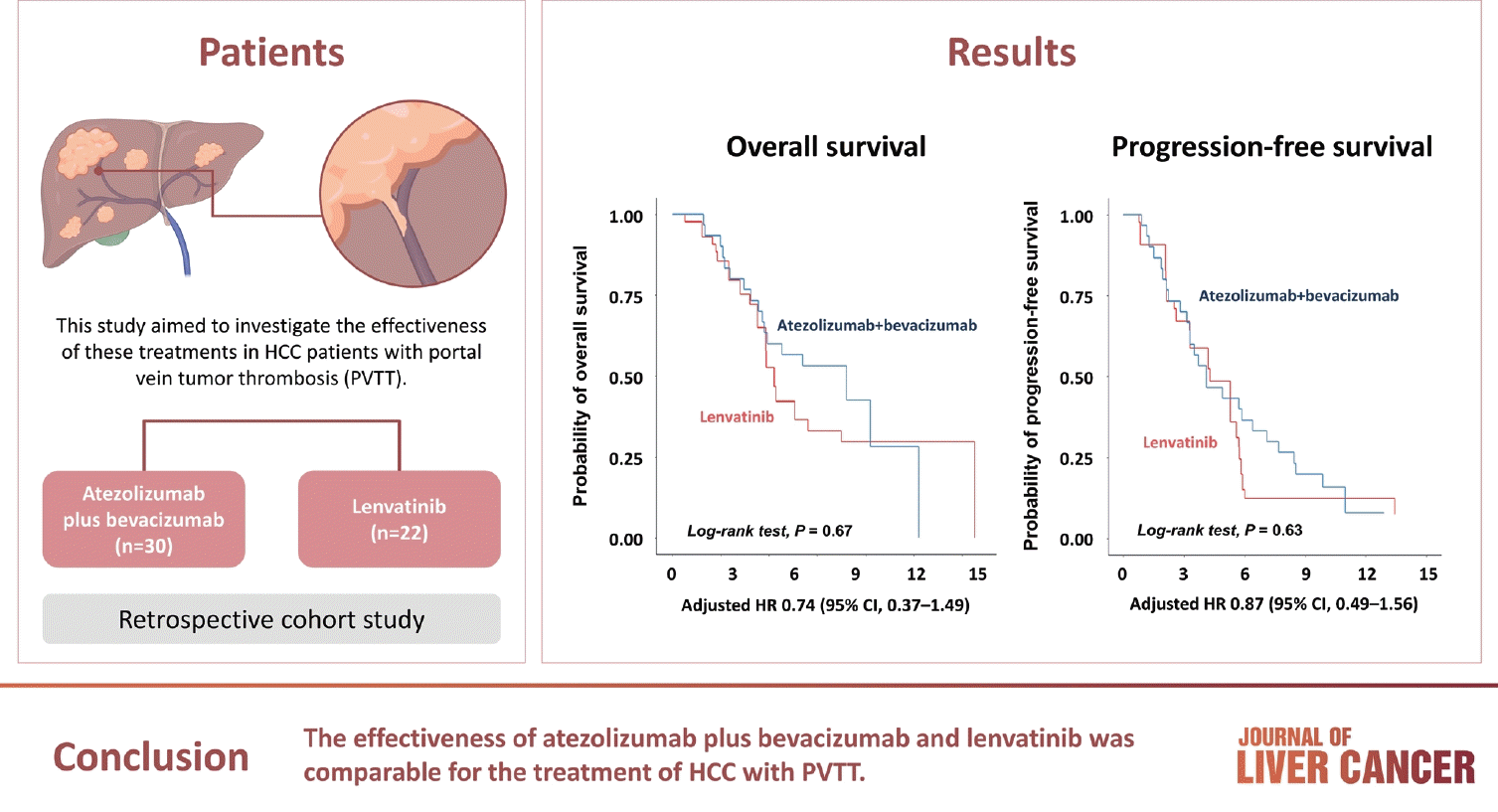
Abstract
Background/Aim
Methods
Results
Notes
Ethics Statement
This study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1805-055-944). The requirement to obtain informed consent from patients was waived owing to the retrospective nature of the study.
Funding Statement
This study was supported by the Scientific Research Fund of the Korean Liver Cancer Association (2021).
Data Availability
The data presented in this study are available upon request from the corresponding author.
Author Contribution
Conceptualization: JP, YBL, DWL
Data curation: JP, YBL, YK, YP, HS, MHH, MKP, EJC, KHL, JHL, SJY, TYK, YJK, TYK, JHY
Formal analysis: JP, YBL
Investigation: JP, YBL, YK, YP, HS, MHH, MKP, DWL, EJC, KHL, JHL, SJY, TYK, YJK, TYK, JHY
Methodology: JP, YBL
Project administration: YBL
Resources: JP
Supervision: JHY
Visualization: JP
Writing - original draft: JP, YBL
Writing - review & editing: JP, YBL, YK, YP, HS, MHH, MKP, DWL, EJC, KHL, JHL, SJY, TYK, YJK, TYK, JHY
Supplementary Material
References
Figure 1.
