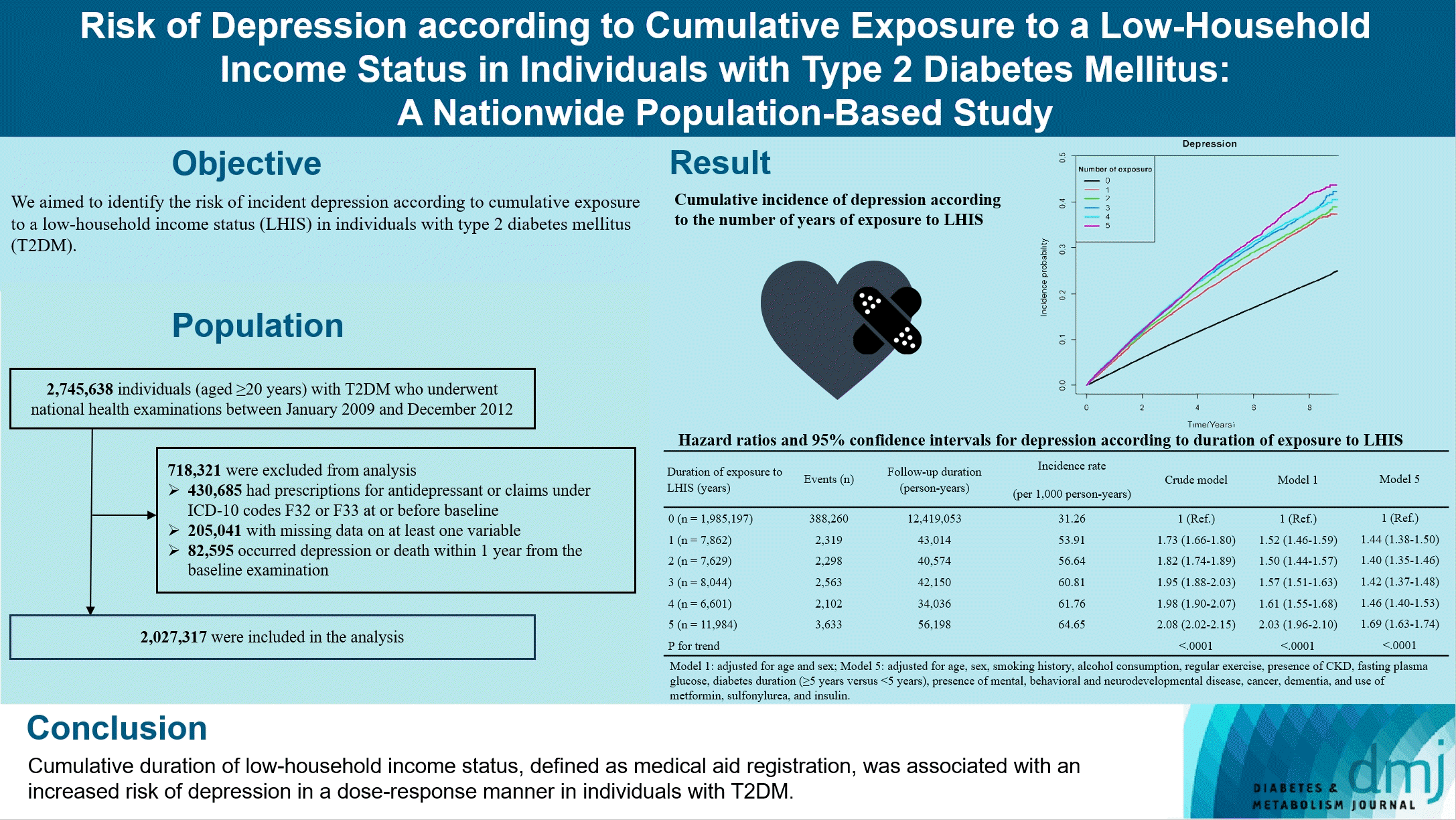
Abstract
Background
Methods
Results
SUPPLEMENTARY MATERIALS
Supplementary Table 2.
