Abstract
This comprehensive review explores the broad landscape of brain–computer interface (BCI) technology and its potential use in intensive care units (ICUs), particularly for patients with motor impairments such as quadriplegia or severe brain injury. By employing brain signals from various sensing techniques, BCIs offer enhanced communication and motor rehabilitation strategies for patients. This review underscores the concept and efficacy of noninvasive, electroencephalogram-based BCIs in facilitating both communicative interactions and motor function recovery. Additionally, it highlights the current research gap in intuitive “stop” mechanisms within motor rehabilitation protocols, emphasizing the need for advancements that prioritize patient safety and individualized responsiveness. Furthermore, it advocates for more focused research that considers the unique requirements of ICU environments to address the challenges arising from patient variability, fatigue, and limited applicability of current BCI systems outside of experimental settings.
Significant communication barriers exist for intensive care unit (ICU) patients, especially those who are quadriplegic or mechanically ventilated. ICU patients often cannot verbally communicate their needs and emotions, leading to increasing likelihood of affective disorders [1]. Traditionally, non-vocal communication methods such as lip reading and gesturing are used as communication approaches for ICU patients [2-4]. However, these traditional methods are not sufficient and often are inefficient [5]. Therefore, an alternative and more efficacious communication modality is needed to enhance patient-provider interactions and to optimize healthcare outcomes. Moreover, for ICU patients, ICU-acquired weakness (ICU-AW) is a prevalent issue [6,7]. ICU-AW is a condition in which ICU patients develop significant muscle weakness during their ICU stay that is not attributed to a pre-existing neuromuscular disorder. To prevent this issue, several management strategies have been employed, including physical therapy, nutritional support, and use of specific medications. Since ICU-AW can hinder recovery and affect the quality of life of ICU survivors, it is a significant concern in critical-care medicine. Therefore, research and clinical practices must explore optimal strategies for prevention, management, and rehabilitation of ICU patients [8] .
In this challenging scenario, brain–computer interfaces (BCIs) can offer a direct communication option by “reading” the patient’s intentions from their brain signals. This approach represents a potential communication tool and rehabilitation solution for ICU patients that enable them to control external devices or communicate merely through brain activity. The brain activity of ICU patients can be detected using well-established measurement tools, such as the electroencephalogram (EEG) and functional near-infrared spectroscopy. If patients require neurosurgery, electrocorticogram assessment through invasive measurement is also possible. Recent development of portable devices of magnetoencephalogram can provide a potential method of reading brain activity in ICU patients [9]. These signals are processed in real time to extract features that are translated into information to be communicated to others (Figure 1).
Because a BCI only needs brain signals to deliver intentions, it has emerged as a focal point of investigation, particularly for its potential in assisting individuals with motor dysfunction [10]. Moreover, using BCIs, patients can capitalize on neural plasticity to possibly reorganize damaged neural circuits based on real-time feedback of their intentions [8,11]. Furthermore, studies have shown that patients who participate in rehabilitation programs with BCIs exhibit larger improvement than those who participate in conventional rehabilitation programs [12,13]. Given the above background, the current review explores the fundamental needs for employing BCI systems for ICU patients. The research underscores the critical role of BCIs in enhancing communication and facilitating comprehensive rehabilitation for ICU patients.
BCI technology has been proposed as a potential communication bridge [14,15] for patients who cannot move or speak without assistance. Studies have shown that intracortical BCIs based on electrode implants have enabled a patient to precisely control a computer cursor to type letters for communication [16]. Recently, for naturalistic communication, a new type of BCI that enables speech synthesis has been developed. Tankus et al. [17] explored how vowels are encoded in the human brain, identifying two coding strategies at the single-neuron level and their implications for BCIs to restore speech in individuals with paralysis. Willett et al. [18] and Dougherty et al. [19] demonstrated that subjects with paralysis can communicate by texting, synthesizing speech, and performing facial expressions via an avatar using BCIs, achieving satisfactory communication speed and accuracy.
Although recent studies have demonstrated the high-quality performance and natural communication interfaces of invasive modalities, these modalities possess limitations in terms of practical application, particularly for ICU patients. These limitations occur owing to the significant risks associated with invasive sensing techniques and the difficulty of applying them to patients who do not have a neurological disorder. For noninvasiveness, affordability, and portability at the expense of lowered signal quality, the EEG has been the most widely used measurement tool in BCI applications. As a noninvasive communication technology, the EEG-based BCI has shown reasonable performance owing to incorporation of advanced machine-learning methods. In the following subsections, detailed BCI techniques and remaining challenges for communication will be discussed.
