Abstract
Bronchopulmonary dysplasia (BPD) and pulmonary hypertension (PH) are potentially fatal complications in prematurely born infants. Extracorporeal membrane oxygenation (ECMO) may be a life-saving option for managing infants with BPD and PH. We present 2 patients who were successfully weaned off mechanical ventilators (MVs) through the application of ECMO. The patients were transferred to our institution after receiving MV care for 8 and 10 months, respectively, for BPD and PH. We were able to remove the patients from MVs after a period of ECMO-mediated lung rest. Although more research is required to determine specific criteria for ECMO use in patients with BPD and PH, our clinical experiences may contribute to the early application of ECMO in MV-dependent patients.
Bronchopulmonary dysplasia (BPD) is a chronic lung condition in preterm infants related to prolonged oxygen therapy. Infants with BPD are particularly susceptible to cardiovascular complications, such as pulmonary hypertension (PH). Limiting the extent of lung injury and allowing normal lung development are the key principles of managing infants with BPD and PH (1). In addition, the use of steroids, diuretics, and pulmonary vasodilators such as inhaled nitric oxide (NO), sildenafil, prostacyclin, and bosentan has been associated with better outcomes (1). Extracorporeal membrane oxygenation (ECMO) may be a therapeutic option for preterm infants with severe BPD and PH, but there has not been a consensus on definitive indications for ECMO in such a population. Early ECMO was generally reserved for patients with extreme cardiorespiratory compromise who would face certain death because of the high mortality associated with ECMO (2,3). However, the use of ECMO in newborn and infantile populations has increased as a result of technological advances in the procedure and years of experience (2-4).
Herein, we present 2 cases of ECMO use in 2022 in preterm infants with severe BPD and PH. They were born in different hospitals, and medical staff at both hospitals believed it was unlikely that they could be taken off mechanical ventilators (MVs) due to the severity of their lung conditions. Nonetheless, the patients were successfully weaned off conventional MVs at our institution with the help of an ECMO. To our knowledge, this is the first article to discuss the effectiveness of ECMO in infants with BPD. This study was reviewed and approved by the institutional review board of the Seoul National University Hospital with informed consent for publication obtained from the guardians of the patients (IRB no. 2307-200-1454).
The boy was born in a tertiary hospital at 27 weeks of gestation, weighing 1,080 g. During the course of 5 months at the neonatal intensive care unit, he was diagnosed with severe BPD and secondary PH, for which supplemental oxygen and oral sildenafil were required at the time of discharge. A few days later, he was hospitalized again with parainfluenza pneumonia. Respiratory symptoms and preexisting PH worsened, which prompted a transfer to the pediatric intensive care unit (PICU) for MV care for the next 3 months. The medical staff recommended cardiopulmonary transplantation as the next mode of treatment, and he was transferred to our institution.
At the time of transfer (December 31, 2021), the boy was 8 months old (postmenstrual age, 61 weeks), weighing 6 kg. he was hypercapnic and hypoxic upon arrival, possibly due to ineffective ventilation during transportation (Table 1). Due to severe desaturation, inhaled NO was needed at 40 μmol/mol even during transportation. A chest radiograph showed severe BPD-related changes (Fig. 1A). Even at the highest treprostinil dose and with inhaled NO at 40 μmol/mol, the oxygen saturation could not be maintained above 80%. In less than 24 hours of hospitalization, the decision was made to put him on venovenous (VV) ECMO using a double-lumen catheter (DLC; Avalon Elite, Maquet).
The notably different compliance and resistance of the lungs resulted in hyperinflation of the right lung and total collapse of the left lung with lung protective ventilator settings (Fig. 1B). On day 43, minimal pressures were applied to accommodate total lung rest to prevent over-inflation and complete deflation (Fig. 1C). On the same day, ECMO was weaned off, and a few days after successful extubation, an elective tracheostomy was done to secure the airway, which had become vulnerable due to a lengthy intubation period. At that time, the boy still needed a certain amount of pressure to avoid periodic desaturation. He was moved to the general ward on day 123 with a home ventilator (HV).
On day 213 (July 30, 2022), the boy was discharged. No major complication occurred during ECMO, and he was put on intensive rehabilitation for marked motor delays but was otherwise doing well. We are considering the removal of the tracheostomy tube after 6 months of follow-up, given the improved lung conditions (Fig. 1D).
The boy was born in a tertiary hospital at 23 weeks of gestation with an initial body weight of 550 g. He was intubated and put on an MV for the next 9 months in the neonatal intensive care unit because of severe BPD and PH. Sildenafil was given up to 4 mg/kg/day, and bosentan was increased up to 3 mg/kg/day; but signs of congestive heart failure worsened, and urinary output decreased substantially even with diuretics. He was transferred to our hospital for a second opinion.
