Abstract
Neutralizing capacity measurement (NCM) of soluble ABH substances (SAS) in plasma was assessed to guide the selection of the appropriate ABO group of fresh-frozen plasma (FFP) for plasma exchange (PE) in blood group O recipients with ABO-incompatible transplantations. Neutralizing capacity was assessed by measuring anti-A and/or anti-B titers in samples comprising one unit of O FFP and 10 O EDTA plasma samples and subtracting the binary logarithm of the titer in each group with a saline dilution. Ten EDTA plasma samples with Lewis b (Leb) antigen positivity and 10 sets of pooled FFP from each blood group were used as diluents. In O FFP, the NCM values (mean±SD) were 3.4±0.52 (2.6±0.52) and 2.6±0.52 (1.5±0.3) in B and AB for IgM (total antibody) anti-B (both P<0.001), and in the 10 O EDTA plasma samples, they were 3.9±0.88 (3.1±0.88) and 3.2±0.79 (2.4±0.97) for IgM (P=0.0013) and total anti-B (P=0.025), respectively. In vitro analysis revealed that B FFP is more effective than AB FFP in reducing IgM and total anti-B antibody titers in O recipients, regardless of Leb antigen positivity.
Therapeutic plasma exchange (TPE) is commonly performed in ABO-incompatible solid organ transplant (iABO-SOT) recipients for desensitization. The American Society for Apheresis (ASFA) recommends using fresh-frozen plasma (FFP) compatible with both the recipient’s and donor’s ABO blood groups when FFP is used as a replacement solution for TPE [1]. Group AB FFP is used not only in recipients with blood group A or B but also in O recipients undergoing iABO-SOT [2]. Despite its limited availability, blood group AB is widely used in Korea, irrespective of the recipient’s ABO group [3]. Soluble ABH substances (SAS) may act as immunogens in ABO hemolytic disease of the newborn [4]. The effect of the ABO group of FFP on desensitization in TPE is unclear [5, 6]. Therefore, neutralizing capacity measurement (NCM) of SAS in plasma was assessed to guide the selection of appropriate ABO group FFP for TPE in group O recipients for iABO-SOT.
This study was conducted in two panels under Institutional Review Board (IRB) approval (AJOUIRB-SMP-2020-300 for Panel I and AJOUIRB-KS-2023-063 for Panel II). Panel I spanned a year from August 2021 and Panel II was conducted from February to August 2023. Neutralizing capacity was assessed by measuring anti-A and/or anti-B antibody titers in one unit of group O FFP (Panel I) and 10 group O EDTA plasma samples (Panel II). The group O FFP with an ABO IgM antibody titer >1:16 used in Panel I was selected from waste FFP, which had expired due to refreezing after transfusion cancellation post-thawing in the blood bank of the hospital. The 10 group O EDTA plasma samples used in Panel II were collected from 10 patients for whom anti-A and/or anti-B antibody titers or ABO blood grouping had been requested, with titers >1:16 after IgM anti-A or/and anti-B antibody titration.
In Panel I, EDTA plasma samples with blood groups A, B, and AB were obtained from apparently healthy adults who underwent medical health check-ups at Ajou University Hospital and tested positive for Lewis b (Leb) antigen using commercial anti-sera (Diagast, Loos, France), indicating the secretion of soluble A, B, and AB substances [7], were used as diluents. In Panel II, 10 sets of 15 pooled FFP solutions of blood groups B, A, and AB served as diluents. Each pooled FFP solution was collected from blood bag segments of 15 FFP units with the same ABO group before release from the blood bank because of the requirement for the use of ≥15 FFP blood units in a single TPE. Two tubes with 0.9% normal saline served as the control diluent in both panels and were titrated in duplicate.
