Abstract
The origin and distribution of median nerve varies among the different individuals. The median nerve variations in axillary region were reported by many authors previously. Understanding of these variations is especially necessary for clinicians to prevent iatrogenic nerve damage. The current work aimed to evaluate the possible anatomical variations of median nerve in the axillary region in a sample of the Iranian cadavers (Shiraz, Fars). We dissected 26 upper limbs from 13 male cadavers to investigate the different variations of median and musculocutaneous nerves according to Venieratos and Anagnostopoulou classification. In 23.07% of specimens (n=6), the medial root united with 2 lateral roots and formed the median nerve proximal to the coracobrachialis muscle. In one case, a communicating branch separated from the musculocutaneous nerve distal to the coracobrachialis and connected to the median nerve in upper arm. Our results suggest that there are anatomical variations of the median nerve in terms of its origin and its communication with the musculocutaneous nerve in the population of southern Iran. The anatomical knowledge of the median nerve variations is important for clinicians to improve patient health outcome. Theses variations of the median nerve should be considered during surgical procedures of the axillary region and nerve block of the infra clavicular part of the brachial plexus.
The median nerve (MN) is the terminal branch of the brachial plexus that receives sensory, motor and sympathetic fibers from the lower cervical and first thoracic spinal nerves [1]. The MN is responsible for pronation of forearm and flexion of wrist joint. It also innervates the thenar muscles and sends sensory branches to palmar surface of thumb, index and middle fingers [2]. The MN is formed by the union of the medial and lateral roots that arise from the medial and lateral cords, respectively [3]. The lateral root contains fibers of ventral rami of C5, C6, C7 spinal nerves and medial root has fibers of C8 and T1. These roots unite inferior to pectoralis minor to form main trunk of MN in anterior lateral relation to axillary artery [4]. The MN courses parallel to the brachial artery along the arm. It originates on the lateral aspect of the artery, traverses to the medial side at approximately the mid-arm level, and persists medially till it approaches the elbow. At that point, the MN gives rise to a branch innervating the pronator teres muscle, in addition to vascular branches to the brachial artery. Furthermore, an articular branch arises at the elbow joint [5].
The atypical formation of MN by 1 medial root and supernumerary lateral roots and other types of variations have been described previously (Table 1) [6-12]. In addition, the communication between MN and musculocutaneous nerve (MCN) is the most frequent of brachial plexus variations [13].
The anatomical variations of MN can be explained from an embryological point of view. The brachial plexus develops as a radicular cone of axons in upper limb bud. These axons form a primary plexus within the mesenchymal tissue of limb bud and then it divides into ventral and dorsal divisions. The MN and ulnar nerve are derived from the ventral divisions and MCN separates from MN later [14]. The outgrowth of peripheral nerve axons into the limb bud and formation of brachial plexus is complex and controlled by different chemical tracers, growth factors and their receptors. So, the alterations of such signaling molecules may contribute to different peripheral nerve formation patterns [15]. These differences are often asymptomatic and observed only during dissection, autopsy or surgery [16]. However, such variations are clinically important for surgical approaches and nerve block in axillary region. In the current work we considered the origin of MN in relation to other anatomical structures within axilla and described the communicating branches between MCN and MN.
The upper extremities of twenty-six human cadaveric specimens (n=26) were dissected based on Grant’s Dissector [17]. The upper limb was abducted to 45°. A longitudinal skin incision was made at mid-axillary line and then the superficial fascia was removed carefully. The pectoralis major muscle was incised and reflected laterally. A longitudinal incision was made at anterior wall of axillary sheath and its contents (axillary vessels and brachial plexus) were separated using blunt dissection. The terminal branches of brachial plexus (MCN, MN, medial, and lateral roots, medial and lateral cords and ulnar nerve) were identified. These nerves usually form an “M”-shaped plexus anterior to third part of axillary artery. The morphology and relations of MCN, MN, and ulnar nerve to adjacent structures were investigated. The variations (communicating branches) were recorded by digital photography and categorized into 3 types according to Venieratos and Anagnostopoulou [18]. 1) Type 1: the communicating branch is proximal to the coracobrachialis. 2) Type 2: the communication branch is distal to the coracobrachialis. 3) Type 3: the MCN doesn’t penetrate the coracobrachialis. Instead the MN gives off a muscular branch to the coracobrachialis (Fig. 1).
The typical formation of the MN was observed in 19 (73.07%) upper limbs. In these specimens, the main branches of the lateral and medial cords were distributed normally inferior to the pectoralis minor and posterior to the pectoralis major muscles. The “M”-shaped configuration between the MCN, MN, and ulnar nerves was obvious. The MCN originated from the lateral cord and reached the coracobrachialis muscle (at the pierce point) and innervated the all muscles of anterior compartment of the arm (coracobrachialis, biceps brachii and brachialis). Thereafter, this nerve continued as the lateral cutaneous nerve of the forearm. The medial root of MN crossed the 3rd part of the axillary artery inferior to the pectoralis minor and it united with the lateral root from lateral cord. The axillary artery was embraced with roots of the MN and after some distance it was continued as the brachial artery. The axillary vein was located in the medial part of the axillary region and it was related to the ulnar nerve and medial cutaneous nerve of the forearm. In addition, some axillary lymph nodes were embedded in axillary adipose tissue (Fig. 2).