Prior to engaging in communication, a preliminary step for application of BCIs to ICU patients involves detection of wakefulness. In ICUs, severe brain injuries often pose a challenge to assessing consciousness and predicting long-term recovery. Measuring consciousness is complicated by its subjective nature and its difficulty to quantify. Several studies have been conducted to assess the consciousness of patients using EEG-based BCIs.
In previous studies, early restoration of consciousness was deemed a pivotal factor in forecasting long-term improvement in functionality [20]. Some patients with brain damage may retain covert consciousness, potentially possessing higher levels of consciousness than what is assessed through behavioral evaluation. Claassen et al. [21] reported that brain activity in response to verbal motor commands, inferred from power spectral density analysis of the EEG, was detectable in 15% of a group of unresponsive patients, indicating a certain level of cognitive function despite apparent unresponsiveness. This finding suggests that adoption of EEG-based BCIs combined with machine learning in ICU environments can enhance the identification of cognitive motor dissociation patients who have potential to recover. Thus, BCI can guide more tailored treatment approaches and prognosis estimation [22]. Edlow et al. [20] advocated for implementation of transcranial magnetic stimulation electroencephalography in ICUs as a revolutionary approach to assess consciousness in severely brain-injured patients. That approach overcomes the limitations of measuring only EEG signals and more effectively guides ethical decision-making in patient care.
Despite the development of neurological assessments to provide a more comprehensive understanding of a patient's condition, reliable assessment of consciousness in ICUs remains an ongoing challenge owing to the need for further validation. Fine-tuned EEG-based BCIs with abundant data, along with advanced machine-learning algorithms, may provide a useful means for consciousness assessment in ICUs.
For direct communication using BCIs, various features and paradigms have been proposed. EEG-based BCIs can be categorized into two broad types: synchronous and asynchronous. Synchronous BCI, also known as the exogenous type, consists of a cue-based paradigm. This type of BCI allows patients to select one of many available messages for communication. Since it relies on EEG responses, such as steady state visually evoked potential (SSVEP) and event-related potential (ERP) triggered by a sensory cue, the cue stimulus is crucial in the system design. However, the method has the limitation of being passive. In contrast, asynchronous BCI enables self-paced control without a cue, utilizing specific brain wave patterns that reflect the user's motor intentions or motor imagery. Additionally, game-type tasks are commonly employed to detect EEG features, encouraging active user engagement in the task. Further details on the EEG features and tasks used in previous studies will be introduced below.
SSVEP occurs when flickering visual stimuli are presented, showing peaks at the frequencies of the stimuli and its harmonics (Figure 2). The physiological mechanism behind SSVEP is not fully understood; however, its amplitude is related to increased synaptic activity. SSVEP-based BCIs have demonstrated high accuracy in detecting a user's intended visual targets. Accuracy rates of 90% or higher have been reported in many studies with healthy volunteers [23-25]. However, the actual accuracy can vary depending on the specific experimental setup, the number of targets to choose from, and the subject's familiarity and fatigue with the BCI system. In a recent study, SSVEP-based BCI was used by two volunteers with brain injuries, achieving a mean accuracy of 57% and 65% for each. It was additionally reported that patients preferred employing a speller tool with SSVEP rather than eye tracking. However, visual fatigue was a significant factor affecting performance using SSVEP [26]. To overcome this issue, recent investigations have experimented with innovative visual stimuli, such as quick response (QR) codes, which have outperformed traditional checkerboard patterns [27]. Furthermore, researchers have adapted SSVEP-based communication to the unique constraints of the ICU environment [23].
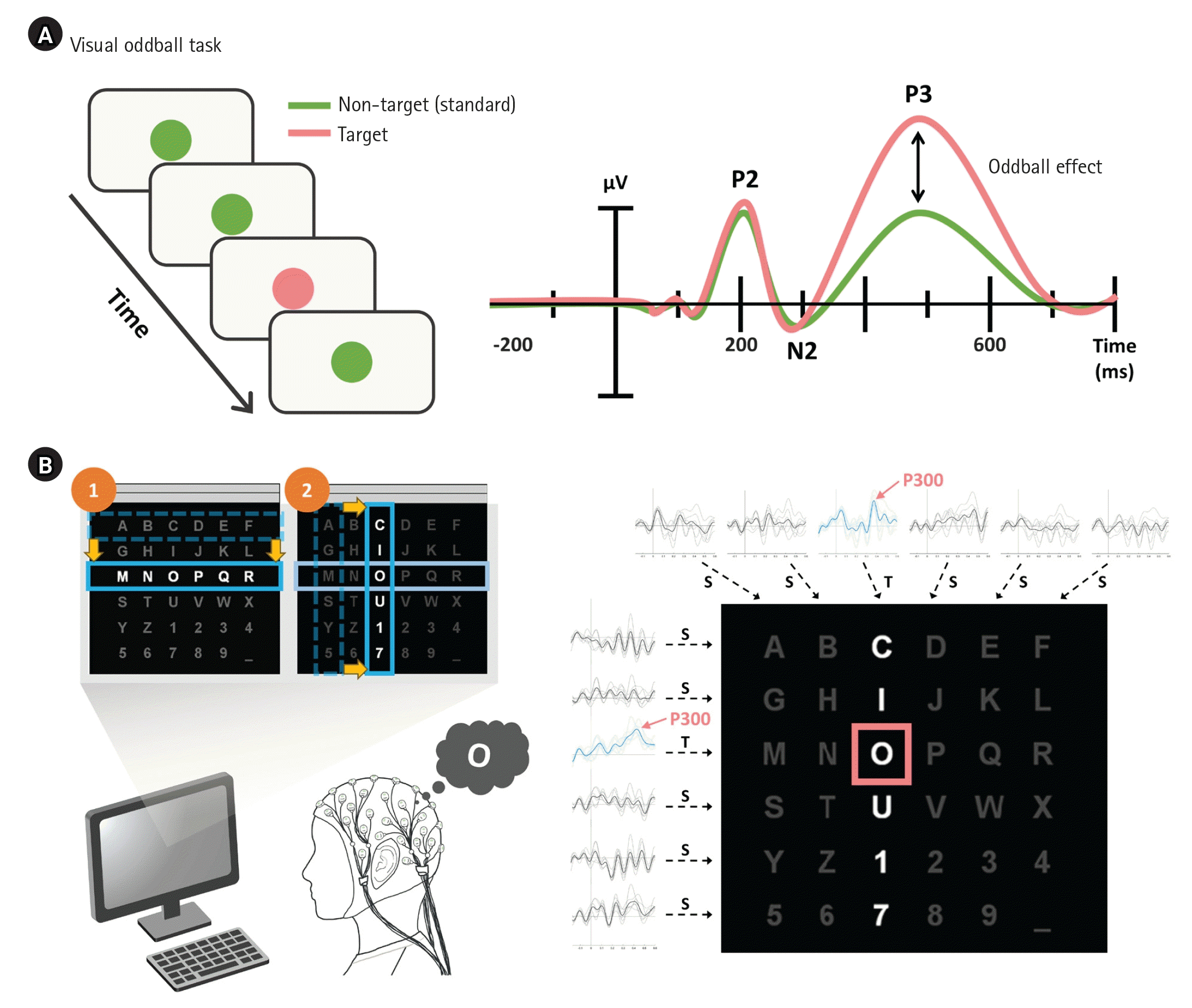
