Abstract
Background: Environmental sanitation plays a significant role on the prevalence of enteropathogenic bacteria. This study aimed to determine the trends in the prevalence and antimicrobial resistance profiles of enteropathogenic bacteria from 2011 to 2019. Methods: A retrospective analysis was performed using data from stool cultures of Salmonella spp., Shigella spp., Plesiomonas shigelloides, Yersinia spp., Vibrio spp., and Campylobacter spp. Samples were obtained between 2011 and 2019 from Severance Hospital. Antimicrobial susceptibility profile was determined using the disk diffusion method for nontyphoidal Salmonella (NTS) and Campylobacter spp., following the Clinical and Laboratory Standards Institute (CLSI) guidelines. Results: The number of specimens obtained for stool culture increased significantly from 13,412 during the period of 19691978, to 48,476 over the past nine years (20112019), whereas the ratio of positive specimens decreased significantly from 1,732 (12.9%) to 449 (0.9%). The proportion of samples positive for Salmonella Typhi decreased from 472 (93.6%, 19691978) to 4 (1.5%, 20112019), whereas the proportion of NTS increased from 14 (2.8%, 19691978) to 261 (96.7%, 20112019). Among all the enteropathogenic bacteria isolated, Shigella spp. accounted for 60.0% (1,039) isolates from 1969 to 1978, but only 1.6% (7) from 2011 to 2019. Campylobacter was the second most prevalent enteropathogenic bacteria, accounting for 29.4% isolates (132). Among the NTS strains isolated from 2016 to 2019, their susceptibility rates to ampicillin and sulfamethoxazole-trimethoprim were 51.1% and 85.2%, respectively. Additionally, the susceptibility rate of Campylobacter to ciprofloxacin was 15.8%. Conclusion: The prevalence of Salmonella Typhi and Shigella spp. significantly decreased, whereas those of NTS and Campylobacter spp. increased. Therefore, continuous monitoring of ciprofloxacin-resistant Campylobacter spp. is of vital importance.
Gastroenteritis refers to stomach and intestinal infections, and most forms of acute gastroenteritis are known as acute diarrhea. Diarrhea in gastroenteritis is commonly characterized as the movement of an increased quantity and frequency of abnormally liquid or formless stools. It is classified as infectious diarrhea when diarrhea is caused by a source of infection and is followed by nausea, vomiting, and abdominal pain. However, in the clinic, microorganisms causing the infection are seldom confirmed [1].
Diarrheal disease is the second leading cause of morbidity and mortality, and mainly occurred in children under 5 years of age, which is one of the global public health issues, especially in developing countries, including Asia, Africa and Latin America [1-3]. According to the World Health Organization (WHO), 1.6 million deaths due to infectious diarrhea were recorded in 2010 [4,5]. The most frequent pathogens causing acute gastroenteritis worldwide are Salmonella spp., Shigella spp., Campylobacter spp., Escherichia coli O157:H7, Listeria monocytogenes, Yersinia enterocolitica, Vibrio cholerae, Rotavirus, Entamoeba histolytica, Cryptosporidium spp., and Giardia lamblia [1,6]. Moreover, the diarrhea-causing pathogens depend on various factors such as atmosphere, climate, season, and region. Therefore, the prevalence of pathogen separation in each region must be considered [7]. In developing countries such as Cambodia, Kenya, and Burkina Faso, where diarrhea rates are high due to low income and undernutrition, the most frequently isolated pathogens were enteroaggregative E. coli [8-10], but the most prevalent in Tehran was Shigella spp. [11]. However, many developed countries have a high standard of health and hygiene, so enteritis is less likely to develop, but infectious diarrhea can also occur in developed countries, as was the case with more than 3,000 infections of the E. coli O104: H4 in Germany in May 2011 [3]. In addition, Salmonella spp. (56%) and Shigella spp. (21%) were the most commonly detected pathogens in Europe and Latin America, as examined by the 2003 global SENTRY Antimicrobial Surveillance Program [12].
Bacteria have been found not to account for a high percentage of cases [1]. In most cases acute gastroenteritis is a self-limiting condition that does not require antibiotic treatment. Inappropriate use of antibiotics may cause antibiotic-associated diarrhea or other complications and may also lead to antibiotic resistance in the long term [1]. With the continuous improvement of health and hygiene, the incidence of infectious enteritis is decreasing, but the resistance rate of infectious enteritis is increasing. In addition, the increase in overseas travel has increased the likelihood of the introduction or epidemic of infectious enteritis caused by contaminated food or water [6,7].