In six specimens, a supernumerary lateral root separated from the medial aspect of MCN and joined the medial root proximal to the pierce point of the coracobrachialis. The bilateral variation of type 2 was observed in case 13 only (Tables 2 and 3). In Case 1, we observed that the left MCN was completely formed posterior to the pectoralis minor muscle and pierced the coracobrachialis. The Y shaped connecting branch was also separated from MCN and its distal part divided into slender first and second lateral roots (LR1 and LR2) (Fig. 3A). The medial root didn’t cross the third part of axillary artery and it united with double lateral roots. Other branches of the medial cord, including the ulnar nerve and the medial cutaneous nerve of the forearm were originated inferior to the pectoralis minor muscle. On the right side, the main branches of the medial and lateral cords were normally distributed.
In case 3, the second lateral root separated from the MCN just proximal to the coracobrachialis. The medial root of the MN crossed the 3rd part of the axillary artery and received lateral roots (Fig. 3B). On the left side, the arrangement of main branches of the brachial plexus was normal and the MCN, MN, and ulnar nerves formed the M-shaped connection as described before. A similar variation was observed in case 4 and on the left side. The medial root of MN originated from the medial cord and crossed the axillary artery. The proximal and distal lateral roots of MN were also separated from the lateral cord and MCN, respectively. In this specimen, the Y shaped roots of MN completely surrounded the 3rd part of the axillary artery (Fig. 3C). We also discovered type 2 variation in the left axillary region of case 5 (Fig. 3D). However, there was no variation in the right axillary region of this case.
In Case 2, we discovered a communicating branch between the right MCN and MN distal to the coracobrachialis muscle (Fig. 4A). The MCN originated from the lateral cord, 5 cm distal to the coracoid process. About 6 cm distal to the pierce point of coracobrachialis, the muscular and communicating branches were separated from MCN. The communicating branch crossed the brachial artery from lateral to medial and joined the MN in the upper part of the arm. We didn’t find any variation in the left upper extremity but the medial and lateral roots were atypically long. The left MCN was separated from the lateral cord near the shoulder joint (4.5 cm distal to the coracoid process) and pierced the coracobrachialis after passing 1 cm. The MN and ulnar nerves also originated 11 and 7 cm distal to the coracoid process respectively (Fig. 4B).
In the present study, we observed unilateral MN supernumerary lateral roots in 23.07% of specimens. The bilateral formation of MN by 2 lateral roots and 1 medial root was observed in a single case. In consistency, Budhiraja et al. [8] studied the MN formation and variations in the Indian population. They observed 3 roots forming the MN from 22.4% upper limbs. In 14.2% of specimens, the supernumerary lateral root was derived from the lateral cord. Whilst, in 8.16% of the upper extremities, the communicating branch separated from the MCN. In addition, the MN formation by 4 roots was noted in 3.57% of the upper extremities [8]. Similarly, Ghosh et al. [19] reported the MN formation by 3 lateral roots in 13 specimens (21.7%) from 60 upper limbs. In addition, they observed MN formation by 4 roots (1 medial and 3 lateral roots) in 3 specimens [19]. Clinically, MN and other branches of brachial plexus are vulnerable to surgical interventions or trauma. If the MN with the additional lateral root is damaged within the axilla, the pronation of forearm and flexion of the wrist joint are lost. The additional lateral root contains fibers of C5 and C6. So, any damage to these fibers may lead to weakness of arm flexion and anesthesia of the lateral half of the forearm [20].
Typically, the medial root of MN crosses the third part of axillary artery and unites with the lateral root to form MN anterior or lateral to the axillary artery. In contrast, the atypical lateral root of MN may cross the axillary artery obliquely and MN forms medially [21, 22]. However, the ischemia of the upper extremity induced by compression of the axillary artery has not been documented in such cases. The present study also revealed that the double lateral roots were in close relation to the third part of the axillary artery. These roots were associated with the anterior aspect of the axillary artery and joined the medial root to form MN.
We found a single specimen in which the MN and MCN had a connecting branch distal to the coracobrachialis muscle (type 2). The MCN is well protected by biceps brachii muscle but it’s prone to damage in shoulder dislocations, coracoid process grafting, arthroscopies and plastic surgeries [23]. If the distal communicating branch is present, any proximal lesion to MCN may impact the MN fibers [24]. In the current study we did not find variation type 3. In this case, the MCN does not innervate the coracobrachialis and instead the lateral cord sends a motor branch to this muscle [25]. The absence of MCN and innervation of coracobrachialis by MN is very rare [26]. Tatar et al. [27] described the innervation of coracobrachialis by a branch from the lateral cord. This branch originated from the lateral root and bifurcated into upper and lower filaments and entered the coracobrachialis [27].
In conclusion, the anatomical knowledge of MN origin and its variations is necessary for clinicians, especially for orthopedic surgeons and anesthesiologists. Understanding of these abnormalities may prevent sensory and motor injuries during surgical approaches in axillary region.
The main limitations of current work are the small sample size and an unknown history of pathologies. In fact, we had access to a limited number of fresh specimens and our study highlights a single center anatomical experience. Therefore, we recommend a meta-analysis of MN variations to acquire more reliable results.
Acknowledgements
We express our gratitude to Fars Legal Medicine Research Center (Shiraz, Fars Province, Iran) for guidance and preparation of specimens. We are also thankful to Shiraz University of Medical Sciences.
Notes
References
1. Bilecenoglu B, Uz A, Karalezli N. 2005; Possible anatomic structures causing entrapment neuropathies of the median nerve: an anatomic study. Acta Orthop Belg. 71:169–76. PMID: 16152850.
2. Soubeyrand M, Melhem R, Protais M, Artuso M, Crézé M. 2020; Anatomy of the median nerve and its clinical applications. Hand Surg Rehabil. 39:2–18. DOI: 10.1016/j.hansur.2019.10.197. PMID: 31816428.