The P300 ERP component is a positive deflection in the EEG waveform that occurs approximately 300 ms after a stimulus (Figure 3A). A notable application is the P300 speller, a BCI system that highlights various columns and rows within an alphabet grid, inducing a response when the patient highlights the specific row or column containing the desired letter—called the row-column paradigm (RCP) (Figure 3B). Early research that introduced the P300 speller with RCP demonstrated a typing speed and peak accuracy of 12 bits/min and 95%, respectively [28]. This type of task engagement has been developed as a single display speller that can reduce the error rate up to 80% and cause less fatigue compared to RCP [29-31]. In a clinical study, Guy et al. [32] demonstrated that 65% of participants, including those with amyotrophic lateral sclerosis (ALS), accurately chose more than 95% of relayed symbols, achieving a typing speed of 3.6/min (without word prediction) and 5.04/min (with word prediction). Additionally, Miao et al. [33] confirmed that ERP-based BCIs are applicable for use by patients with ALS. Other studies attempted to achieve faster and more accurate BCIs by combining other EEG features, such as SSVEP [34,35]. Since the P300 component can be elicited by different modalities of stimuli, including visual, auditory, and tactile, BCI studies using multisensory stimuli [36,37] or multiple intelligent techniques such as 3D interfaces with virtual reality [38,39] have been demonstrated. P300 can be employed not only to send messages, but also to verify consciousness. Li et al. [40] suggested that the P300 can reflect the conscious state of an unresponsive patient, highlighting its usefulness across a broad spectrum of applications.
Brain activity that reflects motor imagery is another commonly used method to detect the intentions of patients who have limited mobility. It involves an EEG signal that can be independently generated by the subject without the need for external stimuli [41]. In practical settings, asynchronous control enhances the user experience and system autonomy by allowing control that is self-initiated. Therefore, it marks a significant trend in the advancement of BCI studies [42]. Liu et al. [43] suggested the self-paced BCI system, which integrates four types of daily assistance tasks in the hospital environment: medical calls, service calls, appliance control, and catering services. The online experiment conducted in that research highlighted the system's efficiency, with a rapid response time of 3.38 seconds, an accuracy rate of 89.2%, and utility in enhancing information processing, easing cognitive load and aiding daily activities for individuals with significant motor restrictions. Motor-imagery-related brain waves can also provide effective feedback by mirroring imagined actions, making it suitable for rehabilitation purposes. Furthermore, numerous studies have focused on rehabilitation BCI systems that utilize brain waves corresponding to motor imagery and intention [13,45,46]. In this regard, motor-imagery-based BCI can also be implemented in ICUs to facilitate active communication and motor function recovery, especially for ICU-AW patients. To date, several studies have attempted to employ motor-imagery-based BCIs not only for consciousness assessment, but also as a rehabilitation tool and communication method in hospitals for acute brain-injury patients, including locked-in-syndrome patients [43,47,48]. The specific EEG features for these applications are detailed in the subsequent section.
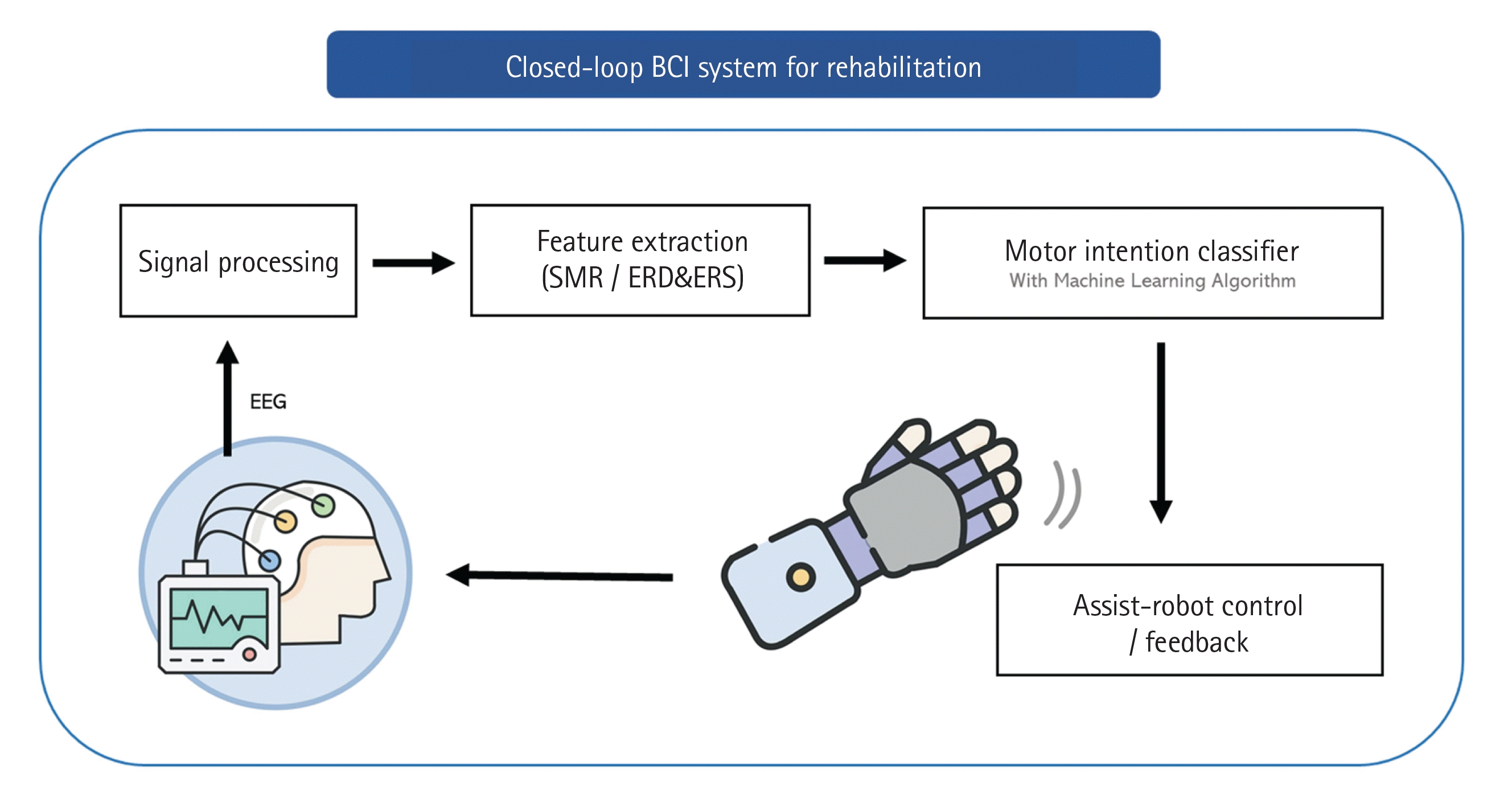
In rehabilitative applications, the EEG is prominent as a suitable measurement for BCI systems because it is noninvasive and easy to use in daily life. Noninvasive BCI-based motor rehabilitation operates as a closed-loop system, continuously stimulating impaired sensorimotor brain areas. This approach enhances functional recovery by recognizing motor intentions using EEG in real time and by providing proprioceptive as well as tactile feedback (Figure 4). Within this framework, motor intentions or motor imagery are identified through noninvasively acquired brainwaves, which are then analyzed to detect specific patterns. Motor rehabilitation commonly employs asynchronous EEG features, such as motor-related cortical potential (MRCP) and sensorimotor rhythm (SMR), both of which are associated with motor intentions. After extraction of these features from the EEG signals, classification algorithms such as support vector machine (SVM) or convolutional neural network are utilized to distinguish between motor intention and non-intention [49-52]. Following the recognition of motor intention, assistive rehabilitation robots or devices are triggered to execute the corresponding movements. This immediate feedback based on motor intention closes the loop from the motor area to the sensory area as somatosensory sensations from movements [53-55].
The detection of motor intentions plays a pivotal role in rehabilitation and responsive neural prosthetics. This process hinges on two key neural elements derived from EEG data: MRCP and SMR. MRCP represents temporal changes in brainwave potentials within the motor cortex when motor intentions are initiated [56,57] . Observable in the time domain, this waveform is characterized by a negative deflection starting at approximately 1.5 seconds prior to the onset of movement [58,59]. Its prevalence is noted primarily in the sensorimotor area. Many studies have reported that MRCP negativity peak amplitude increases with repetitive movements or increased weights owing to peripheral fatigue [60-62]. Furthermore, several studies have indicated a positive correlation between the energy required for movement and the amplitude of the MRCP negativity peak. For instance, stroke patients, who typically exert greater effort to initiate movement, exhibit a larger amplitude in their MRCP negativity peaks [63]. In the context of motor learning, an increase in MRCP amplitude has been observed following motor task training, which may reflect higher cortical loads required to complete the task. Conversely, as motor skills improve through learning, a corresponding reduction is evident in the amplitudes of these peaks [64,65]. This correlation may be observed in patients with ICU-AW or motor impairments who must exert greater energy for movement compared to healthy individuals. The distinction of MRCP in patients not only establishes it as a potential indicator, but also emphasizes the need for careful consideration of these traits to engender critical insights for patient-specific approaches in rehabilitation [66].