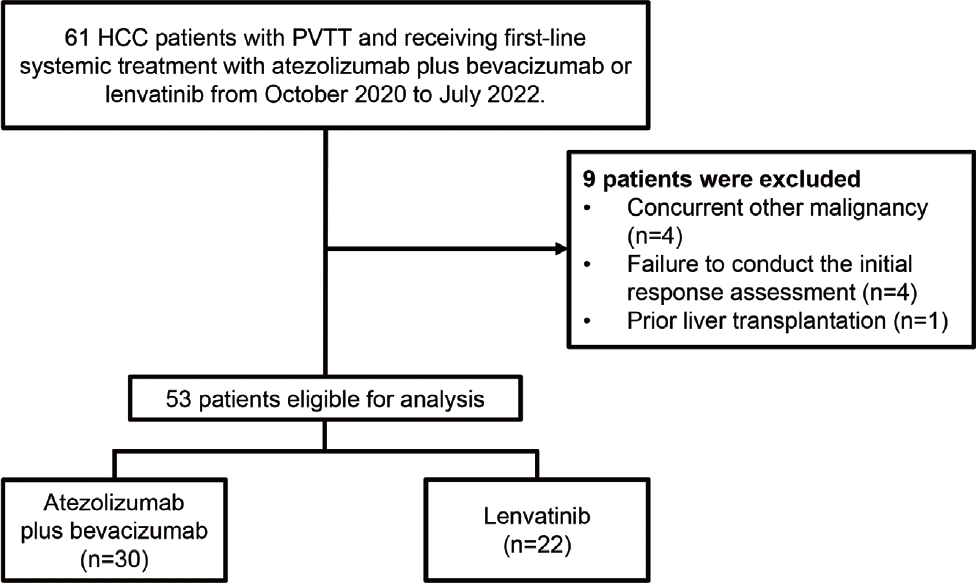
Figure 2.
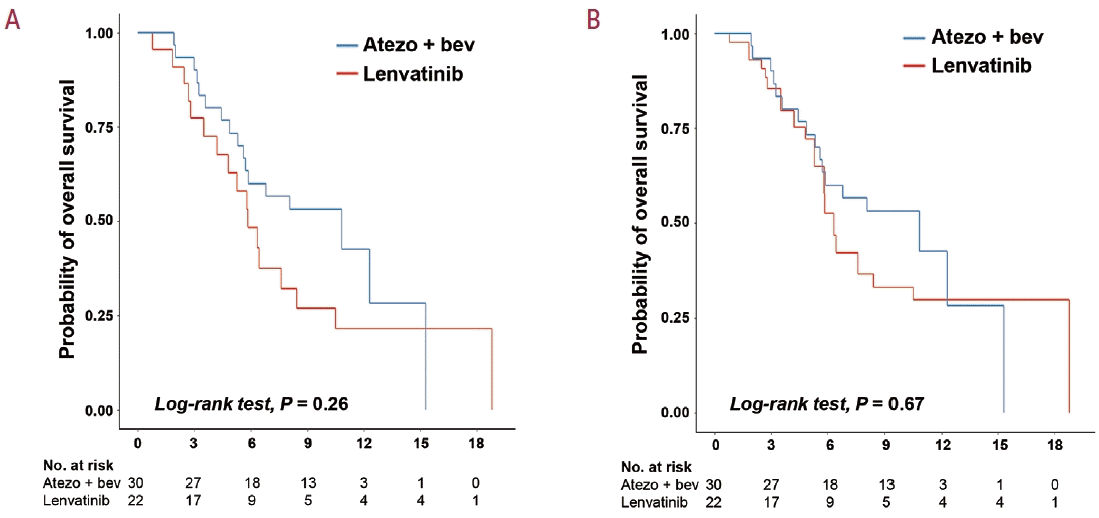
Figure 3.
