INTRODUCTION
THE BILIARY TREE
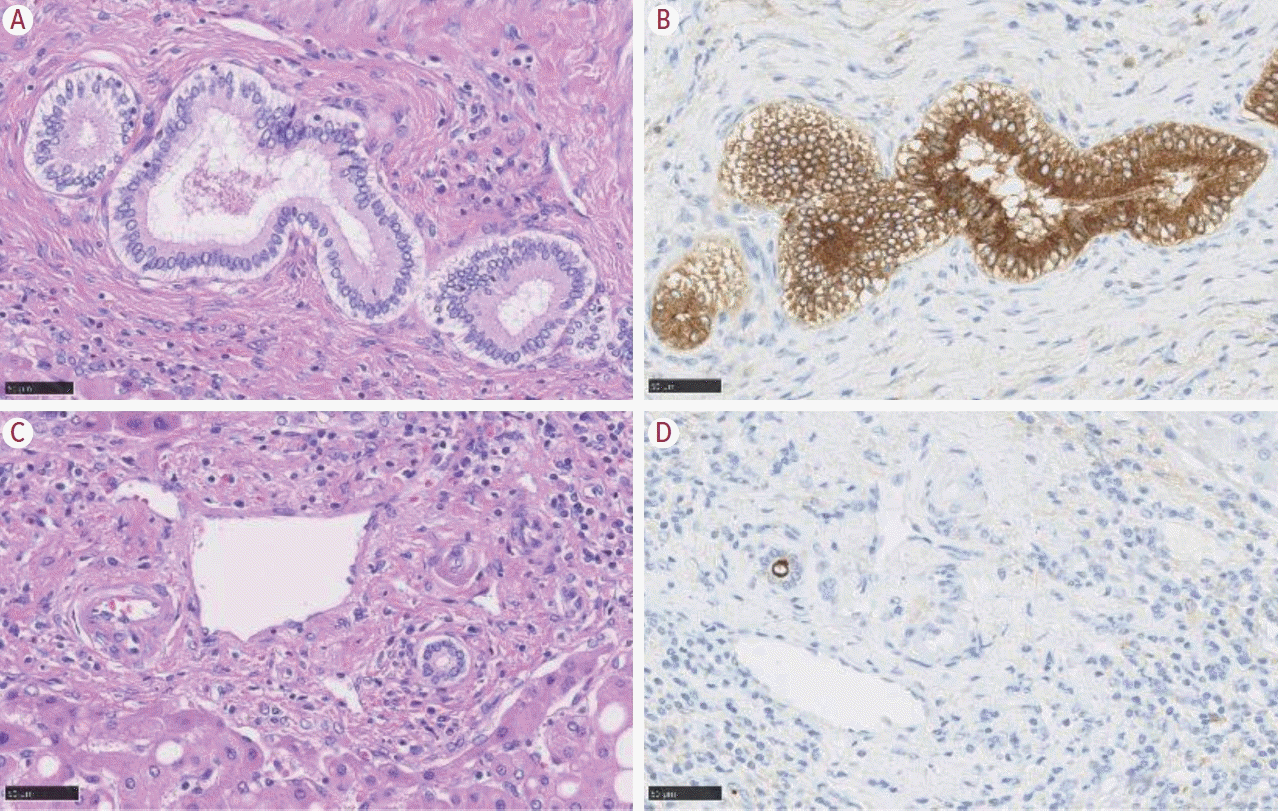 | Figure 1.Heterogeneity of cholangiocytes. The cholangiocytes are mucin-producing, cylindrically shaped cells in the large bile duct (A). In contrast, the small duct contains mucin-negative, cuboidal cholangiocytes (C). The different phenotype is clearly visible with epithelial membrane antigen (EMA) staining. Apical staining in the small duct (D), and cytoplasmic expression in the large bile duct (B) (A, B: hematoxylin- eosin; C, D: EMA immunohistochemistry; A-D: 200×; modified from Komuta12 with permission). |
CCA
Overview and limitations of the anatomy-based classification
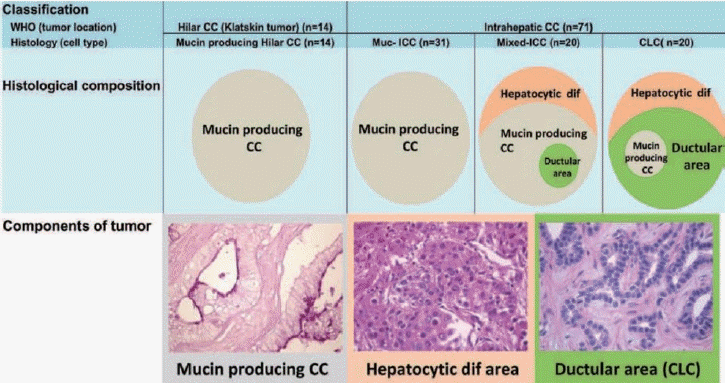 | Figure 2.Histological variation of cholangiocarcinoma (CCA). Hilar CC shows mucin producing CCA, similar to those from intrahepatic cholangiocarcinoma (iCCA) with mucin producing features. In contrast, tumor heterogeneity is a feature of cholangiolocellular carcinoma (CLC) and mixed-iCCA. Reused from Komuta et al.7 with permission. WHO, World Health Organization; CC, cholangiocarcinoma; Muc-iCCA, mucin-production intrahepatic cholangiocarcinoma. |