For NCM, anti-A or/and anti-B antibodies were titrated after incubating the samples and diluents at 37°C for 30 min, using a reported method [8] that was modified to enhance the sensitization of SAS and ABO antibodies, simulating in vivo conditions. Anti-A and/or anti-B antibody titers were measured using gel column agglutination with dedicated centrifuges and incubators, following the manufacturer’s instructions (Grifols, Barcelona, Spain). For ABO antibody titration, 25 μL of serially diluted plasma sample and 50 μL of Serigrup Diana A1/B (Grifols) 0.8% RBC suspension were added to gel card microcolumns. For IgM titer measurement, a DG Gel Neutral card (Grifols) was used, with a 15-min incubation at room temperature. For total antibody titer measurement, a DG Gel Coombs card (Grifols) was used, with a 15-min incubation at 37°C without dithiothreitol. The endpoint titer was defined as 1+ strength, and titer values were converted to binary logarithms (e.g., 1:16 to 4). Neutralizing capacity was calculated by subtracting the binary logarithm of the titer in each group from that in the control group (using 0.9% normal saline).
Statistical analysis was performed using Microsoft Excel (Microsoft, Redmond, WA, USA). Significance was set at P<0.05. Student t-tests and paired t-tests were used to compare mean NCM values between the A and AB or B and AB groups for anti-A or anti-B in Panels I and II, respectively. Student’s t-test was used to compare mean NCM values between anti-A and anti-B in the AB group in Panels I and II.
The diluents, including A, B, and AB group EDTA plasma with Leb antigen positivity (Fig. 1) and pooled A, B, and AB group FFPs (Fig. 2), were more effective in reducing the ABO antibody titer in group O than 0.9% normal saline, except for total anti-A in Panel I. This exception may be attributable to the elevated SD observed in the control titer (N=2), indicating a one-step titer discrepancy rather than indicating a deficiency in the neutralizing capacity of pooled A or AB group FFP. Normal saline was used instead of albumin as a negative control. While complications occur more often with FFP as a replacement solution for TPE than with albumin, FFP is more effective in reducing ABO antibody titers than albumin because of the presence of soluble ABO antigen [2, 6].
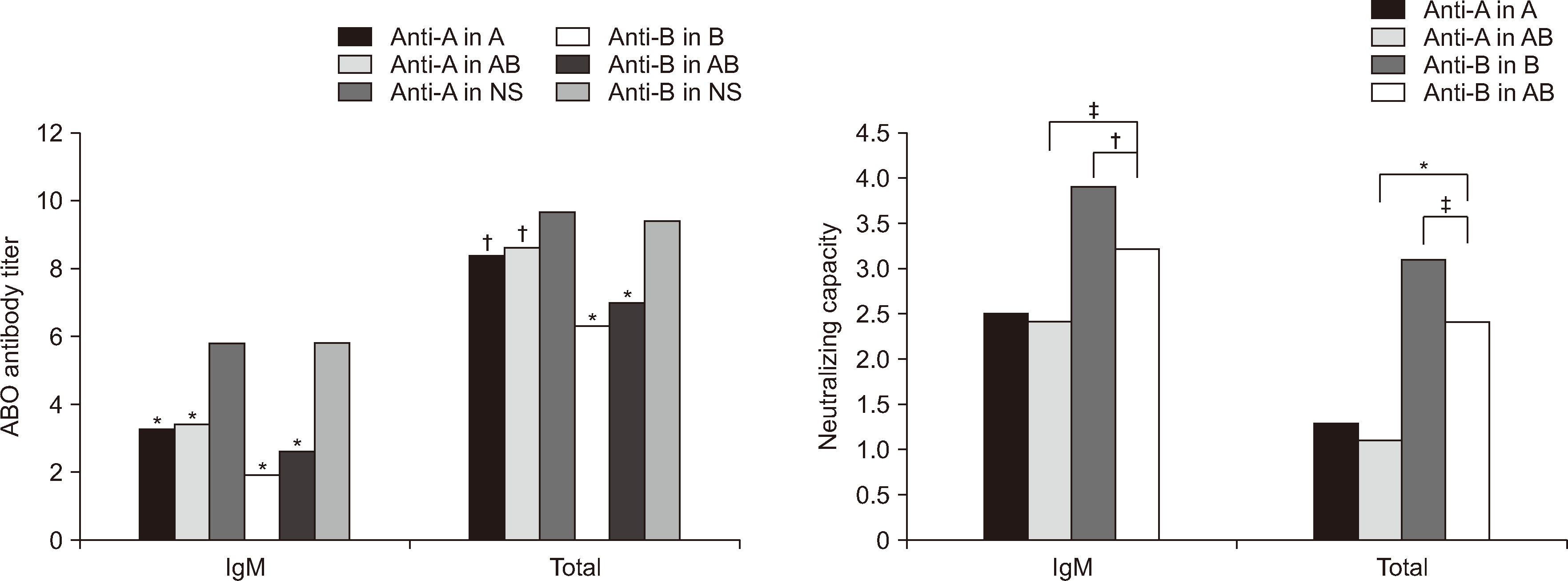
For anti-B antibodies in Panel I, the NCM values for IgM and total antibody were significantly higher in the B group than in the AB group (3.4 vs. 2.4 and 2.6 vs. 1.5, respectively; both P<0.001). This indicated that Leb antigen-positive B group EDTA plasma with group B substance was more effective in neutralizing anti-B antibodies in group O FFP than Leb-positive AB group EDTA plasma with group A and B substances. The NCM value for anti-A antibodies was significantly higher in the Leb-positive AB group than in the Leb-positive A group for total antibody (2.5 vs. 1.2, P<0.001); however, for IgM, there was no significant difference (Fig. 1). In Panel II, for anti-B antibodies, the NCM values for IgM and total antibody were significantly higher in the B group than in the AB group (3.9 vs. 3.2, P=0.001; 3.1 vs. 2.4, P=0.024, respectively) (Fig. 2). However, the NCM values for IgM and total antibody did not significantly differ between the AB and A groups.