3. Budhiraja V, Rastogi R, Asthana AK. 2012; Variations in the formation of the median nerve and its clinical correlation. Folia Morphol (Warsz). 71:28–30. DOI: 10.2139/ssrn.4325366. PMID: 22532182.
4. Akhtar MJ, Kumar S, Chandan CB, Kumar B, Sinha RR, Akhtar MK, Kumar A. 2022; Variations in the formation of the median nerve and its clinical correlation. Maedica (Bucur). 17:878–84. DOI: 10.2139/ssrn.4325366. PMID: 36818254. PMCID: PMC9923088.

5. Chaurasia BD. Human anatomy: regional and applied, dissection and clinical. CBS Publishers & Distributors;2004.
6. Saeed M, Rufai AA. 2003; Median and musculocutaneous nerves: variant formation and distribution. Clin Anat. 16:453–7. DOI: 10.1002/ca.10096. PMID: 12903070.

7. Goyal N, Harjeet , Gupta M. 2005; Bilateral variant contributions in the formation of median nerve. Surg Radiol Anat. 27:562–5. DOI: 10.1007/s00276-005-0023-6. PMID: 16151971.

8. Budhiraja V, Rastogi R, Asthana AK. 2011; Anatomical variations of median nerve formation: embryological and clinical correlation. J Morphol Sci. 28:283–6.
9. Hada S, Kadel M, Pandit TK, Basnet KS. 2020; Variations in formation of median nerve: a cadaveric study. J Chitwan Med Coll. 10:66–8. DOI: 10.3126/jcmc.v10i3.32048.

10. Pandey SK, Shukla VK. 2007; Anatomical variations of the cords of brachial plexus and the median nerve. Clin Anat. 20:150–6. DOI: 10.1002/ca.20365. PMID: 16795062.

11. Bala A, Sinha P, Tamang BK, Sarda RK. 2014; Anatomical variation: median nerve formation - a case vignette. J Clin Diagn Res. 8:AD03–4. DOI: 10.7860/JCDR/2014/7620.4455. PMID: 25120965. PMCID: PMC4129350.

12. Gelmi CAE, Pedrini FA, Fermi M, Mariani GA, Cocco LI, Billi AM. 2018; Communication between median and musculocutaneous nerve at the level of cubital fossa - a case report. Transl Res Anat. 11:1–4. DOI: 10.1016/j.tria.2018.04.001.