Supplementary Table 3.
Supplementary Fig. 1.
Notes
CONFLICTS OF INTEREST
Sang-Man Jin has been associate editor of the Diabetes & Metabolism Journal since 2022. He was not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: S.H.P., Y.B.L., K.H., J.H.K.
Acquisition, analysis, or interpretation of data: K.L., B.K., K.H.
Drafting the work or revising: S.H.P., Y.B.L., J.H.K.
Final approval of the manuscript: S.H.P., Y.B.L., K.L., B.K., S.H.C., S.Y.K., J.P., G.K., S.M.J., K.Y.H., K.H., J.H.K.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1I1A1A0106118813) to Prof. Kyungdo Han. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
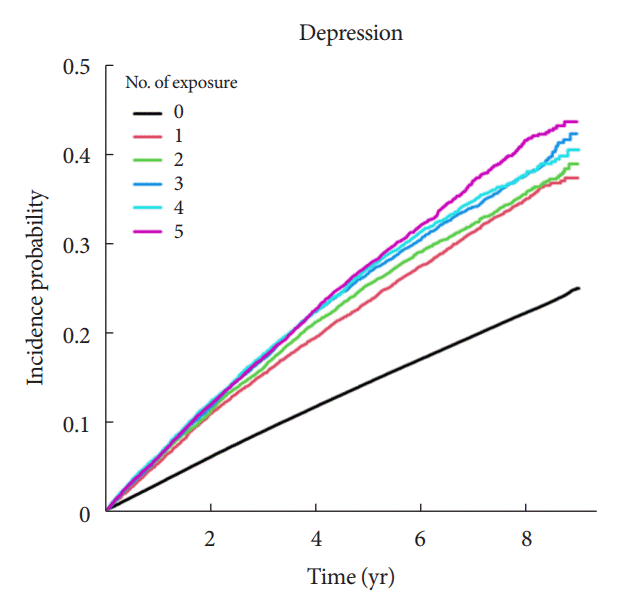
Fig. 2.
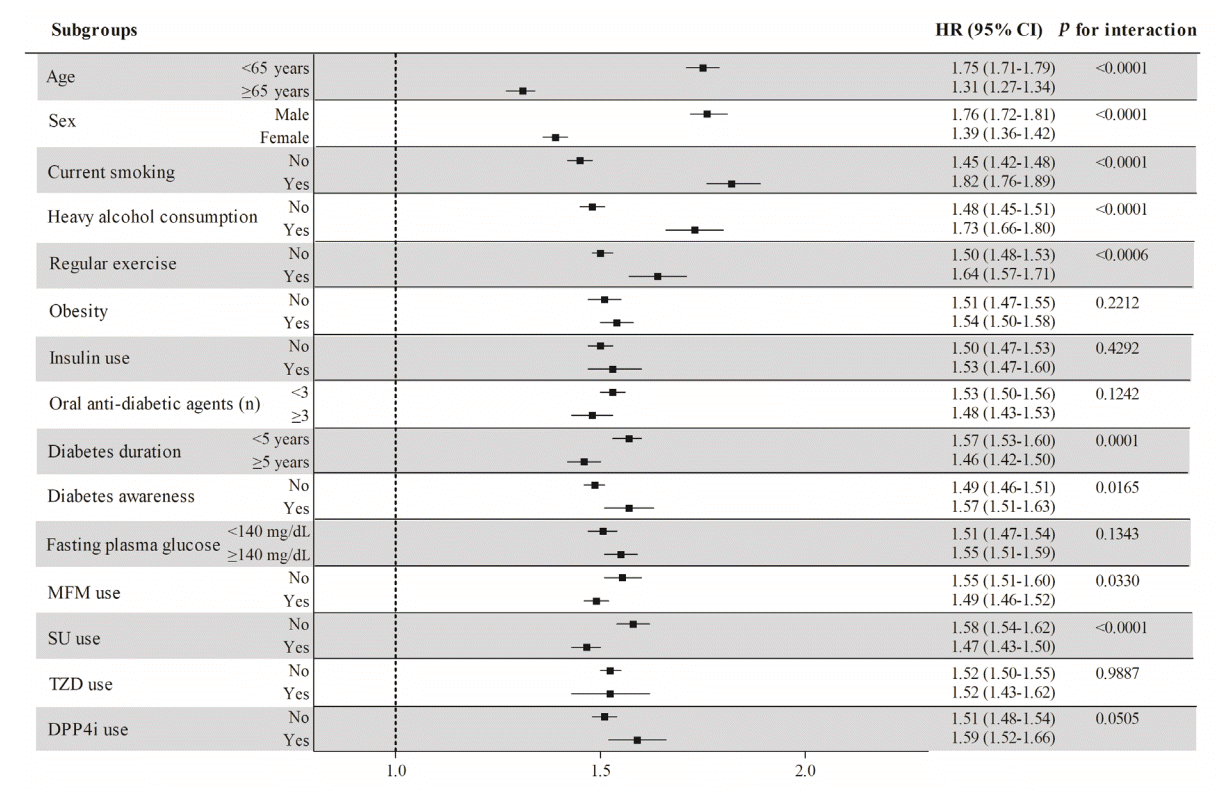
Fig. 3.
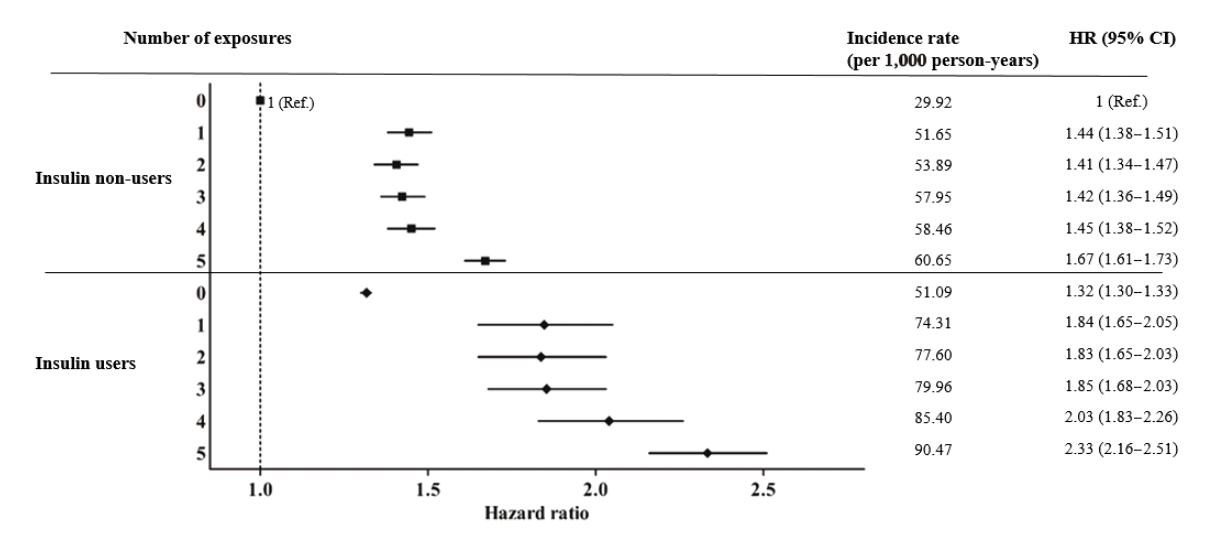
Table 1.
