This article has been
cited by other articles in ScienceCentral.
Abstract
Portal vein tumor thrombosis (PVTT) is an uncommon condition in which tumor cells expand into the vessels, causing blood clot formation in the portal vein. PVTT is mainly associated with hepatocellular carcinoma, leading to an unfavorable prognosis; however, it can also develop in patients with other cancer types. Herein, we report a case of metastatic renal cell carcinoma diagnosed by a blind liver biopsy in a patient with dynamic computed tomography-confirmed portal vein thrombosis and cholangiopathy. This case illustrates the importance of systematic surveillance with routine laboratory tests and contrast-enhanced imaging studies on patients with cancer to detect potential liver infiltration of metastatic cancer.
Keywords: Portal vein tumor thrombosis, Carcinoma, renal cell, Neoplasm metastasis, Liver function tests, Blind liver biopsy
INTRODUCTION
Portal vein thrombosis (PVT) is a rare condition characterized by a blockage of blood flow in the portal vein caused by a blood clot, with an estimated mean age-standardized incidence of 0.7 per 100,000 inhabitants annually.
1 Depending on the severity and duration of PVT, the clinical presentation can vary from asymptomatic to abdominal pain, fever, jaundice, and gastrointestinal bleeding.
2 PVT can be caused by liver cirrhosis, malignancy, and prothrombotic disorders;
3; since its prognoses and treatments differ depending on the underlying cause, the specific etiology of PVT must be identified. Abdominal ultrasonography with Doppler imaging and contrast-enhanced computed tomography (CT) are widely utilized diagnostic methods.
4
Renal cell carcinoma (RCC) is the most common kidney cancer in adults and originates from the tubular epithelium.
5 RCC symptoms often include hematuria, flank mass, and pain; however, this classic triad is seen in only 10% of RCC patients.
5 In addition, RCC is often associated with venous tumor thrombosis; in a cohort study of 647 patients with RCC, the incidence of tumor thrombus at the time of diagnosis was 13.3%.
6
Herein, we report the case of a 63-year-old man who developed PVT caused by a cancer thrombus originating from RCC. The diagnosis was confirmed on a blind liver biopsy performed to identify the cause of high liver enzyme levels and cholangiopathy.
CASE REPORT
A 63-year-old man who complained of gross hematuria and left flank pain for 2 weeks was referred to our hospital for further evaluation and treatment. He had a history of diabetes, hypertension, and a renal cyst detected by CT 5 years ago. He had been taking amlodipine (10 mg/day), hydrochlorothiazide (12.5 mg/day), and olmesartan (40 mg/day) for hypertension for several years. In addition, he had been taking metformin (850 mg/day), empagliflozin (5 mg/day), and glimepiride (3 mg/day) for diabetes and rosuvastatin (10 mg/day) for dyslipidemia. Based on the evidence from CT, which showed a left renal mass measuring 6 cm and renal vein thrombosis, along with relevant laboratory findings, RCC affecting the left kidney was suspected. Open radical nephrectomy was performed, and subsequent histopathological examination confirmed the diagnosis as papillary RCC type 2 with a WHO/ISUP nuclear grade 3. The tumor has invaded the perirenal and sinus fat tissues and the lymphovascular space. The cancer had caused renal vein thrombosis as observed in the CT. Chest CT and a bone scan were conducted on day 21 of hospitalization, and no evidence of lung or bone metastasis was observed. The patient was discharged the next day.