Conversely, SMR embodies rhythmic oscillations predominantly in the alpha (8–12 Hz) and beta (13–30 Hz) frequency bands, originating from the motor and somatosensory cortices [67-69]. The alpha-band SMR, often referred to as the Rolandic mu rhythm [70], varies in amplitude in response to movement or the motor imagery of specific limbs. A power decrement occurs just before the movement begins, which is known as event-related desynchronization (ERD). A subsequent power increment occurs post-movement, which is termed event-related synchronization (ERS) [71,72]. Individuals with motor cortex impairment have shown abnormal SMR patterns, such as reduced mu ERD or delayed ERD. Höhne et al. [48] developed a motor-imagery-based BCI system for a locked-in syndrome patient, utilizing oscillatory features, including ERD and ERS, as a class-discriminant feature. They consequently achieved successful communication through left-hand and right-hand motor imagery despite challenges with artifacts and involuntary movements. This study validated the effectiveness of a customized motor-imagery-based BCI system for severely impaired individuals, significantly improving communication capabilities and potentially enhancing their quality of life.
Current BCI systems that capture motor intentions usually focus on movement initiation, whereas the intention of stopping movement has been rarely studied with respect to BCIs. Movement cancellation, or stop, entails the capability of ceasing ongoing or imminent movements as required. This function assumes critical importance in rehabilitation that involves external devices owing to its implications for safety and sophisticated feedback [73-76]. Instances may occur in rehabilitation tasks or everyday activities in which the subject may need to suddenly discontinue their actions. Unintended movements cause a risk of injury, potentially resulting from muscular overexertion. Furthermore, in the context of neuroplasticity, incorporating refined "stop" feedback may enhance recovery processes, presenting an advantage over conventional rehabilitation BCI systems that solely emphasize initiation. Therefore, detection of movement cancellation emerges as a significant consideration in the field of rehabilitation [74]. However, most motor-imagery-based BCIs implement a stop function that relies on a rest state rather than an authentic stop intention. Alternatively, some systems use a combination of specific motor-imagery commands, such as initiating a left-hand command immediately after a right-hand one to signal a stop command [77,78]. Nevertheless, more intuitive methods should be investigated.
Contemporary BCI technology is commonly constrained by its user-dependent nature. Some EEG studies have indicated that patients have greater variability in EEG features or BCI performance compared to healthy subjects. Thus, comprehensive understanding of EEG features in patients with certain disorders remains essential and requires further research and validation in specific patient populations. Moreover, patients tend to experience fatigue more readily when concentrating on tasks, unlike healthy individuals. However, research concerning the impact of fatigue, attention, engagement, and mood on patient performance using BCIs has been minimally studied. Additionally, owing to the highly constrained environment of the ICU, few studies have demonstrated meaningful communication using BCIs in ICUs or other hospital environments, outside of experimental laboratory settings. Therefore, it is crucial to consider the specific characteristics of ICU patients and the complexities of their surrounding environments when implementing BCIs in ICUs.
BCI technology has marked a transformative phase in motor rehabilitation by leveraging noninvasive EEG to facilitate an understanding and manipulation of neural circuits for enhanced motor function recovery. While current systems excel in initiating movements, a notable research gap exists concerning the stop mechanism, the latter of which is critical for ensuring user safety and optimizing rehabilitative outcomes. The need for a more intuitive, patient-responsive stop command warrants much greater research in this area.
▪ Brain–computer interface (BCI) technology represents a significant breakthrough in communication and motor rehabilitation by leveraging brain signals to perceive subject intention, with potential applications to intensive care unit (ICU) patients.
▪ Current BCI applications in motor rehabilitation lack sophisticated “stop” mechanisms; therefore, further research is needed to focus on intuitive, patient-specific responses to support safety and optimal therapeutic outcomes.
▪ An urgent need exists for expanded research and trials of communicative BCIs in actual ICUs that consider patient-specific factors such as fatigue and attention variability to validate the clinical efficacy of BCI systems.
Notes
FUNDING
This research was supported by the U-K Brand Research Fund (1.230016.01) of UNIST (Ulsan National Institute of Science and Technology).
REFERENCES
1. Happ MB. Interpretation of nonvocal behavior and the meaning of voicelessness in critical care. Soc Sci Med. 2000; 50:1247–55.