Due to poor oxygenation, inhaled NO was started as soon as the boy arrived at our PICU on February 3, 2022, followed by intravenous treprostinil and bosentan (Table 2). A radiograph indicated severe BPD (Fig. 2A). The boy was maintained under heavy sedation and received a very low tidal volume to reduce hyperinflation. However, air trapping and carbon dioxide retention remained, and worsening PH led to steadily declining right ventricular function. The oxygen concentration of MV was increased to 100% by the end of the first month of PICU hospitalization, but his oxygen saturation seldom went above 90%, requiring the application of VV ECMO. A DLC was inserted to implement the ECMO. At this point, he was 10 months old (postmenstrual age of 63 weeks) and weighed 5 kg. Adequate oxygenation was possible even in a moderate MV setting with the help of ECMO (Fig. 2B, Table 2). Medications for PH were reduced as the lung condition improved by allowing the lungs to rest.
After 68 days on ECMO (day 96), the boy was weaned from it (Fig. 2C). A tracheostomy was done 1 week later, and on day 165, the conventional MV was converted to an HV. On day 251 (October 11, 2022), he was discharged. He tolerated ECMO without notable issues, except the need for frequent repositioning of the DLC due to its large size for him. Despite global developmental delays and feeding difficulties requiring extensive rehabilitation and training, he was seen thriving at 6-month follow-up, and needs to be on the HV for only a few hours a day (Fig. 2D).
We have been expanding the indications for ECMO as its use has grown in our center, particularly since the introduction of DLC in 2009. The conventional method of peripheral VV ECMO uses large cannulae, which are often unsuitable for the small femoral veins of pediatric patients (5). This issue was solved by cannulating the DLC (5). Premature infants with BPD may have been disregarded as candidates for ECMO in the past due to their small sizes. However, infants with BPD and PH are at high risk of cardiovascular and respiratory complications, such as asthma, bronchiolitis, and respiratory-related hospitalization during the first few years of life, leading to a mortality up to 50% (6-8). In our cases, the 2 infants with BPD and PH who had previously been considered hopeless were treated with ECMO, overcoming potentially fatal circumstances. It has given the lungs time to recover from being subjected to high pressures, which made it possible to successfully extubate the patient.
There are only a handful of studies regarding the efficacy of ECMO in infants with BPD. In a total of 143 patients aged 60 days-18 years managed with ECMO from 2004 to 2017, the overall survival rate of the patients with isolated BPD and with BPD and PH was 86.7% (13 of 15) and 68.0% (87 of 128), respectively (P = 0.23) (9). One study performed from 1990 to 1999 at the Children’s Hospital of Philadelphia presents a 78% (7 of 9) survival for BPD patients with cardiopulmonary complications (10). The Extracorporeal Life Support Organization registry analysis shows a median ECMO duration of 272 and 258.5 hours in children and adolescents with isolated BPD and in those with BPD and PH, respectively. The other study reported a mean ECMO duration of 368 hours in infants with BPD (9,10).
The total durations on ECMO for patients 1 and 2 were 42 and 68 days, respectively, which are longer than the abovementioned durations of ECMO. Although patients in both cases experienced early-onset cardiopulmonary problems, the ECMO was started at the age of 8-10 months. We hypothesize that the duration of ECMO treatment could be curtailed if it is introduced in the earlier stages of pulmonary dysplasia. Also, minimizing the duration of sedation and aggressive rehabilitation during ECMO could result in better outcomes even with a long duration of the ECMO. The application of VV ECMO using the DLC, as used in this case, is feasible for infants weighing 2 kg or more. Therefore, it is also practical to consider the early use of ECMO (11). Our clinical experiences have led us to advocate earlier initiation of ECMO for BPD patients with refractory respiratory failure in efforts to reduce the duration of MVs or hospitalization.
Although no substantial ECMO-related problems occurred in the case patients, prolonged application of ECMO not only raises the risk of adverse events, such as thrombosis or coagulopathy, but is also linked to poor neurodevelopmental outcomes (12). Furthermore, even though previous studies shed positive light on the efficacy of ECMO use in patients with BPD, the survival benefit remains unknown. A future prospective multicenter trial could contribute to increased survival and the identification of specific indications for the use of ECMO in patients with BPD and PH.
In conclusion, we present 2 cases of applying ECMO to MV-dependent patients with severe BPD and PH. ECMO-related complications can be fatal, but technological advances and accumulation of clinical experiences suggest that ECMO may be effective in overcoming the high mortality rate of BPD and PH. Early ECMO use may be advantageous for patients with BPD and PH who are vulnerable to prolonged MV dependence. In rare cases, when patients with BPD and PH present to emergency departments with severe respiratory distress, the need for early use of ECMO may be discussed with medical staff of PICUs.
Notes
Author contributions
Conceptualization and Methodology: W Jang and B Lee
Data curation, Software, and Visualization: YH Jeon and W Jang
Formal analysis and Funding acquisition: not applicable
Investigation and Validation: all authors
Project administration and Supervision: B Lee
Resources: YH Jeon, W Jang, and B Lee
Writing-original draft: YH Jeon and W Jang
Writing-review and editing: all authors
All authors read and approved the final manuscript.
References
1. Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013; 37:124–31.