In the study, a method simpler than flow cytometry [6] was employed for NCM of SAS in plasma, capable of detecting both IgG and IgM of ABO antibodies. ELISA is another method to measure SAS [9, 10].
A previous study demonstrated that both group AB and group A or B FFP can be infused into group O recipients, as there was no significant difference in NCM of FFP between groups A and AB [6]. This finding is consistent with our Panel II results, obtained using similar study conditions; however, the previous study did not assess NCM of FFP between groups B and AB in group O recipients.
It is unclear why only group B plasma was more effective in reducing the ABO antibody titer than group A plasma. One possibility is that group B has a higher secretor frequency than group AB, consistent with findings in Karachi, Pakistan (79.5% vs. 45.5%) [7], and Korea (73.3% vs. 63.6%) [11]. Other previous reports for groups A, B, AB, and O show varying frequencies: 72.1%, 73.3%, 63.6%, and 86.7% in Korea [11]; 70.1%, 67.8%, 67.9%, and 88.3% in Iraq [12]; and 87.1%, 73.3%, 94.2%, and 85.0% in Rajasthan, India [13], respectively. However, this does not fully explain why group A plasma was not as effective in reducing ABO antibody titers as group B plasma, despite the similar frequency of Leb positivity between groups A and B in Korea. Further studies are needed to clarify this.
The second possibility is that FFP from secretor individuals in the AB group may have a lower neutralizing capacity for soluble B substances than for soluble A substances. In the AB group with Leb positivity in Panel I, NCM values for anti-B were significantly lower than those for anti-A for IgM (2.4 vs. 2.9, P=0.019) and total antibody (1.5 vs. 2.5, P<0.001), providing support for this hypothesis. However, the AB group in Panel II, irrespective of Leb positivity, showed contrasting results.
As a limitation of this study, only one unit of FFP was used as a common sample in Panel I because of the scarcity of waste FFP in our hospital during the study. Had a pooled FFP sample been used, the results may have been more reliable. However, this limitation does not appear to be significant, as Panel II yielded consistent results.
In summary, in vitro analysis showed that FFP from donor group A or B is not inferior to AB FFP. Notably, B FFP is more effective than AB FFP in reducing IgM and total anti-B antibody titers in group O recipients, regardless of Leb antigen positivity. Further research is needed to determine whether these results can be applied in vivo and to understand why B FFP is more effective than AB FFP in O recipients.
ACKNOWLEDGEMENTS
I thank Cho HS, MT of the Department of Laboratory Medicine of the University Hospital for ABO antibody titration and Unionlab for providing gel column cards for the study.