2. Carroll SM. Nonvocal ventilated patients perceptions of being understood. West J Nurs Res. 2004; 26:85–103.

3. Leathart AJ. Communication and socialisation (1): an exploratory study and explanation for nurse-patient communication in an ITU. Intensive Crit Care Nurs. 1994; 10:93–104.

4. Menzel LK. Factors related to the emotional responses of intubated patients to being unable to speak. Heart Lung. 1998; 27:245–52.

5. Eliseyev A, Gonzales IJ, Le A, Doyle K, Egbebike J, Velazquez A, et al. Development of a brain-computer interface for patients in the critical care setting. PLoS One. 2021; 16:e0245540.

7. Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020; 46:637–53.

8. Frolov AA, Bobrov PD. Brain-computer interfaces: neurophysiological bases and clinical applications. Neurosci Behav Physiol. 2018; 48:1033–40.

9. Boto E, Holmes N, Leggett J, Roberts G, Shah V, Meyer SS, et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature. 2018; 555:657–61.

10. Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008; 7:1032–43.

11. Cervera MA, Soekadar SR, Ushiba J, Millán JD, Liu M, Birbaumer N, et al. Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis. Ann Clin Transl Neurol. 2018; 5:651–63.

12. Ang KK, Guan C, Chua KS, Ang BT, Kuah C, Wang C, et al. A clinical study of motor imagery-based brain-computer interface for upper limb robotic rehabilitation. Annu Int Conf IEEE Eng Med Biol Soc. 2009; 2009:5981–4.
13. Cheng N, Phua KS, Lai HS, Tam PK, Tang KY, Cheng KK, et al. Brain-computer interface-based soft robotic glove rehabilitation for stroke. IEEE Trans Biomed Eng. 2020; 67:3339–51.

14. Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002; 113:767–91.

15. Kübler A, Kotchoubey B, Kaiser J, Wolpaw JR, Birbaumer N. Brain-computer communication: unlocking the locked in. Psychol Bull. 2001; 127:358–75.

16. Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006; 442:164–71.

17. Tankus A, Fried I, Shoham S. Structured neuronal encoding and decoding of human speech features. Nat Commun. 2012; 3:1015.

18. Willett FR, Kunz EM, Fan C, Avansino DT, Wilson GH, Choi EY, et al. A high-performance speech neuroprosthesis. Nature. 2023; 620:1031–6.

19. Dougherty MP, Poch AM, Chorich LP, Hawkins ZA, Xu H, Roman RA, et al. Unexplained female infertility associated with genetic disease variants. N Engl J Med. 2023; 388:1055–6.

20. Edlow BL, Fecchio M, Bodien YG, Comanducci A, Rosanova M, Casarotto S, et al. Measuring consciousness in the intensive care unit. Neurocrit Care. 2023; 38:584–90.

21. Claassen J, Doyle K, Matory A, Couch C, Burger KM, Velazquez A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019; 380:2497–505.

22. Egbebike J, Shen Q, Doyle K, Der-Nigoghossian CA, Panicker L, Gonzales IJ, et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol. 2022; 21:704–13.

23. Dehzangi O, Farooq M. Portable brain-computer interface for the intensive care unit patient communication using subject-dependent SSVEP identification. Biomed Res Int. 2018; 2018:9796238.

24. Zhang R, Xu Z, Zhang L, Cao L, Hu Y, Lu B, et al. The effect of stimulus number on the recognition accuracy and information transfer rate of SSVEP-BCI in augmented reality. J Neural Eng. 2022; 19:036010.

25. Ladouce S, Darmet L, Torre Tresols JJ, Velut S, Ferraro G, Dehais F. Improving user experience of SSVEP BCI through low amplitude depth and high frequency stimuli design. Sci Rep. 2022; 12:8865.

26. Peters B, Bedrick S, Dudy S, Eddy B, Higger M, Kinsella M, et al. SSVEP BCI and eye tracking use by individuals with late-stage ALS and visual impairments. Front Hum Neurosci. 2020; 14:595890.