At the 6th month of outpatient follow-up, the patient brought a non-contrasted abbreviated pancreas magnetic resonance imaging (MRI) from an outside health checkup center, which was acquired using a 1.5-T scanner (Optima MR360, GE Healthcare, Milwaukee, WI, USA) and consisted of only pancreas-centered, fat-suppressed T1-weighted and T2-weighted images, and the liver was partially scanned. It showed gallbladder and pancreas edema and intrahepatic duct dilatation. Liver function tests revealed abnormal increases in the levels of alanine transaminase, aspartate transaminase, alkaline phosphatase, and gamma-glutamyl transferase (
Table 1). His medications had not been changed within 6 months. Blood tests for autoimmune markers showed an increase in serum immunoglobulin G levels; however, both antinuclear antibody and anti-mitochondrial antibody levels were within the normal range (
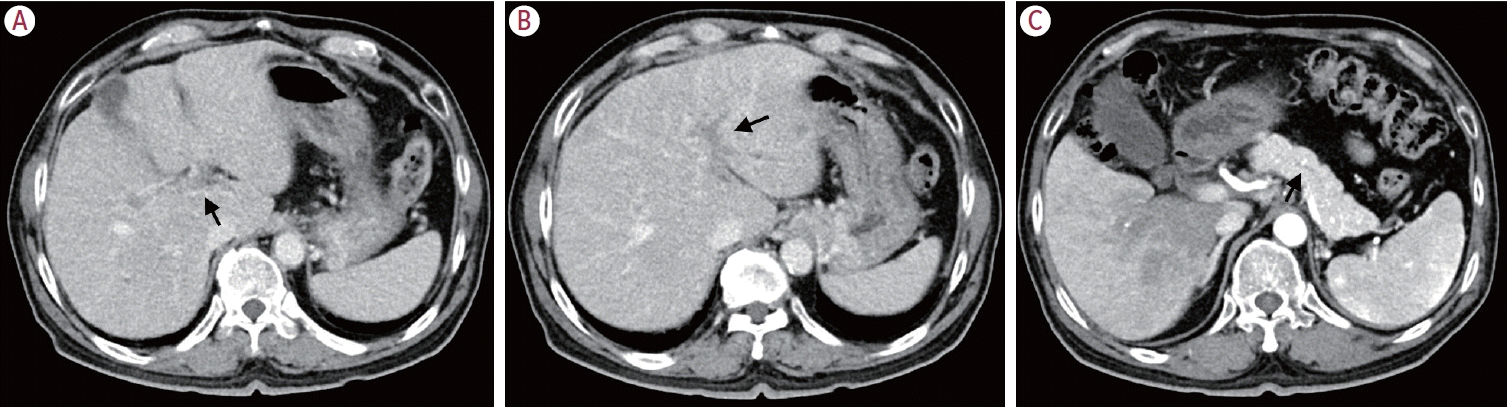
Table 1). Chest CT showed several tiny nodules in the lung, suggestive of reactive or inflammatory change. Liver dynamic CT detected thrombi in the portal vein branches (
Fig. 1A), thickening of the wall of the hilar duct (
Fig. 1B) suggestive of the possibility of primary sclerosing cholangitis, and mild dilatation of the main pancreatic duct (
Fig. 1C). For differential diagnosis of autoimmune liver diseases, a blind percutaneous liver biopsy was performed.