In Korea, 14.7% of enteropathogenic bacteria were isolated based on acute infectious diarrhea surveillance in 2017 [6]. However, studies in South Korea over the past 10 years have primarily focused on advanced genotypic and phenotypic characteristics of enteropathogenic bacterial strains and as a result very few studies have been done on conventional surveillance of these pathogens leading to a gap in data for monitoring the trends in isolation and resistance patterns. Conventional method, however, remains as a gold standard for the confirmation of bacteria to monitor the trend and resistance patterns of enteropathogenic bacteria. This study aims to determine the trends in the isolation and antimicrobial resistance patterns of enteropathogenic bacteria between 2011 and 2019 at Severance Hospital. Also, to compare the current pattern and trends of enteropathogenic bacterial antimicrobial susceptibility with previous reports in Severance Hospital.
Study design
A retrospective analysis was performed using existing data of stool culture from the patients hospitalized at Severance Hospital from 2011 to 2019.
Specimen collection
The stool samples were collected and placed it in transport mini sputum container. Bacteria to be cultured belong to Salmonella spp., Shigella spp., Plesiomonas shigellosides, Yersinia spp., Vibrio spp., and Campylobacter spp..
Bacterial culture media and condition
MacConkey agar base, Salmonella Shigella agar, selenite broth (BBL, Becton, Dickinson and Company, Sparks, MD, USA) were used for Enterobacteriaceae. Thiosulfate citrate bile sucrose agar base (BBL) was used for Vibrio species. Campylobacter selective supplement, Campylobacter growth supplement (Oxoid Ltd, Basingstoke, UK) and blood agar base (BBL) were used for Campylobacter species culture. Enterobacteriaceae and Vibrio spp. cultures were incubated in aerobic condition at 35oC for 24 hours. For Campylobacter spp. specimen-inoculated plates were incubated in a microaerophilic atmosphere at 42°C for 48 hours [13].
Species identifications and antimicrobial susceptibility testing (AST)
Colony shape, biochemical testing, matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Bremen, Germany), and VITEK 2 ID cards (bioMerieux, Inc., Durham, NC, USA) were used for bacteria identification. Moreover, serological identification of Salmonella or Shigella spp. was performed by using a slide aggregation reaction Salmonella and Shigella group diagnostic sera (National Health Research Institute, Osong, Korea). The AST was tested by VITEK 2 AST card, disc diffusion method and E-test following the Clinical Laboratory Standard Institute (CLSI) guideline [14]. A standardized 0.5 McFarland suspension was prepared, and VITEK 2 cards for AST were inoculated following the manufacturer recommendation. For AST testing of Salmonella or Shigella spp. were tested with N224 VITEK 2 card, and additionally ciprofloxacin disc susceptibility test. Erythromycin, ciprofloxacin, and tetracycline discs were tested at the same time for Campylobacter spp. Quality control of species identification and AST were performed according to CLSI guideline and manufacturer’s instruction [14].
Data registration and statistical analysis
Data stored in Severance Hospital information management system. Microsoft Excel 2010 (16.0.13328.20262) was used for statistical analysis.
Ethical considerations
Ethical approval was granted from Yonsei Institutional Review Board at the Severance Hospital, Yonsei University, Seoul, Republic of Korea (IRB No. 4-2020-1031). Patients were identified with a unique hospital number. No other data besides those noted in the routine medical files were used for the clinical and epidemiological data.
Comparison of trends in isolation of enteropathogenic bacteria at a tertiary-care hospital
There were 48,476 specimens were requested for stool culture from 2011 to 2109. Among the total requested specimens, 449 specimens were positive accounted to 0.9%. The enteropathogenic bacteria isolated from the stool culture during 2011 to 2019 were distributed as 270 (60.1%) Salmonella spp., 7 (1.6%) Shigella spp., 132 (29.4%) Campylobacter spp., 7 (1.6%) Vibrio parahaemolyticus, 1 (0.2%) Yersinia enterocolitica, and 32 (7.1%) others isolates.