27. Siribunyaphat N, Punsawad Y. Steady-state visual evoked potential-based brain-computer interface using a novel visual stimulus with quick response (QR) code pattern. Sensors (Basel). 2022; 22:1439.

28. Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988; 70:510–23.

29. Guan C, Thulasidas M, Wu J. High performance P300 speller for brain-computer interface. In : IEEE International Workshop on Biomedical Circuits and Systems; 2004 Dec 1-3; Singapore. p. S3/5/INV–S3/13.

30. Pan J, Chen X, Ban N, He J, Chen J, Huang H. Advances in P300 brain-computer interface spellers: toward paradigm design and performance evaluation. Front Hum Neurosci. 2022; 16:1077717.

31. Pan J, Li Y, Gu Z, Yu Z. A comparison study of two P300 speller paradigms for brain-computer interface. Cogn Neurodyn. 2013; 7:523–9.

32. Guy V, Soriani MH, Bruno M, Papadopoulo T, Desnuelle C, Clerc M. Brain computer interface with the P300 speller: usability for disabled people with amyotrophic lateral sclerosis. Ann Phys Rehabil Med. 2018; 61:5–11.

33. Miao Y, Yin E, Allison BZ, Zhang Y, Chen Y, Dong Y, et al. An ERP-based BCI with peripheral stimuli: validation with ALS patients. Cogn Neurodyn. 2020; 14:21–33.

34. Jalilpour S, Hajipour Sardouie S, Mijani A. A novel hybrid BCI speller based on RSVP and SSVEP paradigm. Comput Methods Programs Biomed. 2020; 187:105326.

35. Santamaria-Vazquez E, Martinez-Cagigal V, Gomez-Pilar J, Hornero R. Asynchronous control of ERP-based BCI spellers using steady-state visual evoked potentials elicited by peripheral stimuli. IEEE Trans Neural Syst Rehabil Eng. 2019; 27:1883–92.

36. Lu Z, Li Q, Gao N, Yang J, Bai O. A novel audiovisual P300-speller paradigm based on cross-modal spatial and semantic congruence. Front Neurosci. 2019; 13:1040.

37. Pires G, Barbosa S, Nunes UJ, Gonçalves E. Visuo-auditory stimuli with semantic, temporal and spatial congruence for a P300-based BCI: an exploratory test with an ALS patient in a completely locked-in state. J Neurosci Methods. 2022; 379:109661.

38. Korkmaz OE, Aydemir O, Oral EA, Ozbek IY. An efficient 3D column-only P300 speller paradigm utilizing few numbers of electrodes and flashings for practical BCI implementation. PLoS One. 2022; 17:e0265904.

39. Noorzadeh S, Rivet B, Jutten C. 3-D Interface for the P300 speller BCI. IEEE Trans Hum Mach Syst. 2020; 50:604–12.

40. Li R, Song WQ, Du JB, Huo S, Shan GX. Connecting the P300 to the diagnosis and prognosis of unconscious patients. Neural Regen Res. 2015; 10:473–80.

41. Rao RP. Brain-computer interfacing: an introduction. Cambridge University Press;2013.
42. Yu Y, Zhou Z, Liu Y, Jiang J, Yin E, Zhang N, et al. Self-paced operation of a wheelchair based on a hybrid brain-computer interface combining motor imagery and P300 potential. IEEE Trans Neural Syst Rehabil Eng. 2017; 25:2516–26.

43. Liu Y, Liu Y, Tang J, Yin E, Hu D, Zhou Z. A self-paced BCI prototype system based on the incorporation of an intelligent environment-understanding approach for rehabilitation hospital environmental control. Comput Biol Med. 2020; 118:103618.

44. Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004; 51:1034–843.

45. Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil. 2015; 96(3 Suppl):S79–87.

46. Benzy VK, Vinod AP, Subasree R, Alladi S, Raghavendra K. Motor imagery hand movement direction decoding using brain computer interface to aid stroke recovery and rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2020; 28:3051–62.

47. Chatelle C, Spencer CA, Cash SS, Hochberg LR, Edlow BL. Feasibility of an EEG-based brain-computer interface in the intensive care unit. Clin Neurophysiol. 2018; 129:1519–25.

48. Höhne J, Holz E, Staiger-Sälzer P, Müller KR, Kübler A, Tangermann M. Motor imagery for severely motor-impaired patients: evidence for brain-computer interfacing as superior control solution. PLoS One. 2014; 9:e104854.

49. Amin SU, Alsulaiman M, Muhammad G, Mekhtiche MA, Hossain MS. Deep learning for EEG motor imagery classification based on multi-layer CNNs feature fusion. Future Gener Comput Syst. 2019; 101:542–54.

50. Chen Z, Wang Y, Song Z. Classification of motor imagery electroencephalography signals based on image processing method. Sensors (Basel). 2021; 21:4646.

51. Dong E, Li C, Li L, Du S, Belkacem AN, Chen C. Classification of multi-class motor imagery with a novel hierarchical SVM algorithm for brain-computer interfaces. Med Biol Eng Comput. 2017; 55:1809–18.

52. León J, Escobar JJ, Ortiz A, Ortega J, González J, Martín-Smith P, et al. Deep learning for EEG-based Motor Imagery classification: accuracy-cost trade-off. PLoS One. 2020; 15:e0234178.

53. Cantillo-Negrete J, Carino-Escobar RI, Carrillo-Mora P, Barraza-Madrigal JA, Arias-Carrión O. Robotic orthosis compared to virtual hand for Brain-Computer Interface feedback. Biocybern Biomed Eng. 2019; 39:263–72.

54. Cantillo-Negrete J, Carino-Escobar RI, Carrillo-Mora P, Rodriguez-Barragan MA, Hernandez-Arenas C, Quinzaños-Fresnedo J, et al. Brain-computer interface coupled to a robotic hand orthosis for stroke patients’ neurorehabilitation: a crossover feasibility study. Front Hum Neurosci. 2021; 15:656975.