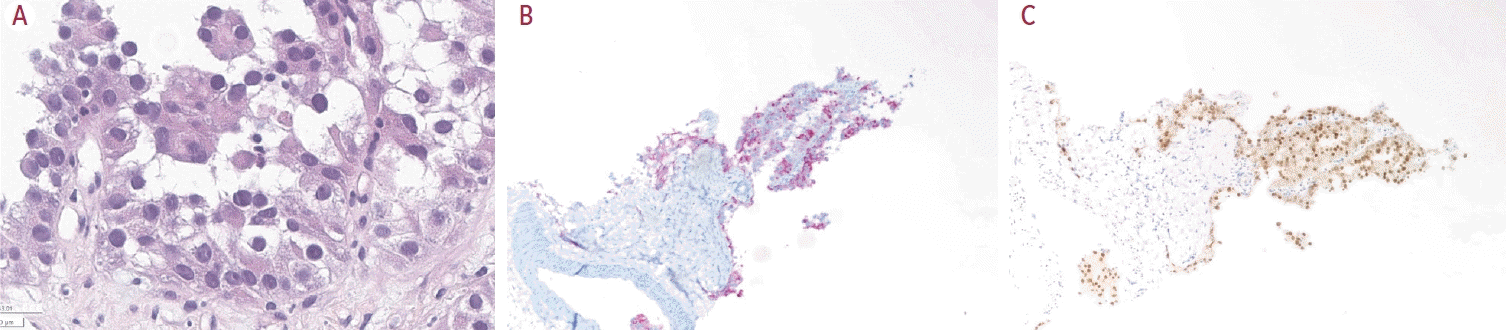
Finally, pathologic examinations confirmed the presence of cancer cells (
Fig. 2); the tissue stained positive for paired box 8 and alpha-methylacyl-coenzyme A racemase and showed a frameshift mutation in the
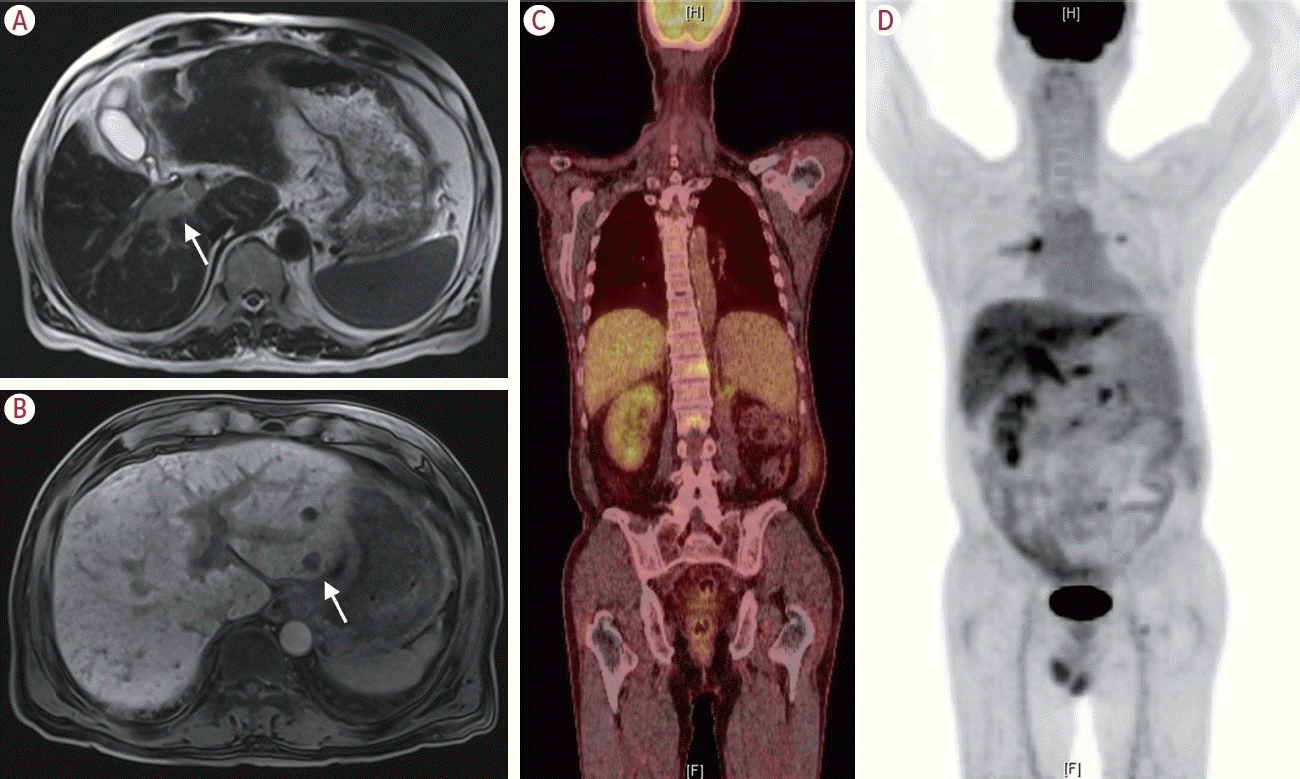
SETD2 gene on next-generation sequencing analysis, signifying metastasis from RCC. Dynamic liver MRI revealed diffuse portal vein thrombosis and potential hepatic metastases in the S2 segment of the liver (
Fig. 3A,
B). Contrast-enhanced chest CT revealed pulmonary thromboembolism, likely caused by tumor thrombi, in the bilateral pulmonary arteries. In addition, it revealed an increase in the size and number of nodules in the pleural and parenchymal regions of both lungs. Positron emission tomography (PET) showed increased
18F-fluorodeoxyglucose (FDG) uptake in multiple sites, including the vertebrae and portal vein, suggesting metastasis (
Fig. 3C,
D).