13. Chauhan R, Roy TS. 2002; Communication between the median and musculocutaneous nerve - a case report. J Anat Soc India. 51:72–5.
14. Natsis K, Piagkou M, Totlis T, Kapetanakis S. 2020; A prefix brachial plexus with two trunks and one anterior cord. Folia Morphol (Warsz). 79:402–6. DOI: 10.5603/FM.a2019.0081. PMID: 31322725.

15. Leijnse JN, de Bakker BS, D'Herde K. 2020; The brachial plexus - explaining its morphology and variability by a generic developmental model. J Anat. 236:862–82. DOI: 10.1111/joa.13123. PMID: 31814126. PMCID: PMC7163732.

16. Sontakke BR, Tarnekar AM, Waghmare JE, Ingole IV. 2011; An unusual case of asymmetrical formation and distribution of median nerve. Int J Anat Var. 4:57–60.
17. Tank PW, Grant JCB. Grant's dissector. Wolter Kluwer Health/Lippincott Williams & Wilkins;2013.
18. Venieratos D, Anagnostopoulou S. 1998; Classification of communications between the musculocutaneous and median nerves. Clin Anat. 11:327–31. DOI: 10.1002/(SICI)1098-2353(1998)11:5<327::AID-CA6>3.0.CO;2-M. PMID: 9725577.

19. Ghosh B, Dilkash MNA, Prasad S, Sinha SK. 2022; Anatomical variation of median nerve: cadaveric study in brachial plexus. Anat Cell Biol. 55:130–4. DOI: 10.5115/acb.22.022. PMID: 35718802. PMCID: PMC9256496.

20. Benes M, Kachlik D. 2021; Atypical branching of the musculocutaneous and median nerves with associated unusual innervation of muscles in the anterior compartment of the arm: case report and plea for extension of the current classification system. Surg Radiol Anat. 43:671–8. DOI: 10.1007/s00276-021-02731-8. PMID: 33689004.

21. Singhal S, Rao VV, Ravindranath R. 2007; Variations in brachial plexus and the relationship of median nerve with the axillary artery: a case report. J Brachial Plex Peripher Nerve Inj. 2:21. DOI: 10.1186/1749-7221-2-21. PMID: 17915015. PMCID: PMC2082021.

22. Das S, Paul S. 2005; Anomalous branching pattern of lateral cord of brachial plexus. Int J Morphol. 23:289–92. DOI: 10.4067/S0717-95022005000400001.

23. Darvishi M, Moayeri A. 2019; Anatomical variations of the musculocutaneous and median nerves: a case report. Folia Med (Plovdiv). 61:327–31. DOI: 10.2478/folmed-2018-0080. PMID: 31301650.

24. Guerri-Guttenberg RA, Ingolotti M. 2009; Classifying musculocutaneous nerve variations. Clin Anat. 22:671–83. DOI: 10.1002/ca.20828. PMID: 19637305.