iCCA
Etiology-based classification
Macroscopic classification
Histological classification
Table 1.
Reused from komuta [12] with permission.
iCCA, intrahepatic cholangiocarcinoma; PSC, primary sclerosing cholangitis; PI, periductal infiltrating; MF, mass-forming; IDH, isocitrate dehydrogenase; FGFR, fibroblast growth factor.
Large duct type
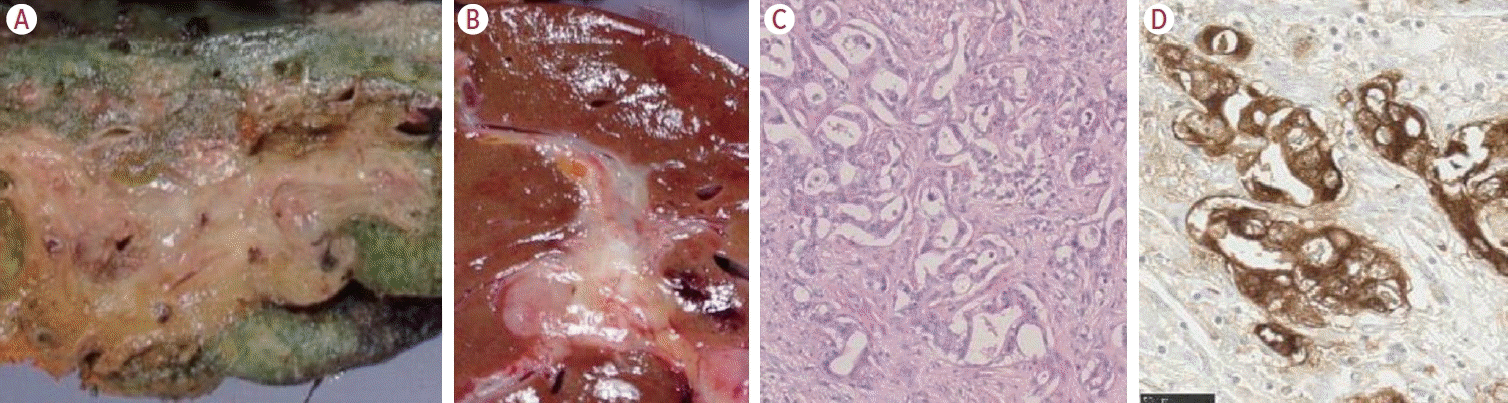 | Figure 3.The large duct type. Macroscopically, the tumor demonstrates periductal infiltrating type (PI) (A), and PI and mass-forming type (B). The tumor exhibits a clear glandular structure with mucin production (C), and cytoplasmic expression with epithelial membrane antigen (EMA) staining (D) (C: hematoxylin-eosin; D: EMA immunohistochemistry; C: 40×; D: 100×; modified from Komuta12 with permission). |
Small duct type
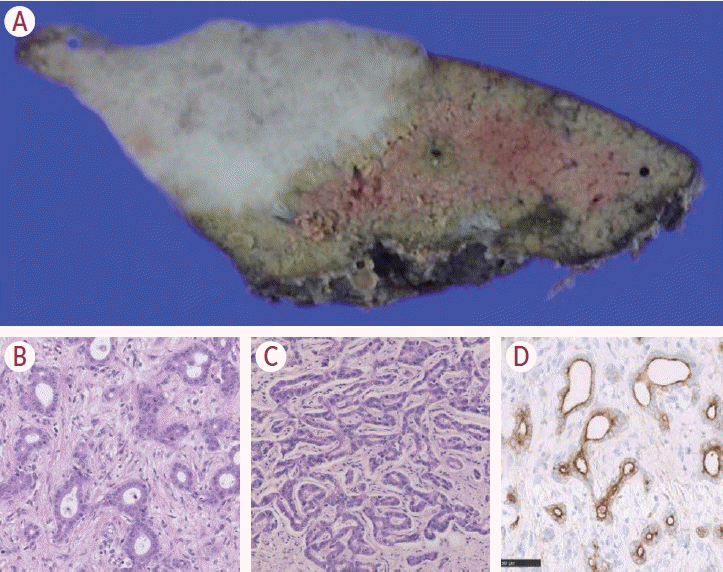 | Figure 4.The small duct type. The tumor shows mass-forming aspect (A), often arising from chronic liver diseases. The tumor is characterized by small uniform glandular structure (B) or ductular