| Characteristic |
Duration of exposure to low-household income, yr |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 (n=1,985,197) | 1 (n=7,862) | 2 (n=7,629) | 3 (n=8,044) | 4 (n=6,601) | 5 (n=11,984) | P value | P for trend | |
| Age, yr | 56.3±12.33 | 58.15±13.3 | 59.63±13.37 | 60.13±12.67 | 59.79±12.58 | 56.31±8.96 | <0.0001 | <0.0001 |
| Male sex | 1,282,720 (64.61) | 3,906 (49.68) | 3,471 (45.5) | 3,563 (44.29) | 2,894 (43.84) | 5,860 (48.9) | <0.0001 | <0.0001 |
| Current smoker | 556,739 (28.04) | 2,128 (27.07) | 1,872 (24.54) | 1,920 (23.87) | 1,607 (24.34) | 3,331 (27.8) | <0.0001 | <0.0001 |
| Heavy alcohol consumption | 219,216 (11.04) | 651 (8.28) | 543 (7.12) | 560 (6.96) | 451 (6.83) | 840 (7.01) | <0.0001 | <0.0001 |
| Regular exercise | 415,454 (20.93) | 1,237 (15.73) | 1,207 (15.82) | 1,309 (16.27) | 1,080 (16.36) | 1,925 (16.06) | <0.0001 | <0.0001 |
| Body mass index, kg/m2 | 25.1±3.39 | 24.96±3.8 | 25±3.86 | 25.04±3.91 | 24.99±3.92 | 25.29±4.11 | <0.0001 | <0.0001 |
| Waist circumference, cm | 85.53±8.83 | 85±9.43 | 85.19±9.46 | 85.21±9.51 | 85.02±9.55 | 85.85±9.98 | <0.0001 | <0.0001 |
| Systolic BP, mm Hg | 129.22±15.79 | 128.95±16.76 | 128.94±16.53 | 128.87±16.71 | 128.59±16.63 | 126.91±16.53 | <0.0001 | <0.0001 |
| Diastolic BP, mm Hg | 79.34±10.29 | 78.79±10.5 | 78.65±10.33 | 78.57±10.38 | 78.38±10.5 | 77.95±10.4 | <0.0001 | <0.0001 |
| Fasting plasma glucose, mg/dL | 146.23±46.73 | 147.41±53.32 | 146.25±53.43 | 145.6±54.79 | 146.37±56.67 | 142.56±53.27 | <0.0001 | <0.0001 |
| Insulin usea | 140,139 (7.06) | 897 (11.41) | 1,008 (13.21) | 1,170 (14.55) | 921 (13.95) | 1,800 (15.02) | <0.0001 | <0.0001 |
| Diabetes duration ≥5 years | 565,984 (28.51) | 2,408 (30.63) | 2,604 (34.13) | 3,111 (38.67) | 2,359 (35.74) | 5,006 (41.77) | <0.0001 | <0.0001 |
| Diabetes unawareness | 848,952 (42.76) | 2,549 (32.42) | 2,270 (29.75) | 1,909 (23.73) | 1,495 (22.65) | 2,427 (20.25) | <0.0001 | <0.0001 |
| Oral anti-diabetic agents ≥3 | 266,013 (13.40) | 1,399 (17.79) | 1,458 (19.11) | 1,825 (22.69) | 1,548 (23.45) | 2,858 (23.85) | <0.0001 | <0.0001 |
| Type of oral anti-diabetic agents | ||||||||
| MFM | 895,859 (45.13) | 4,163 (52.95) | 4,233 (55.49) | 4,848 (60.27) | 4,038 (61.17) | 7,961 (66.43) | <0.0001 | <0.0001 |
| SU | 782,992 (39.44) | 3,744 (47.62) | 3,718 (48.74) | 4,434 (55.12) | 3,644 (55.20) | 6,210 (51.82) | <0.0001 | <0.0001 |
| TZD | 122,299 (6.16) | 519 (6.60) | 510 (6.69) | 624 (7.76) | 559 (8.47) | 853 (7.12) | <0.0001 | <0.0001 |
| DPP4i | 161,049 (8.11) | 776 (9.87) | 810 (10.62) | 1,008 (12.53) | 886 (13.42) | 2,397 (20.00) | <0.0001 | <0.0001 |