25. Abhaya A, Khanna J, Prakash R. 2003; Variation of the lateral cord of brachial plexus piercing coracobrachialis muscle. J Anat Soc India. 52:168–70.
26. Aydin ME, Kale A, Edizer M, Kopuz C, Demir MT, Corumlu U. 2006; Absence of the musculocutaneous nerve together with unusual innervation of the median nerve. Folia Morphol (Warsz). 65:228–31.
27. Tatar I, Brohi R, Sen F, Tonak A, Celik H. 2004; Innervation of the coracobrachialis muscle by a branch from the lateral root of the median nerve. Folia Morphol (Warsz). 63:503–6. PMID: 15712151.
Fig. 1
(A) Schematic representation of brachial plexus, (B) typical formation of median nerve and (C) three different atypical communications between median and musculocutaneous nerves. MN, median nerve; LR, lateral root (1 and 2); MCN, musculocutaneous nerve; CRDs, cords; LC, lateral cord; DIVs, divisions; TNKs, trunks; RTs, roots; MR, medial root; UN, ulnar nerve; MC, medial cord; PC, posterior cord; PMi, pectoralis minor; CP, coracoid process; AA, axillary artery; Dt, deltoid muscle; CB, coracobrachialis; PM, pectoralis major; SB, short head of biceps; LB, long head of biceps; SJ, shoulder joint; LCF, lateral cutaneous nerve of forearm; COM, communicating branch; NCB, nerve to coracobrachialis.
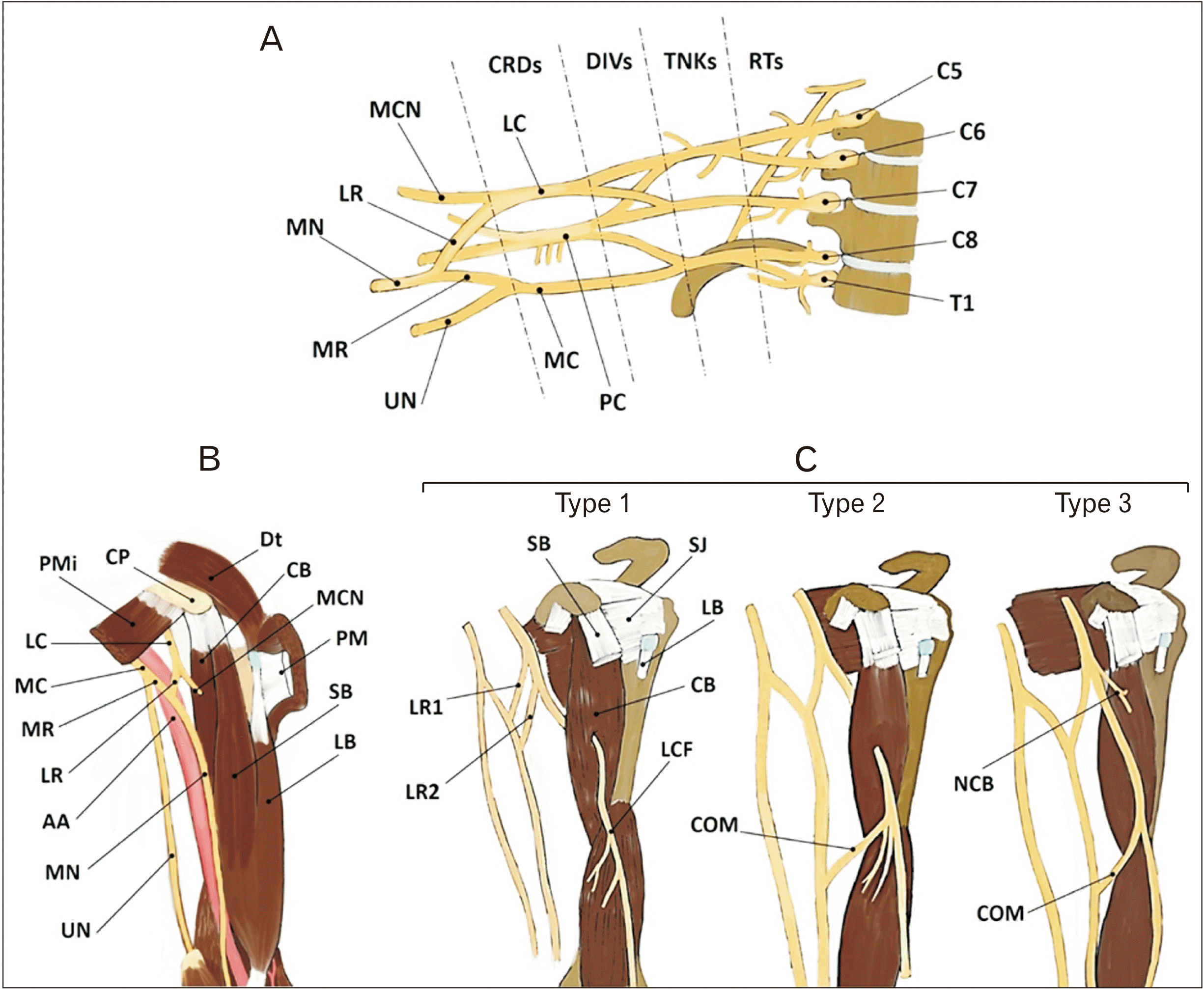
Fig. 2
The normal distribution of branches from lateral and medial cords in right axillary region. PMi, pectoralis minor; LC, lateral cord; MCN, musculocutaneous nerve; PM, pectoralis major; LR, lateral root of median nerve; MR, medial root of median nerve; CB, coracobrachialis; MN, median nerve; BA, brachial artery; AA, axillary artery; AV, axillary vein; MC, medial cord; MCF, medial cutaneous nerve of forearm; UN, ulnar nerve; LN, axillary lymph nodes.
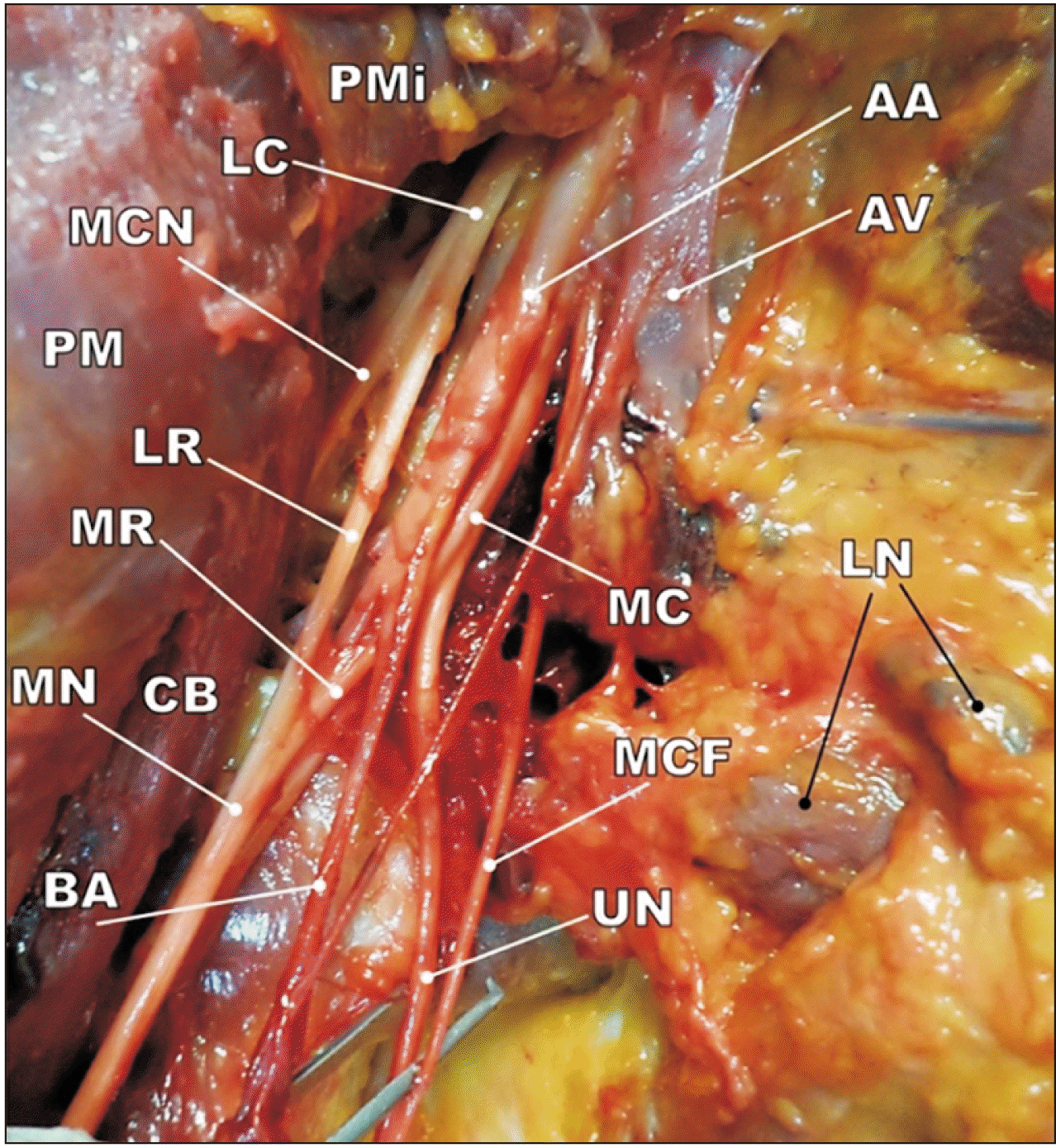
Fig. 3
(A–D) Photographs depicting the 2 lateral roots of median nerve in case 1, 3, 4, and 5. LR1, first lateral root; LR2, second lateral root; MN, median nerve; AA, axillary artery; Pmi, pectoralis minor muscle; MC, medial cord; MR, medial root; MCF, medial cutaneous nerve of forearm; AV, axillary vein; UN, ulnar nerve; LC, lateral cord; PM, pectoralis major muscle; MCN, musculocutaneous nerve; CB, coracobrachialis muscle; RN, radial nerve.
