standard deviation; IV, inverse variance; CI, confidence interval; ERCP, endoscopic retrograde cholangiopancreatography.
Table 1
Characteristics of included studies
| Author (yr) | Country | Ethnicity | Type of subject | Type of investigation | Type of article | Outcome | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| De Filippo et al. (2008) [15] | Italy | European | Living patient | MRCP | Prospective study | Ductal variation | *** | **** | *** | 10 |
| Adibelli et al. (2017) [9] | Turkey | Asian | Living patient | MRCP | Retrospective cohort study | Ductal variation | ** | *** | *** | 8 |
| Bülow et al. (2014) [11] | Germany | European | Healthy volunteers | MRCP | Prospective study | Ductal variation | ** | *** | ** | 7 |
| Bang et al. (2006) [16] | Korea | Asian | Living patient | ERCP | Retrospective cohort study | Ductal variation | *** | ** | *** | 8 |
| Kamisawa et al. (2010) [17] | Japan | Asian | Living patient | ERCP | Retrospective cohort study | Ductal variation | *** | *** | *** | 9 |
| Shahriah et al. (2014) [18] | Bangladesh | Asian | Cadaver | Dissection | Cross-sectional study | Ductal variation | ** | ** | ** | 6 |
| Kim et al. (2002) [19] | Korea | Asian | Living patient | ERCP | Retrospective cohort study | Ductal variation | *** | *** | **** | 10 |
| Oracz et al. (2006) [20] | Poland | European | Living patient | MRCP | Retrospective cohort study | Ductal variation | ** | *** | **** | 9 |
| Uomo et al. (1995) [21] | Italy | European | Living patient | ERCP | Retrospective cohort study | Ductal variation | ** | *** | ** | 7 |
| Prasanna et al. (2015) [22] | India | Asian | Cadaver | Duct injection | Cross-sectional study | Ductal variation | *** | ** | *** | 8 |
| Prasad et al. (2019) [23] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal variation | *** | *** | *** | 9 |
| Prasad et al. (2019) [23] | India | Asian | Living patient | MRCP | Prospective study | Ductal variation | ** | ** | ** | 6 |
| Abdelkareem et al. (2019) [3] | Palestine | Asian | Living patient | MRCP | Retrospective study | Ductal variation | *** | *** | **** | 10 |
| Koshariya et al. (2019) [24] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal variation | ** | *** | **** | 9 |
| Gonoi et al. (2013) [25] | Japan | Asian | Living patient | MRCP | Prospective study | Ductal variation | *** | *** | *** | 9 |
| Darnis et al. (1984) [26] | France | European | Living patient | ERCP | Prospective study | Ductal variation | ** | ** | ** | 6 |
| Schmitt et al. (2010) [27] | France | European | Autopsy | Duct injection | Cross-sectional study | Ductal variation | *** | *** | **** | 10 |
| Smanio (1969) [28] | Brazil | South American | Autopsy | Duct injection | Cross-sectional study | Ductal variation | ** | *** | **** | 9 |
| Dawson and Langman (1961) [29] | Canada | North American | Autopsy | Duct injection | Cross-sectional study | Ductal variation | ** | *** | ** | 7 |
| Berman et al. (1960) [30] | USA | North American | Autopsy | Duct injection | Cross-sectional study | Ductal variation | *** | ** | **** | 9 |
| Ishaque et al. (2020) [31] | Pakistan | Asian | Living patient | MRCP | Prospective study | Ductal variation | *** | *** | *** | 9 |
| Johansson et al. (2022) [32] | Finland | European | Living patient | MRCP | Prospective study | Ductal variation | ** | ** | ** | 6 |
| Covantsev et al. (2022) [4] | Russia | European | Cadaver | Dissection | Cross-sectional study | Ductal variation & dimension | *** | *** | **** | 10 |
| Malathi and Kishan Reddy (2019) [33] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal variation | ** | *** | **** | 9 |
| Shu et al. (2006) [34] | Chinese | Asian | Living patient | MRCP | Prospective study | Ductal variation & dimension | *** | ** | ** | 7 |
| Kasugai et al. (1972) [35] | Japan | Asian | Living patient | ERCP | Prospective study | Ductal variation & dimension | *** | *** | **** | 9 |
| Kreel and Sandin (1973) [36] | UK | European | Cadaver | Pancreatogram | Prospective study | Ductal variation & dimension | ** | *** | *** | 9 |
| Kang et al. (1989) [37] | Korea | Asian | Living patient | ERCP | Prospective study | Ductal variation & dimension | *** | ** | ** | 6 |
| Vinay Kumar and Lokanadham (2019) [38] | India | Asian | Cadaver | Dissection | Prospective study | Ductal variation & dimension | ** | *** | **** | 10 |
| Hayashi et al. (2016) [39] | Japan | Asian | Living patient | MRCP | Prospective study | Ductal dimension | *** | *** | **** | 9 |
| Govindraj and Shabna (2017) [40] | India | Asian | Cadaver | Dissection | Prospective study | Ductal dimension | ** | ** | ** | 6 |
| Liessi et al. (2010) [41] | Italy | European | Living patient | MRCP | Prospective study | Ductal dimension | *** | *** | **** | 10 |