55. Spüler M, López-Larraz E, Ramos-Murguialday A. On the design of EEG-based movement decoders for completely paralyzed stroke patients. J Neuroeng Rehabil. 2018; 15:110.

56. Karimi F, Kofman J, Mrachacz-Kersting N, Farina D, Jiang N. Detection of movement related cortical potentials from EEG using constrained ICA for brain-computer interface applications. Front Neurosci. 2017; 11:356.

57. Shibasaki H, Barrett G, Halliday E, Halliday AM. Cortical potentials associated with voluntary foot movement in man. Electroencephalogr Clin Neurophysiol. 1981; 52:507–16.

58. Shakeel A, Navid MS, Anwar MN, Mazhar S, Jochumsen M, Niazi IK. A review of techniques for detection of movement intention using movement-related cortical potentials. Comput Math Methods Med. 2015; 2015:346217.

59. Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006; 117:2341–56.

60. Berchicci M, Menotti F, Macaluso A, Di Russo F. The neurophysiology of central and peripheral fatigue during sub-maximal lower limb isometric contractions. Front Hum Neurosci. 2013; 7:135.

61. Schillings ML, Kalkman JS, van der Werf SP, Bleijenberg G, van Engelen BG, Zwarts MJ. Central adaptations during repetitive contractions assessed by the readiness potential. Eur J Appl Physiol. 2006; 97:521–6.

62. de Morree HM, Klein C, Marcora SM. Perception of effort reflects central motor command during movement execution. Psychophysiology. 2012; 49:1242–53.

63. Wiese H, Stude P, Sarge R, Nebel K, Diener HC, Keidel M. Reorganization of motor execution rather than preparation in poststroke hemiparesis. Stroke. 2005; 36:1474–9.

64. Berchicci M, Quinzi F, Dainese A, Di Russo F. Time-source of neural plasticity in complex bimanual coordinative tasks: juggling. Behav Brain Res. 2017; 328:87–94.

65. Jochumsen M, Rovsing C, Rovsing H, Cremoux S, Signal N, Allen K, et al. Quantification of movement-related EEG correlates associated with motor training: a study on movement-related cortical potentials and sensorimotor rhythms. Front Hum Neurosci. 2017; 11:604.

66. Xu R, Jiang N, Vuckovic A, Hasan M, Mrachacz-Kersting N, Allan D, et al. Movement-related cortical potentials in paraplegic patients: abnormal patterns and considerations for BCI-rehabilitation. Front Neuroeng. 2014; 7:35.

67. Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, He B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: an EEG and fMRI study of motor imagery and movements. Neuroimage. 2010; 49:2596–606.

68. Yuan H, He B. Brain-computer interfaces using sensorimotor rhythms: current state and future perspectives. IEEE Trans Biomed Eng. 2014; 61:1425–35.

69. Tsuchimoto S, Shibusawa S, Mizuguchi N, Kato K, Ebata H, Liu M, et al. Resting-state fluctuations of EEG sensorimotor rhythm reflect BOLD activities in the pericentral areas: a simultaneous EEG-fMRI study. Front Hum Neurosci. 2017; 11:356.

70. Okada S, Urakami Y, Kato T, Tsuji M, Inoue R. The Rolandic mu rhythm: a clinical study of the atypical group. Clin Electroencephalogr. 1992; 23:10–8.

71. Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999; 110:1842–57.

72. Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva FH. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage. 2006; 31:153–9.

73. Nann M, Peekhaus N, Angerhöfer C, Soekadar SR. Feasibility and safety of bilateral hybrid EEG/EOG brain/neural-machine interaction. Front Hum Neurosci. 2020; 14:580105.

74. Araujo RS, Silva CR, Netto SP, Morya E, Brasil FL. Development of a low-cost EEG-controlled hand exoskeleton 3D printed on textiles. Front Neurosci. 2021; 15:661569.

75. Massardi S, Pinto-Fernandez D, Babič J, Dežman M, Trošt A, Grosu V, et al. Relevance of hazards in exoskeleton applications: a survey-based enquiry. J Neuroeng Rehabil. 2023; 20:68.

76. Onose G, Grozea C, Anghelescu A, Daia C, Sinescu CJ, Ciurea AV, et al. On the feasibility of using motor imagery EEG-based brain-computer interface in chronic tetraplegics for assistive robotic arm control: a clinical test and long-term post-trial follow-up. Spinal Cord. 2012; 50:599–608.