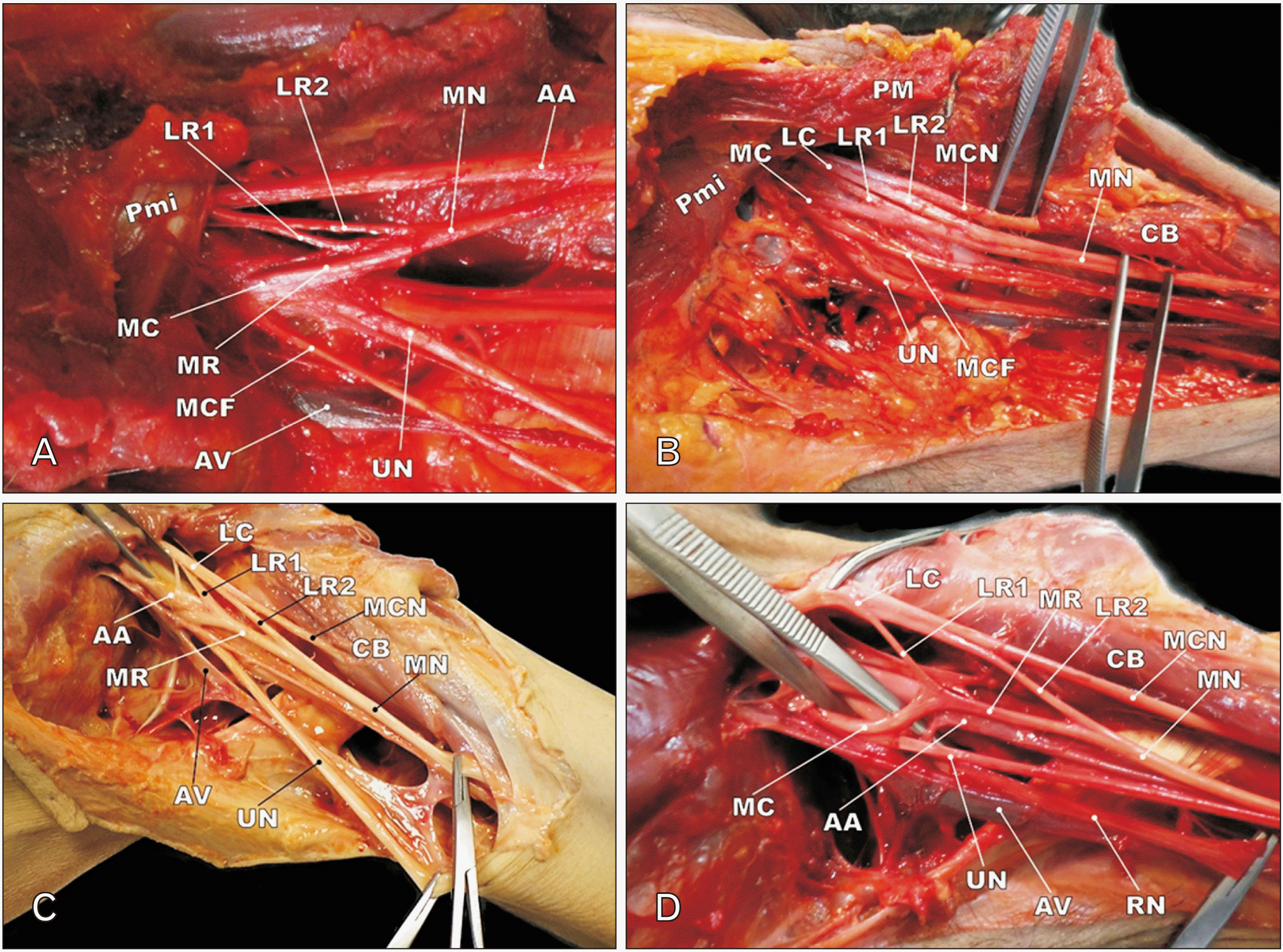
Fig. 4
(A) Dissection of right and (B) left axillary regions in case 2. COM, communicating branch; BM, biceps muscle; CB, coracobrachialis muscle; PM, pectoralis major muscle; MCN, musculocutaneous nerve; MN, median nerve; MCF, medial cutaneous nerve of forearm; AA, axillary artery; LC, lateral cord; LR, lateral root (1 and 2); MC, medial cord; AV, axillary vein; MR, medial root; UN, ulnar nerve.
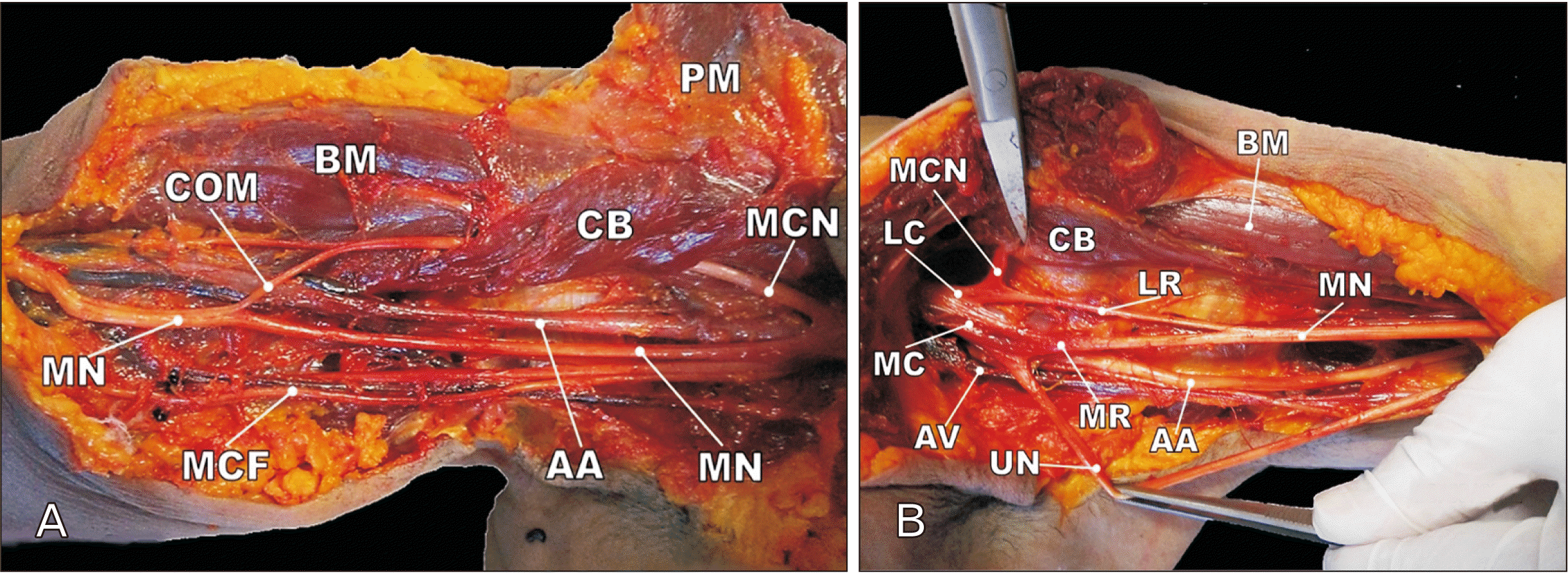
Table 1
Atypical variations of median nerve and musculocutaneous nerve
| Variation | Variables | Authors (yr) |
|---|---|---|
| Formation of median nerve by additional roots in axilla | 2 Lateral and 1 medial roots | Saeed (2003) [6] |
| 2 Lateral roots with reccurent branch to medial root | Goyal (2005) [7] | |
| 3 Lateral roots and 1 medial root | Budhiraja (2011) [8] | |
| Hada (2020) [9] | ||
| Atypical roots of median nerve | Medial root received a branch from posterior cord | Pandey (2007) [10] |
| A connecting branch between medial root and lateral cord | Bala (2014) [11] | |
| Atypical connection between MN and MCN | A long communicating branch from axilla to cubital fossa | Gelmi (2018) [12] |
Table 2
The classification of musculocutaneous and median nerve communicating branches
| Case | Right (R)/left (L) | Normal | Variation | ||
|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | |||
| 1 | R | + | |||
| L | + | ||||
| 2 | R | + | |||
| L | + | ||||
| 3 | R | + | |||
| L | + | ||||
| 4 | R | + | |||
| L | + | ||||
| 5 | R | + | |||
| L | + | ||||
| 6 | R | + | |||
| L | + | ||||
| 7 | R | + | |||
| L | + | ||||
| 8 | R | + | |||
| L | + | ||||
| 9 | R | + | |||
| L | + | ||||
| 10 | R | + | |||
| L | + | ||||
| 11 | R | + | |||
| L | + | ||||
| 12 | R | + | |||
| L | + | ||||
| 13 | R | + | |||
| L | + | ||||
Table 3
The length of medial and lateral roots of median nerve and musculocutaneous nerve (from its origin to coracobrachialis)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download