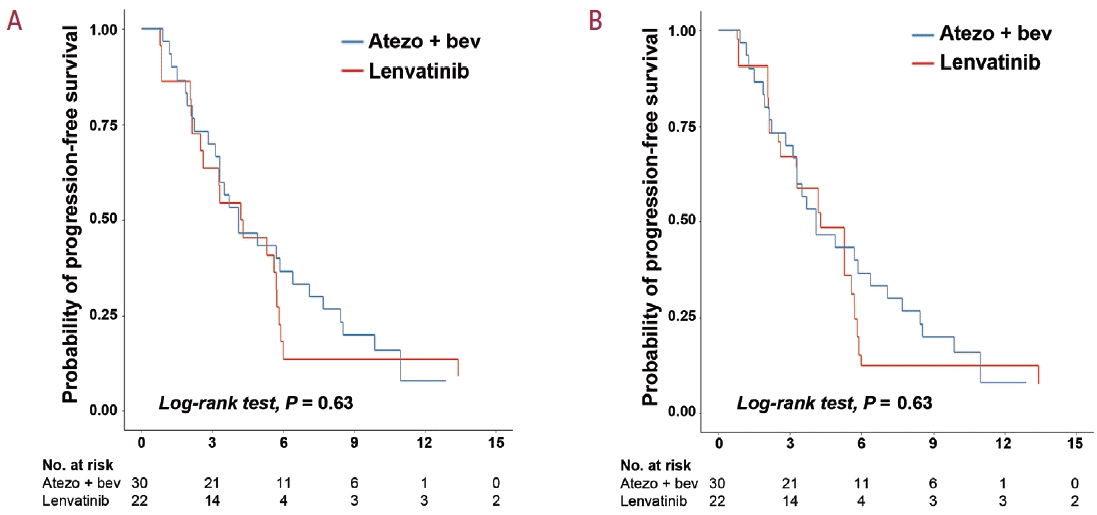
Table 1.
Values are presented as median (range) or number (%).
IPTW, inverse probability of treatment weighting; SMD, standardized mean difference; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; PVTT, portal vein tumor thrombosis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Table 2.
| Variable |
Before IPTW |
After IPTW |
||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | Crude HR(95% CI) | P-value | Adjusted HR(95% CI) | P-value | |
| Treatment | 0.26 | 0.37 | 0.67 | 0.40 | ||||
| Lenvatinib | 1* | 1* | 1* | 1* | ||||
| Atezolizumab plus bevacizumab | 0.67 (0.34-1.34) | 0.71 (0.34-1.49) | 0.84 (0.38-1.86) | 0.74 (0.37-1.49) | ||||
| Age (years) | 0.84 | 0.48 | ||||||
| <65 | 1* | 1* | ||||||
| ≥65 | 1.08 (0.53-2.20) | 1.31 (0.63-2.72) | ||||||
| Sex | 0.90 | 0.97 | ||||||
| Female | 1* | 1* | ||||||
| Male | 1.06 (0.44-2.57) | 1.02 (0.40-2.60) | ||||||
| Etiology of HCC | 0.63 | 0.66 | ||||||
| Other etiology | 1* | 1* | ||||||
| HBV or HCV | 0.75 (0.23-2.47) | 0.73 (0.17-3.05) | ||||||
| Esophageal varix | 0.14 | 0.11 | ||||||
| Absent | 1* | 1* | ||||||
| Present | 1.71 (0.83-3.52) | 1.88 (0.86-4.07) | ||||||
| Child-Pugh score | <0.001 | 0.003 | <0.001 | 0.003 | ||||
| A5 | 1* | 1* | 1* | 1* | ||||
| A6 | 4.42 (1.89-10.35) | 3.57 (0.74-1.90) | 5.17 (2.08-12.84) | 3.57 (0.74-1.90) | ||||
| AFP (ng/mL) | 0.68 | 0.44 | ||||||
| <200 | 1* | 1* | ||||||
| ≥200 | 1.16 (0.57-2.36) | 1.38 (0.61-3.13) | ||||||
| PIVKA-II (mAU/mL) | 0.28 | 0.17 | ||||||
| <1,000 | 1* | 1* | ||||||
| ≥1,000 | 1.47 (0.73-2.97) | 1.70 (0.80-3.62) | ||||||
| Number of hepatic masses | 0.82 | 0.55 | ||||||
| Single | 1* | 1* | ||||||
| Multiple | 0.92 (0.46-1.86) | 0.79 (0.37-1.70) | ||||||
| LN metastasis | 0.49 | |||||||
| Absent | 1* | 1* | 0.66 | |||||
| Present | 0.72 (0.72-1.87) | 0.77 (0.24-2.48) | ||||||
| Extrahepatic metastasis | 0.27 | |||||||
| Absent | 1* | 1* | 0.22 | |||||
| Present | 1.48 (0.74-2.97) | 1.61 (0.76-3.43) | ||||||
| Extent of PVTT | 0.01 | 0.08 | 0.01 | |||||
| No main PV invasion | 1* | 1* | 1* | 1* | 0.09 | |||
| Main PV invasion | 6.31 (1.51-26.44) | 3.69 (0.85-16.04) | 7.88 (1.53-40.70) | 4.45 (0.81-24.30) | ||||
| Hepatic vein invasion | 0.89 | 0.81 | ||||||
| Absent | 1* | 1* | ||||||
| Present | 0.90 (0.21-3.79) | 0.83 (0.17-4.00) | ||||||
OS, overall survival; IPTW, inverse probability of treatment weighting; HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; LN, lymph node; PVTT, portal vein tumor thrombosis; PV, portal vein.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download