Salmonella spp. and Campylobacter spp. were the most common pathogen 60.1% and 29.4%, respectively. Followed by Salmonella spp., there were separated 4 (1.5%) Salmonella Typhi, 0 (0.0%) Salmonella Paratyphi A, 111 (41.1%) Salmonella serogroup B, 82 (30.4%) Salmonella serogroup C, 63 (23.3%) Salmonella serogroup D, 5 (1.9%) Salmonella serogroup E and 5 (1.9%) Salmonella another serogroup (Table 1).
Number of patients with nontyphoidal Salmonella and Campylobacter spp. isolation by month in 2011-2019
The findings were figured out of the number of patients with nontyphoidal Salmonella (NTS) and Campylobacter spp. isolation by month in 2011-2019. Among 261 strains of NTS, most of them were isolated in summer-autumn season: August (16.5%), September (15.3%), October (15.3%) and July (10.0%). Moreover, 61 of 132 Campylobacter spp. were also isolated in the hot season, June (11.4%), July (13.6%) and August (21.2%) (Table 2).
Number of patients with nontyphoidal Salmonella and Campylobacter spp. by age group
The patient under 5 years and older than 60 years of age were found highly infected by NTS and Campylobacter spp. However, Campylobacter was isolated, not only under the age of 5 and more than 60 but also found highly infected at 10-19 and 20-29 (Table 3).
Comparison of trends in antimicrobial susceptibility of nontyphoidal Salmonella
As shown in Table 4, the susceptibility rate of NTS between 2011 and 2015 was ampicillin (53%), sulfamethoxazole-trimethoprim (93%), cefotaxime (92%), ceftazidime (92%), and fluoroquinolone (84%). Similarly, the susceptibility rate of NTS between 2016 to 2019 was ampicillin (51%), sulfamethoxazoletrimethoprim (85%), cefotaxime (74%), ceftazidime (81%), and fluoroquinolone (73%).
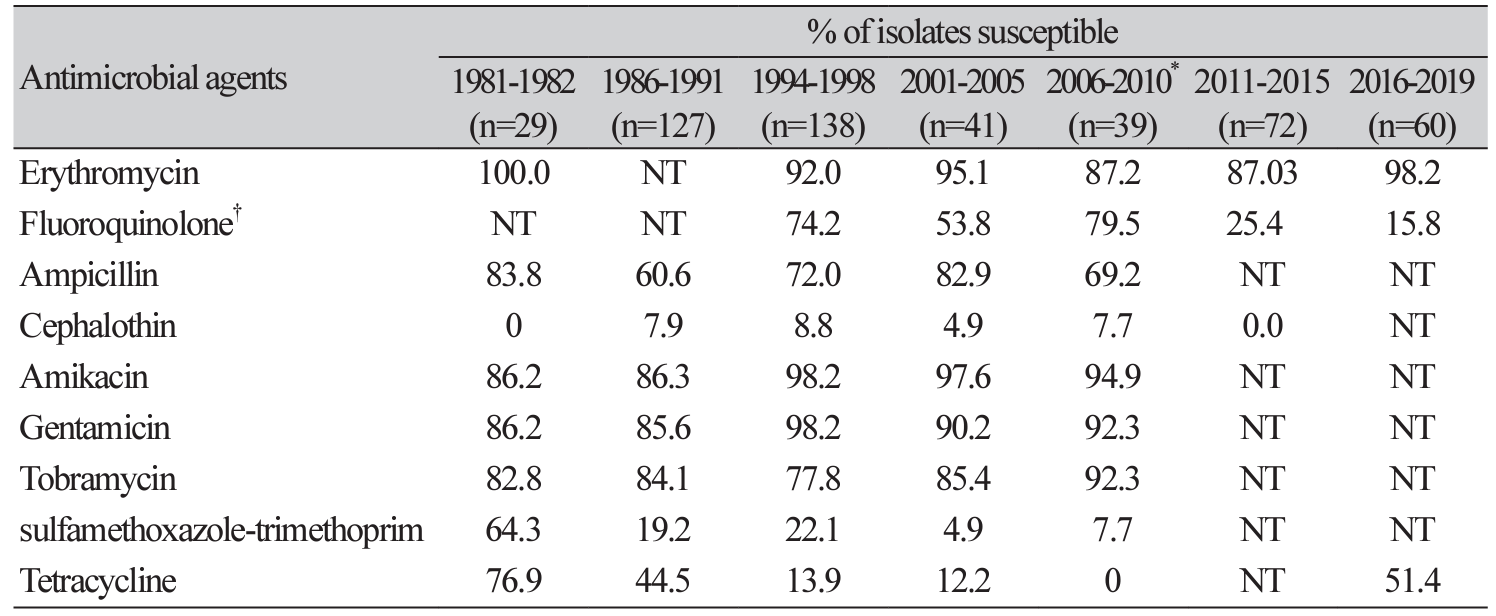
Comparison of trends in antimicrobial susceptibility of Campylobacter spp.