reaction-like structure (C) without mucin production. Epithelial membrane antigen (EMA) staining shows an apical expression pattern (D) (B, C: hematoxylin-eosin; D: EMA immunohistochemistry; B-D: 40×; modified from Komuta12 with permission). |
Mixed type
Genetic alteration-based classification
Prognosis
CLC
DIAGNOSIS
Differential diagnosis between iCCA and metastatic adenocarcinoma to the liver
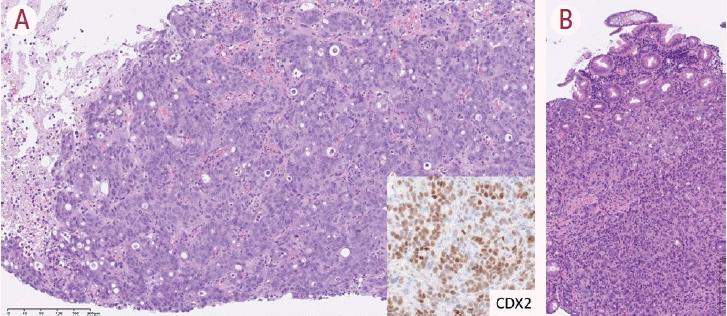 | Figure 5.A case of metastatic colon cancer of the liver. A liver tumor biopsy from a patient with a liver tumor and a clinical diagnosis of intrahepatic cholangiocarcinoma (iCCA). The tumor biopsy shows trabecular/glandular structures composed of tumor cells with enlarged nuclei. Necrosis is also seen (A). Immunohistochemically, the tumor cells are positive for CDX2, indicating its colorectal origin (insert). A colonoscopy revealed a tumor whose morphology mimicked that of the liver tumor, confirming metastatic liver cancer from the colon (B) (A, B: hematoxylin-eosin stain, 40×; inset: CDX2, 100×). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download