Figure 1.
Illustration of a brain–computer interface (BCI) system. ECoG: electrocorticogram; EEG: electroencephalogram; fNIRS: functional near-infrared spectroscopy; MEG: magnetoencephalogram.
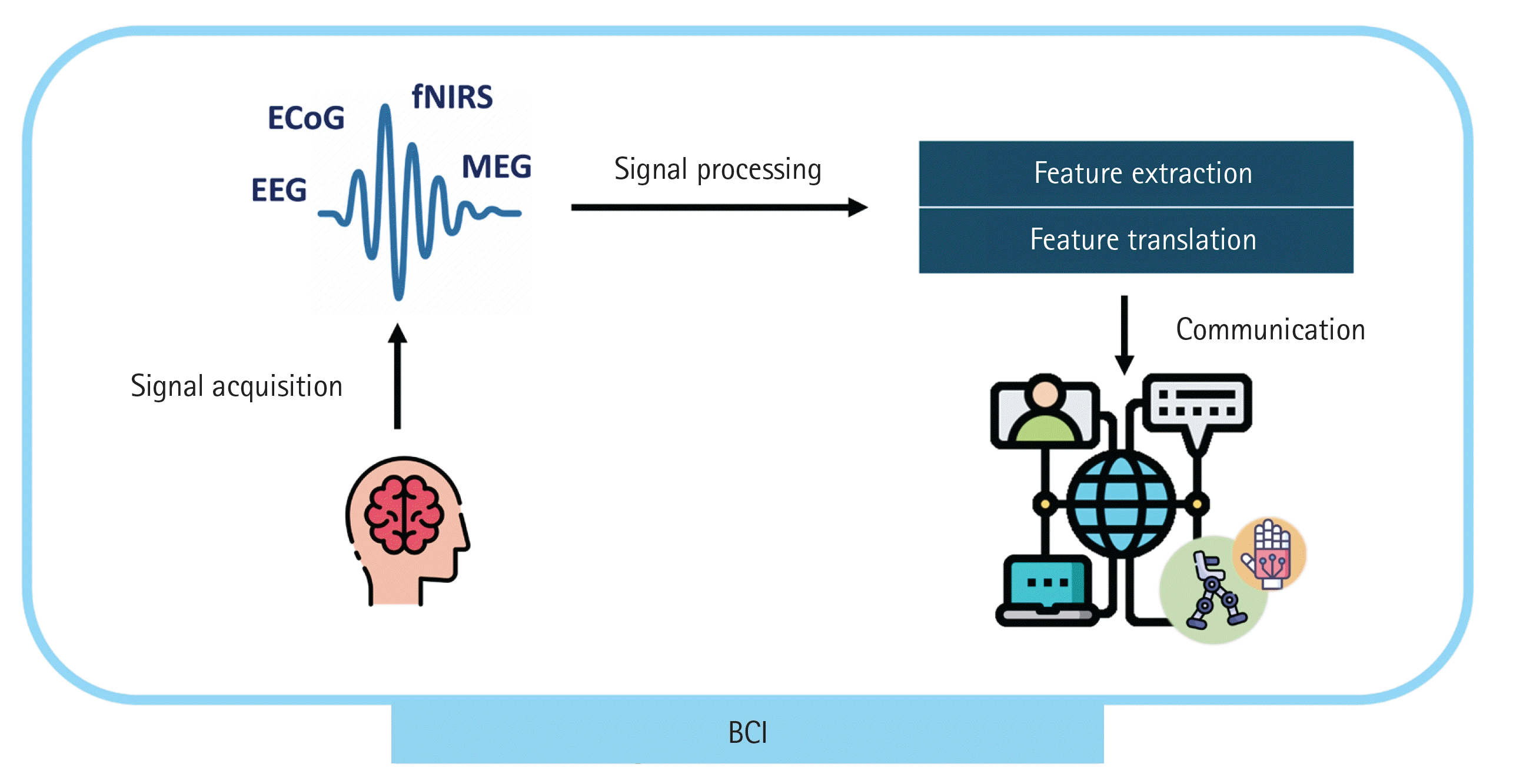
Figure 2.
The steady state visually evoked potential paradigm and typical frequency encoding from acquired electroencephalogram (EEG) signals. PSD: power spectral density.
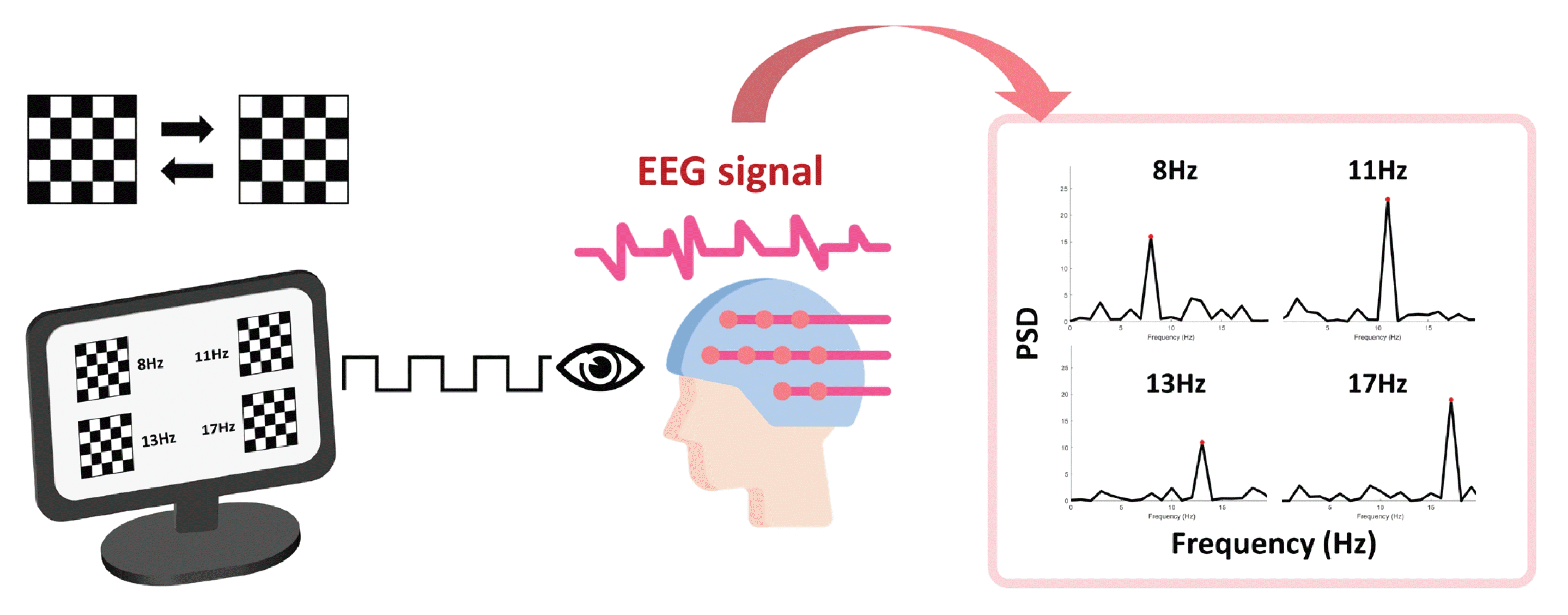
Figure 3.
P300-based brain–computer interface [44]. (A) Oddball paradigm and P300 or P3 in the event-related potential family. (B) P300-based speller with the row-column paradigm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download