Notes
References
1. Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, et al. 2023; Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American Society for Apheresis: the ninth special issue. J Clin Apher. 38:77–278. DOI: 10.1002/jca.22043. PMID: 37017433.

2. Parmentier SP, Rosenkranz E, Schirutschke H, Opgenoorth M, Quick C, Hoelig K, et al. 2017; Comparing the efficacy of three techniques to reduce isoagglutinin titers in ABO incompatible kidney transplant recipients. Atheroscler Suppl. 30:253–6. DOI: 10.1016/j.atherosclerosissup.2017.05.017. PMID: 29096846.
3. Chung Y, Ko DH, Lim J, Kim KH, Kim H. 2022; Choice of ABO group for blood component transfusion in ABO-incompatible solid organ transplantation: a questionnaire survey in Korea and guideline proposal. Ann Lab Med. 42:105–9. DOI: 10.3343/alm.2022.42.1.105. PMID: 34374356. PMCID: PMC8368232.

4. Krog GR, Lorenzen H, Clausen FB, Hansen AT, Donneborg ML, Dziegiel MH. 2022; ABO haemolytic disease of the newborn: improved prediction by novel integration of causative and protective factors in newborn and mother. Vox Sang. 117:415–23. DOI: 10.1111/vox.13195. PMID: 34409614.
5. Yang JJ, Ryu KS, Kim JS, Chung Y, Kim H, Hwang SH, et al. 2021; Evaluation of safety of using incompatible plasma for therapeutic plasma exchange during shortage of AB plasma. J Clin Apher. 36:628–33. DOI: 10.1002/jca.21904. PMID: 33950554.
6. Won DI, Ham JY, Kim CD, Suh JS, Kim BC. 2015; Benefits of fresh-frozen plasma as a replacement fluid to neutralize ABO antibodies. J Clin Apher. 30:288–96. DOI: 10.1002/jca.21378. PMID: 25546477.
7. Saboor M, Ullah A, Qamar K, Mir A, Monuddin . 2014; Frequency of ABH secretors and non secretors: a cross sectional study in Karachi. Pak J Med Sci. 30:189–93. DOI: 10.12669/pjms.301.4194. PMID: 24639859. PMCID: PMC3955570.
8. Fung MK, Eder AF, Spitalnik SL, Westhoff CM. 2017. Technical manual. 19th ed. American Association of Blood Banks;Bethesda: p. Methods 2–8. DOI: 10.4160/9789290604808.
9. Parigian MJ. 1995; An ELISA procedure for the detection of soluble ABH blood group substance in semen, saliva, and vaginal samples. J Forensic Sci. 40:122–5. DOI: 10.1520/JFS13774J. PMID: 7876793.

10. Zhou B, Guo JY, Wang CX, Chen J. 1990; The rapid determination of the ABO group from body fluids (or stains) by dot enzyme-linked immunosorbent assay (dot-ELISA) using enzyme-labeled monoclonal antibodies. J Forensic Sci. 35:1125–32. DOI: 10.1520/JFS12934J. PMID: 2230686.

11. Song SU, An SS, Ryu SW, Kim JS, Suh IB. 2008; Evaluation of the genotypes of the Lewis blood group in a Korean population using direct sequencing. Korean J Hematol. 43:34–42. DOI: 10.5045/kjh.2008.43.1.34.

12. Jaff MS. 2010; Higher frequency of secretor phenotype in O blood group - its benefits in prevention and/or treatment of some diseases. Int J Nanomedicine. 5:901–5. DOI: 10.2147/IJN.S13980. PMID: 21116330. PMCID: PMC2990383.
13. Rajawat G, Ramalingam K, Pareek R, Singh G, Narula H, Aggarwal A. 2023; Assessment of salivary ABO blood group antigens and secretor status in Srinagar, Rajasthan: a correlational analysis of 300 samples. Cureus. 15:e37415. DOI: 10.7759/cureus.37415. PMID: 37182010. PMCID: PMC10172881.