As shown in Table 5, the rates of susceptibility to erythromycin and fluoroquinolone were 87.03% and 25.4%, respectively, for Campylobacter spp.; they were isolated from 2011 to 2015. On the other hand, the rates of susceptibility to erythromycin, fluoroquinolone and tetracycline were 98.2%, 15.8% and 51.4%%, respectively, for Campylobacter spp.; they were isolated from 2016 to 2019.
In this study, we compared the results of the isolation of enteropathogenic bacteria in 2011-2019 with the results of isolates in 1969-2010. Moreover, we analyzed the trend of enteropathogenic bacteria and examined the antibiotic resistance pattern. The number of requests for stool culture testing during this study period increased by 3.6 times compared to 13,412 samples from 1969-1978. However, the numbers of samples positive for culture decreased significantly from 1,732 (positive rate 12.9%) to 449 (positive rate 0.9%). These might be due to an increase in the number of patients with an increase in the number of beds, an increase in hospitalized infection monitoring culture, and improved health and hygiene [15]. Shigella, Vibrio, and Salmonella are mainly isolated among enterocolitis in developing countries, but Shigella and Salmonella Typhi are rare in developed countries [16]. In developed countries, Campylobacter and NTS became the major isolates. A USA study reported that 42% of the enteritis bacterial causative agents were Campylobacter 32%, NTS 32%, and Shigella 13%, supporting the changes in these isolates [16,17]. Another report regarding traveler diarrhea investigated tourisms from South East Asia countries including Thailand where commonly identified Campylobacter and Aeromonas spp. [18].
Particularly, Salmonella enteritidis belonging to the Salmonella serotype D group, is one of the most common Salmonella serotypes prevalent all over the world and is known to be the main causative agent of foodborne salmonellosis [19]. During the study period, 2011-2019, NTS was detected in 261 patients, and it was the most isolated isolate, accounting for 58%. Salmonella isolated in 1969-1978, 472 (93.6%) were Salmonella Typhi, but in 2011-2019, there were only 4 (1.5%) isolated. The number of Salmonella Paratyphi A was 101 (9.2%) in 1979-1988, but it decreased significantly to 0 (0.0%) in 2001-2010. On the other hand, NTS’s trend gradually increased and isolated from 14 cases in 1969-1978 to 261 cases in 2011-2019. In the past, there were many Salmonella serogroup B and D among NTS, but the isolates started to be decreased since 2010. Despite, compared to developing countries were remained highly infected as a massive typhoid fever outbreak in Pakistan resulted in 5,372 extensively drug-resistant (XDR) Salmonella Typhi cases stated during 2016-2018, and five travel-related cases in the United States [20].
Shigella spp. was the most common enteritis bacterium, accounting for more than 50% of the total number of isolates, separated from a total of 2,547 isolated in 1969-1988, but the trend decreased significantly after 1989. In this study, a total of 7 (1.6%) isolates were isolated from 2011-2019. In the developing countries, there were many S. dysenteriae and S. flexneri infections, but in developed countries, there were many S. sonnei infections [21]. In the recent years, S. dysenteriae infection has been rare in Korea [22], the number of S. flexneri was the most prevalent until the late 1980s, and S. sonnei has been the most prevalent since 1990 [23]. Nonetheless, even after the late 1990s, it was decreased, Shigella infections often occurred, it is requiring continuous culture [23]. On the other hand, Thailand reported that the most commonly isolated Shigella species over the past two decades have been S. flexneri (79%) and S. sonnei (15%) and the incidence of S. sonnei were predominated over 80% in children under 5 in their country [24]. Even though, Thailand is a developed country, Shigella ratio remains high, if compare to Korea’s current situation.