| Varley et al. (1976) [42] | USA | North American | Living patient | ERCP | Prospective study | Ductal dimension | *** | *** | *** | 9 |
| Classen et al. (1973) [43] | Germany | European | Living patient | ERCP | Prospective study | Ductal dimension | ** | ** | ** | 6 |
| Kasugai et al. (1972) [35] | Japan | Asian | Living patient | ERCP | Prospective study | Ductal variation & dimension | *** | *** | **** | 10 |
| Cotton (1974) [44] | UK | European | Living patient | ERCP | Prospective study | Ductal dimension | ** | *** | **** | 9 |
| Seifert et al. (1973) [45] | Germany | European | Living patient | ERCP | Prospective study | Ductal dimension | ** | *** | ** | 7 |
| Okuda et al. (1973) [46] | Japan | Asian | Living patient | ERCP | Prospective study | Ductal dimension | *** | ** | *** | 8 |
| Sivak and Sullivan (1976) [47] | USA | North American | Living patient | ERCP | Prospective study | Ductal dimension | *** | *** | *** | 9 |
| Ara et al. (2011) [48] | Bangladesh | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | ** | ** | ** | 6 |
| Kochhar et al. (1996) [49] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | *** | *** | **** | 10 |
| Sahni et al. (2001) [50] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | ** | *** | **** | 9 |
| Fatima (2010) [51] | Bangladesh | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | *** | *** | *** | 9 |
| Wilasrusmee and Pongchairerks (1999) [52] | Thailand | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | ** | ** | ** | 6 |
| Pina (2016) [53] | Argentina | South American | Cadaver | Dissection | Cross-sectional study | Ductal dimension | *** | *** | **** | 10 |
| Anand et al. (1989) [1] | India | Asian | Living patient | ERCP | Prospective study | Ductal dimension | ** | *** | **** | 9 |
| Hastier et al. (1998) [54] | France | European | Living patient | Pancreatogram | Prospective study | Ductal dimension | ** | *** | ** | 7 |
| Akochi et al. (2018) [55] | Rwanda | African | Living patient | USG | Prospective study | Ductal dimension | *** | *** | **** | 10 |
| Karak et al. (1991) [56] | India | Asian | Living patient | ERCP | Prospective study | Ductal dimension | ** | *** | ** | 7 |
| Hadidi (1983) [57] | Iran | Asian | Living patient | USG | Prospective study | Ductal dimension | *** | ** | *** | 8 |
| Cremer et al. (1976) [69] | Belgium | European | Living patient | ERCP | Prospective study | Ductal dimension | NA | NA | NA | NA |
| Millbourn (1960) [58] | Sweden | European | Living patient | Pancreatogram | Prospective study | Ductal dimension | *** | *** | *** | 9 |
| Trapnell and Howard (1966) [59] | UK | European | Living patient | Pancreatogram | Prospective study | Ductal dimension | ** | ** | ** | 6 |
| Hand (1963) [60] | USA | North American | Cadaver | Dissection | Cross-sectional | Ductal dimension | *** | *** | **** | 10 |
| Ogoshi et al. (1973) [61] | Japan | Asian | Living patient | ERCP | Prospective study | Ductal variation & dimension | *** | *** | *** | 9 |
| Birnstingl (1959) [62] | UK | European | Living patient | Pancreatogram | Prospective study | Ductal dimension | ** | ** | ** | 6 |
| MacCarty et al. (1975) [63] | India | Asian | Living patient | Pancreatogram | Prospective study | Ductal dimension | *** | *** | **** | 10 |
| Toda et al. (1998) [64] | Japan | Asian | Living patient | ERCP | Prospective study | Ductal dimension | ** | *** | **** | 9 |
| Aubé et al. (2003) [65] | France | European | Living patient | MRCP | Prospective study | Ductal variation & dimension | ** | *** | ** | 7 |
| Arora et al. (2011) [2] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | *** | *** | **** | 10 |
| Mchonde and Gesase (2014) [66] | Tanzania | African | Cadaver | Dissection | Cross-sectional study | Ductal dimension | ** | *** | **** | 9 |
| Jirasiritham et al. (2016) [67] | Thailand | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | ** | *** | ** | 7 |
| Malathi and Kishan Reddy (2019) [33] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | *** | *** | **** | 10 |
| Gosavi and Gaikwad (1980) [68] | India | Asian | Cadaver | Dissection | Cross-sectional study | Ductal dimension | ** | *** | ** | 7 |
Table 2
Pooled estimates of morphometric parameters and morphological subtypes of accessory pancreatic duct
Table 3
Pooled estimates of morphometric parameters and morphological subtypes of main pancreatic duct
Table 4
The distribution of variants of the pancreatic ductal system and pooled estimates of the length of the main pancreatic duct and accessory pancreatic in different populations




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download