2. Keckler SJ, Laituri CA, Ostlie DJ, St Peter SD. A review of venovenous and venoarterial extracorporeal membrane oxygenation in neonates and children. Eur J Pediatr Surg. 2010; 20:1–4.

3. Rush B, Wiskar K, Berger L, Griesdale D. Trends in extracorporeal membrane oxygenation for the treatment of acute respiratory distress syndrome in the United States. J Intensive Care Med. 2017; 32:535–9.

4. Kim W, Kwon HW, Min J, Cho S, Kwak JG, Kim WH. Extracorporeal membrane oxygenation in pediatric patients with respiratory failure: early experience with the double-lumen cannula over 2 years. Korean J Thorac Cardiovasc Surg. 2020; 53:132–9.

5. Jarboe MD, Gadepalli SK, Church JT, Arnold MA, Hirschl RB, Mychaliska GB. Avalon catheters in pediatric patients requiring ECMO: placement and migration problems. J Pediatr Surg. 2018; 53:159–62.

6. Jo HS, Cho KH, Cho SI, Song ES, Kim BI. Recent changes in the incidence of bronchopulmonary dysplasia among very-low-birth-weight infants in Korea. J Korean Med Sci. 2015; 30 Suppl 1:S81–7.

7. Kim HR, Jung YH, Kim BI, Kim SY, Choi CW. Differences in comorbidities and clinical burden of severe bronchopulmonary dysplasia based on disease severity. Front Pediatr. 2021; 9:664033.

8. Arjaans S, Haarman MG, Roofthooft MT, Fries MW, Kooi EM, Bos AF, et al. Fate of pulmonary hypertension associated with bronchopulmonary dysplasia beyond 36 weeks postmenstrual age. Arch Dis Child Fetal Neonatal Ed. 2021; 106:45–50.

9. Pena Hernandez A, Carr NR, McCurnin D, Armijo-Garcia V. Extracorporeal life support in pediatric patients with bronchopulmonary dysplasia and associated pulmonary hypertension. ASAIO J. 2020; 66:1063–7.

10. Hibbs A, Evans JR, Gerdes M, Hunter JV, Cullen JA. Outcome of infants with bronchopulmonary dysplasia who receive extracorporeal membrane oxygenation therapy. J Pediatr Surg. 2001; 36:1479–84.