C. jejuni is a major cause of bacterial diarrheal illness in the USA and in many other countries, with an estimated 845,000 foodborne cases per year in the USA alone, third in the number of estimated bacterial foodborne disease cases after Salmonella spp. and Clostridium perfringens [21]. This hospital began to culture Campylobacter spp. from 1981 and identified 239 isolates in 1989-1998, 83 isolates in 2001-2010 and 132 isolates in 2011-2019. Among the 132 isolates of Campylobacter spp. in 2011-2019, C. jejuni was the most common pathogen. By the way, a study in Cambodia found in multiplex polymerase chain reaction (PCR) among 681 stool samples were 82 (12%) tested positive followed by C. jejuni in 66 samples and C. coli in 16 [25] with Campylobacter positive rates for Vietnam and Laos were below 5% [26]. Thus, it showed that the rate of Campylobacter spp. infection in developed country is higher than in developing countries.
V. parahemolyticus was isolated 7 (1.6%) during the investigation period. The number was small because patients with this enteritis recovered naturally after 2-3 days of infection without treatment, so there was little opportunity to isolate the bacteria from tertiary medical institutions. In Korea, it is projected that the number of infected patients is high because Korean coast often reproduce fish and shellfish [27]. In fact, with the food poisoning from 2003-2016 in 2017 Korea Ministry of Food and Drug Safety report, 225 cases and 4,256 patients were determined in the outbreaks by V. parahaemolyticus, which was accounting for 5.8% of total outbreaks and 4.1% of total patients, respectively, based on all food-borne illnesses occurring in Korea [27].
Some studies have been reported on Y. enterocolitica distribution, mostly in Northern Europe and northern states of the USA, reflected its increased rate because of cold temperatures [28]. In Korea, have been reported 47 cases of Y. enterocolitica and 15 cases of P. shigelloides in 1979-1998 [15], In this study, only one Y. enterocolitica was found.
NTS and Campylobacter spp. were mainly isolated in patients under five years of age, similar to those of other domestic and foreign researchers [16,29]. However, Campylobacter spp. was isolated, not only under the age of 5 but also at 10-19 and 20-29. While infection with C. jejuni or C. coli can occur in patients of all ages [30], a recent study from Denmark showed that infection is more common in children (1 to 4 years) and young adults (15 to 24 years) than in other age groups [31]. Also, slightly older patients (34.6 years versus 27.5 years) and those who traveled abroad were at a greater risk of being infected with C. coli than with C. jejuni [32]. Moreover, infections with C. jejuni and C. coli are more common during the summer [31,33]. Similarly, this study found that most of Campylobacter spp. was isolated in June, July, and August.
In this study, ampicillin and sulfamethoxazole-trimethoprim resistance in NTS was higher than in the previous period. Furthermore, in the last four years in 2016-2019, the susceptibility ratio of cefotaxime, ceftazidime and fluoroquinolone decreased significantly. The generation of extended-spectrum β-lactamase (ESBL) resistant to third-generation cephalosporin in Korea was first reported in 2003. It had been increasing since then, presumably due to CTX-M-type ESBL [34,35]. Even though, ciprofloxacin resistance rate has good activity against Salmonella spp.; continuous monitoring is still necessary because ciprofloxacin-resistant Salmonella has increased in foreign countries [36]. According to NTS analyzed by Korea Centers for Disease Control and Prevention (KCDC) from 2006 and 2008, the resistance ratios to ampicillin, nalidixic acid, ciprofloxacin, and trimethoprim-sulfamethoxazole (TMP/SMX) were at 49%, 50%, < 1%, and 8%, respectively [37].