| Dyslipidemia | 785,882 (39.59) | 3,378 (42.97) | 3,329 (43.64) | 3,782 (47.02) | 3,191 (48.34) | 6,217 (51.88) | <0.0001 | <0.0001 |
| Lipid-lowering agents | 589,342 (29.69) | 2,678 (34.06) | 2,687 (35.22) | 3,178 (39.51) | 2,689 (40.74) | 5,519 (46.05) | <0.0001 | <0.0001 |
| Total cholesterol, mg/dL | 197.68±46.15 | 195.28±44.54 | 193.47±50.63 | 191.66±43.68 | 191.95±52.92 | 188.51±45.37 | <0.0001 | <0.0001 |
| HDL-C, mg/dL | 52.18±29.68 | 52.32±27.05 | 51.66±19.31 | 52.22±57.87 | 51.71±23.82 | 51.02±21.1 | 0.0005 | 0.0005 |
| LDL-C, mg/dL | 113.13±86.12 | 110.16±49.72 | 109.7±55.81 | 111±160.01 | 107.62±51.18 | 105.35±43.12 | <0.0001 | <0.0001 |
| eGFR, mL/min/1.73 m2 | 85.68±36.5 | 84.11±34.03 | 83.37±39.21 | 82.97±34.64 | 83.55±35.22 | 86.96±34.81 | <0.0001 | <0.0001 |
| CKD (eGFR <60 mL/min/1·73 m2) | 202,864 (10.22) | 1,183 (15.05) | 1,309 (17.16) | 1,345 (16.72) | 1,147 (17.38) | 1,624 (13.55) | <0.0001 | <0.0001 |
| Mental, behavioral and neurodevelopmental disease | 295,144 (14.87) | 1,925 (24.48) | 2,016 (26.43) | 2,359 (29.33) | 1,979 (29.98) | 4,561 (38.06) | <0.0001 | <0.0001 |
| Cancer | 92,747 (4.67) | 541 (6.88) | 499 (6.54) | 559 (6.95) | 412 (6.24) | 733 (6.12) | <0.0001 | <0.0001 |
| Dementia | 21,735 (1.09) | 190 (2.42) | 245 (3.21) | 272 (3.38) | 220 (3.33) | 383 (3.20) | <0.0001 | <0.0001 |
Values are presented as mean±standard deviation or number (%).
BP, blood pressure; MFM, metformin; SU, sulfonylurea; TZD, thiazolidinedione; DPP4i, dipeptidyl peptidase-4 inhibitor; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease.
Table 2.
Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, smoking history, alcohol consumption, regular exercise, and presence of chronic kidney disease (CKD); Model 4: adjusted for age, sex, smoking history, alcohol consumption, regular exercise, and presence of CKD, fasting plasma glucose, and diabetes duration (≥5 years vs. <5 years); Model 5: adjusted for age, sex, smoking history, alcohol consumption, regular exercise, and presence of CKD, fasting plasma glucose, diabetes duration (≥5 years vs. <5 years), presence of mental, behavioral and neurodevelopmental disease, cancer, dementia, and use of metformin, sulfonylurea, and insulin.
Table 3.
Adjusted for age, sex, smoking history, alcohol consumption, regular exercise, presence of chronic kidney disease, fasting plasma glucose, diabetes duration (≥5 years vs. <5 years), presence of mental, behavioral and neurodevelopmental disease, cancer, and dementia.
HR, hazard ratio; CI, confidence interval; MFM, metformin; SU, sulfonylurea; TZD, thiazolidinedione; DPP4i, dipeptidyl peptidase-4 inhibitor.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download