Fig. 1.
Serial chest radiographs of patient 1. (A) The radiograph shows multiple atelectases, chiefly in the right upper lung, and cystic lesions with an endotracheal tube (arrowhead), left internal jugular central venous catheter (arrow), and nasojejunal tube (asterisk) inserted (day 1, hospitalized to the pediatric intensive care unit). (B) It shows hyperinflated right lung and collapsed left lung with a double-lumen catheter (arrowhead) for venovenous ECMO in the early stage of ECMO use (day 7). (C) It shows similarly inflated lungs, right after cessation of ECMO (day 43). (D) It is the most recent radiograph taken at the outpatient clinic (day 495). ECMO: extracorporeal membrane oxygenation.
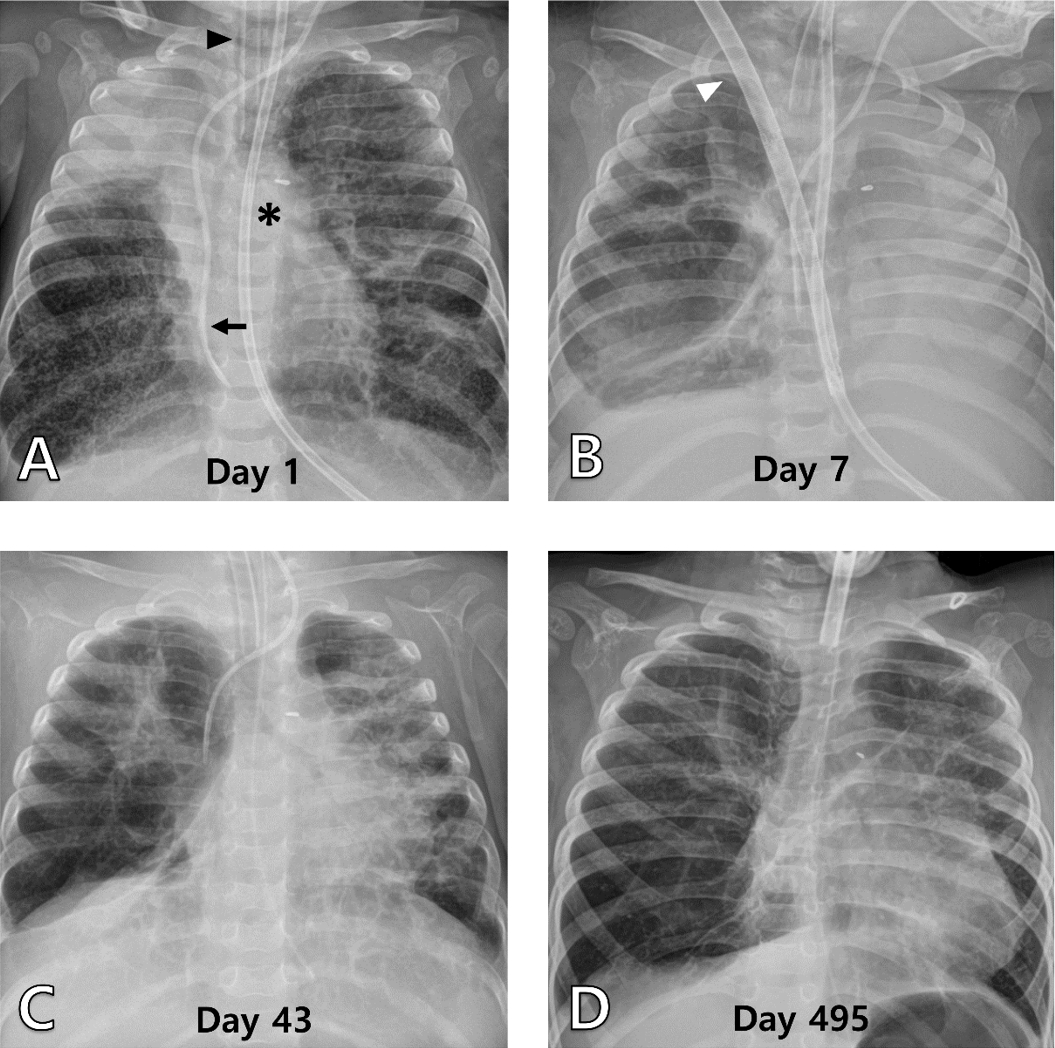
Fig. 2.