Erythromycin and ciprofloxacin are recommended for the treatment of C. enteritis, and recently increased resistance to these drugs has been reported. Although, it is difficult to make a direct comparison according to the change of the standard for analyzing the antimicrobial susceptibility test of Campylobacter spp., the ciprofloxacin susceptibilities were 79.5% in 2006-2010 [7], 25.4% in 2011-2015 and 15.8% in 2016-2019. Thus, we observed the resistance rapidly increased. On the other hand, the erythromycin and tetracycline susceptibility in 2016-2019 were gradually decreased to 98.2% and 51.4%, respectively, compared to the previous period. In other studies in Europe in 2012, ciprofloxacin resistance of Campylobacter spp. was reported to be as high as 44% in some European countries [38]. Fluoroquinolone resistance of Campylobacter spp. has also been reported to be high in Mexico (56%) and Thailand (> 92%) [39,40]. Despite, in Japan showed that macrolide resistance was in a low rate in C. jejuni and C. coli isolated, but quinolones-resistant Campylobacter spp. has emerged [41]. Considering these, macrolides including azithromycin may be considered for empirical antibiotic therapy in areas where Campylobacter spp. is common and has a high resistance to fluoroquinolone [42].
In summary, these results showed an increase in the incidence of enteropathogenic bacterial infection in Severance Hospital throughout the study. The pathogens with the most significant increase in incidence were nontyphoidal Salmonella and Campylobacter spp., even though there was a decrease in Salmonella Typhi and Shigella’s incidence. Enteropathogenic bacteria resistance to commonly prescribed antibiotics is steadily on the rise, as shown in this study. Nontyphoidal Salmonella resistance increased to ampicillin, sulfamethoxazole-trimethoprim, cefotaxime, ceftazidime, and fluoroquinolone.
Campylobacter resistance to fluoroquinolone increased significantly over the study period, but Campylobacter isolates remained susceptible to erythromycin. Continuous monitoring of resistant enteropathogenic bacteria is necessary.
REFERENCES
1. Kim YJ, Park KH, Park DA, Park J, Bang BW, Lee SS, et al. Guideline for the antibiotic use in acute gastroenteritis. Infect Chemother 2019;51:217-43.
2. Zhang H, Pan F, Zhao X, Wang G, Tu Y, Fu S, et al. Distribution and antimicrobial resistance of enteric pathogens in Chinese paediatric diarrhoea: a multicentre retrospective study, 20082013. Epidemiol Infect 2015;143:2512-9.
3. King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003;52:1-16.
4. Kirk MD, Angulo FJ, Havelaar AH, Black RE. Diarrhoeal disease in children due to contaminated food. Bull World Health Organ 2017;95:233-4.
5. Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223.
6. Korean Society of Infectious Diseases, Korean Society for Chemotherapy, and Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of gastrointestinal infections. Infect Chemother 2010;42:323-61.
7. Cho IJ, Yim J, Lee Y, Kim MS, Seo Y, Chung HS, et al. Trends in isolation and antimicrobial susceptibility of enteropathogenic bacteria in 2001-2010 at a Korean tertiary care hospital. Ann Clin Microbiol 2013;16:45-51.
8. Meng CY, Smith BL, Bodhidatta L, Richard SA, Vansith K, Thy B, et al. Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr Infect Dis J 2011;30:331-5.
9. Sang WK, Oundo V, Schnabel D. Prevalence and antibiotic resistance of bacterial pathogens isolated from childhood diarrhoea in four provinces of Kenya. J Infect Dev Ctries 2012;6:5728.
10. Bonkoungou IJ, Haukka K, Österblad M, Hakanen AJ, Traoré AS, Barro N, et al. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr 2013;13:16.
11. Jafari F, Garcia-Gil LJ, Salmanzadeh-Ahrabi S, Shokrzadeh L, Aslani MM, Pourhoseingholi MA, et al. Diagnosis and prevalence of enteropathogenic bacteria in children less than 5 years of age with acute diarrhea in Tehran children's hospitals. J Infect 2009;58:21-7.
12. Streit JM, Jones RN, Toleman MA, Stratchounski LS, Fritsche TR. Prevalence and antimicrobial susceptibility patterns among gastroenteritis-causing pathogens recovered in Europe and Latin America and Salmonella isolates recovered from bloodstream infections in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (2003). Int J Antimicrob Agents 2006;27:367-75.
13. Murray PR, Baron EJ, et al. eds. Manual of clinical microbiology. Washington, D.C.; ASM Press, 2007.
14. Clinical and Laboratory Standards Institute (CLSI). Performance standard for antimicrobial susceptibility testing, 29th ed. CLSI supplement M100. Wayne; PA, 2019.