The patient was referred to the department of oncology, and cancer therapy was initiated with cabozantinib and nivolumab. Besides treatment for metastatic RCC, the patient received conservative treatments to address pulmonary thromboembolism and manage iron deficiency anemia. The patient is still receiving inpatient treatment without additional oncological intervention since hospitalization.
DISCUSSION
RCC consists of several histological subtypes with distinct genetic and clinical features;
7; the papillary type, which accounts for 10-15% of all RCCs, is the second most common subtype after clear-cell RCC.
7 After nephrectomy, metastasis to various tissues and organs, such as the lung, bone, and liver will eventually occur in 3-16% of patients with RCC.
8 Wells et al.
9 reported that patients with metastatic papillary RCC had a relatively poor prognosis compared with those with metastatic clear-cell RCC. In our patient with abnormal liver enzyme levels, metastatic papillary RCC was incidentally diagnosed from a blind liver biopsy performed for differential diagnosis of autoimmune liver diseases. Contrast-enhanced CT and PETCT revealed portal vein thrombosis and intense FDG uptake, indicating that the thrombus had originated from the RCC and disseminated around the portal vein.
Portal vein tumor thrombosis (PVTT) is a common complication occurring in 44.0-62.2% of patients with hepatocellular carcinoma
10 and is associated with a poor prognosis.
11 Moreover, thrombosisinduced portal hypertension and intestinal infarction can lead to fatal complications,
3 which hinder the immediate initiation of cancer therapy. Several studies have also reported PVTT in patients with liver metastasis of various cancers, including RCC.
12 Therefore, imaging studies should be performed to detect tumor thrombosis during the follow-up period, even in asymptomatic cases. PVTT can be differentiated from bland thrombosis using contrast-enhanced CT, an imaging modality with high accessibility and availability, as generalized thrombus enhancement and neovascularity are findings suggestive of malignant portal vein thrombosis.
13 According to Sakamoto et al.,
12 dynamic CT was useful for evaluating PVTT and assessing the extent of the disease in a patient with hepatic metastasis from RCC.
Currently, the latest National Comprehensive Cancer Network guidelines for stage III kidney cancer recommend performing abdominal (preferably CT or MRI) and chest imaging (preferably CT) every 3-6 months for at least 3 years after nephrectomy.
14 However, non-enhanced CT may not be sufficient to evaluate hepatic metastasis and tumor thrombosis in several types of cancers.
13,
15 As observed in our case, despite monitoring the patient with non-enhanced abdominal and chest CT at 3-month intervals, PVT and metastasis were not detected. Therefore, speculatively, the adoption of contrast-enhanced CT during follow-up after nephrectomy would at least enable the prompt detection of abnormal PVT, which can facilitate the final diagnosis of intrahepatic disseminated metastasis from RCC, leading to more effective management and timely intervention.
In summary, we report a unique case of hepatic metastasis caused by massive PVTT originating from RCC, confirmed by a blind liver biopsy. This case demonstrates that high liver enzyme levels in a patient with cancer may be caused by liver infiltration of metastatic cancer, highlighting the importance of systematic surveillance with routine laboratory tests and imaging studies of other organs, including the liver. Given the limitations in identifying PVT and metastasis with non-enhanced CT, dynamic CT monitoring appears to be a good option for patients with cancer.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download