Serial chest radiographs of patient 2. (A) It shows extensive reticular opacity with patchy hyperinflated areas, with an endotracheal tube (arrowhead) and nasojejunal tube (asterisk) inserted (day 1). (B) It shows improved lung conditions 1 month after ECMO modulated lung resting, with a double-lumen catheter (arrowhead) and left internal jugular hemodialysis catheters (arrow) (day 58). (C) It shows hyperinflated lungs immediately after the removal of the double-lumen catheter (day 96). (D) It is the most recent radiograph taken at the outpatient clinic (day 476). ECMO: extracorporeal membrane oxygenation.
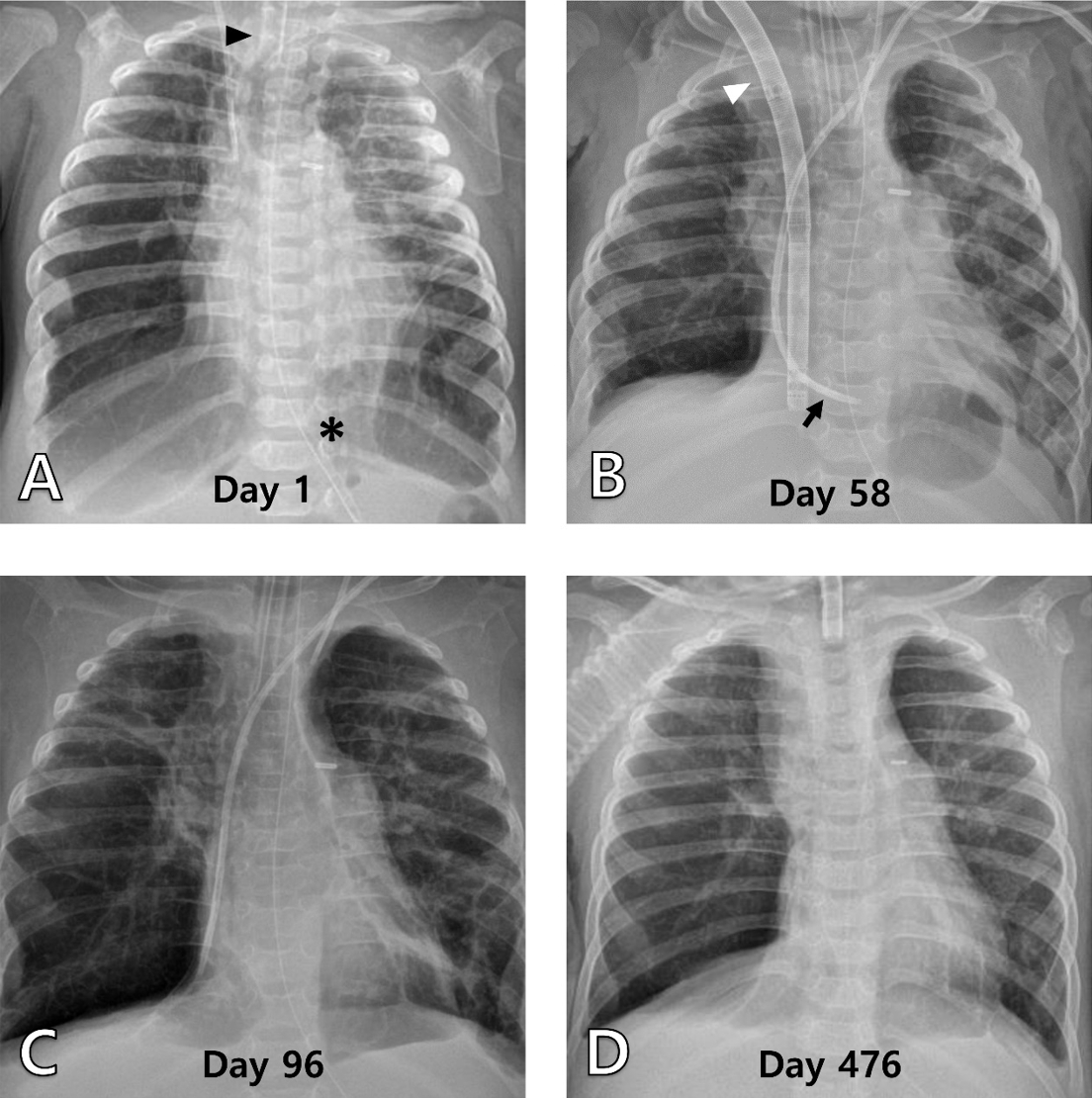
Table 1.
Changes in vital signs and blood gas analysis during the hospital course of patient 1
Table 2.
Changes in vital signs and blood gas analysis during the hospital course of patient 2




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download