Fig. 1
Mean values of ABO antibody titers or neutralizing capacities in group O fresh frozen plasma (FFP) converted to binary logarithms according to the diluents in Panel I. EDTA plasmas with Lewis b antigen positivity were used as diluents for the group O FFP. The black lines and asterisk (*) above the bars in ‘ABO antibody titer’ represent the mean value of 0.9% of normal saline of control group and a significant difference vs. the control group, respectively. *P<0.001; †P<0.05. Neutralizing capacity was the titer differences assessed by subtracting the binary logarithm value of the titer in each blood group from that in 0.9% normal saline.
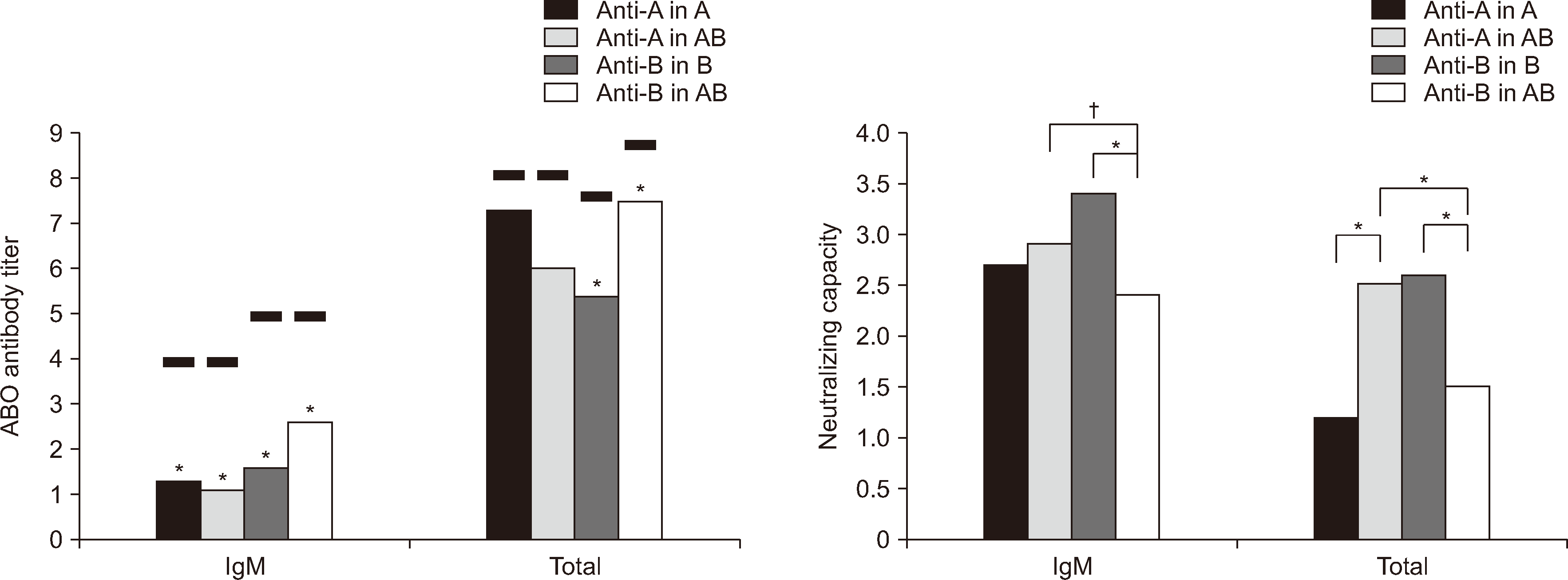
Fig. 2
Mean values of ABO antibody titers or neutralizing capacities in ten blood group O EDTA samples converted to binary logarithms according to diluents in Panel II. Pooled fresh frozen plasmas (FFP) were utilized as diluents for the O EDTA samples, with each pooled FFP comprising blood bag segments from 15 FFP samples of the same ABO blood group. The symbols above bars in ‘ABO antibody titer’ repsent a significant difference compared to normal saline (NS). *P<0.001; †P<0.01; ‡P<0.05. Refer to Fig. 1. for information on neutralizing capacity.
Abbreviation: NS, 0.9% normal saline.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download