15. Shin HB, Jeong SH, Kim M, Kim WH, Lee K, Chong Y. Isolation trend of enteropathogenic bacteria in 1969-1998. Korean J Clin Microbiol 2001;4:87-95.
16. Thapar N and Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet 2004;363:641-53.
17. Kendall ME, Crim S, Fullerton K, Han PV, Cronquist AB, Shiferaw B, et al. Travel-associated enteric infections diagnosed after return to the United States, foodborne diseases active surveillance network (FoodNet), 2004-2009. Clin Infect Dis 2012;54:S480-7.
18. Tribble DR, Sanders JW, Pang LW, Mason C, Pitarangsi C, Baqar S, et al. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycinbased regimens with a 3-day levofloxacin regimen. Clin Infect Dis 2007;44:338-46.
19. Mezal EH, Sabol A, Khan MA, Ali N, Stefanova R, Khan AA. Isolation and molecular characterization of Salmonella enterica serovar Enteritidis from poultry house and clinical samples during 2010. Food Microbiol 2014;38:67-74.
20. Tanmoy AM, Westeel E, De Bruyne K, Goris J, Rajoharison A, Sajib MSI, et al. Salmonella enterica serovar Typhi in Bangladesh: exploration of genomic diversity and antimicrobial resistance. MBio 2018;9:e02112-18.
21. Todd ECD. Foodborne diseases: overview of biological hazards and foodborne diseases. In: Motarjemi Y, ed. Encyclopedia of food safety. Waltham; Academic Press, 2014:221-42.
22. Pai H. History and epidemiology of bacillary dysentery in Korea: from Korean war to 2017. Infect Chemother 2020;52:123-31.
23. Lee JC, Jeong YS, Oh JY, Kang HY, Kim KH, Kim J, et al. Epidemiology of shigellosis in Korea. J Bacteriol Virol 2006;36:41-9.
24. Chompook P, Samosornsuk S, Lorenz von S, Jitsanguansuk S, Sirima N, Sudjai S, et al. Estimating the burden of shigellosis in Thailand: 36-month population-based surveillance study. Bull World Health Organ 2005;83:739-46.
25. Osbjer K, Tano E, Chhayheng L, Mac-Kwashie AO, Fernström LL, Ellström P, et al. Detection of Campylobacter in human and animal field samples in Cambodia. Apmis 2016;124:508-15.
26. Nguyen NH, Nguyen TNM, Hotzel H, El Adawy H, Nguyen AQ, Tran HT, et al. Thermophilic Campylobacter - neglected foodborne pathogens in Cambodia, Laos and Vietnam. Gastroenterol Hepatol 2017;8:00279.
27. Park K, Mok JS, Kwon JY, Ryu AR, Kim SH, Lee HJ. Food-borne outbreaks, distributions, virulence, and antibiotic resistance profiles of Vibrio parahaemolyticus in Korea from 2003 to 2016: a review. Fish Aquatic Sci 2018;21:3.
28. Drancourt M. Acute diarrhea. Infectious Diseases. https://doi.org/10.1016/B978-0-323-045797.00035-6 [Online] (last visited on 2 December 2021).
29. Cho SH, Shin HH, Choi YH, Park MS, Lee BK. Enteric bacteria isolated from acute diarrheal patients in the Republic of Korea between the year 2004 and 2006. J Microbiol 2008;46:32530.
30. Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 2015;28:687-720.
31. Nielsen HL, Ejlertsen T, Engberg J, Nielsen H. High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: a population-based study. Clin Microbiol Infect 2013;19:445-50.
32. Bessède E, Lehours P, Labadi L, Bakiri S, Mégraud F. Comparison of characteristics of patients infected by Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus. J Clin Microbiol 2014;52:328-30.
33. Rao MR, Naficy AB, Savarino SJ, Abu-Elyazeed R, Wierzba TF, Peruski LF, et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol 2001;154:166-73.
34. Lee K, Yong D, Yum JH, Kim HH, Chong Y. Diversity of TEM-52 extended-spectrum β-lactamase-producing non-typhoidal Salmonella isolates in Korea. J Antimicrob Chemother 2003;52:493-6.
35. Yong D, Lim YS, Yum JH, Lee H, Lee K, Kim EC, et al. Nosocomial outbreak of pediatric gastroenteritis caused by CTX-M-14-type extended-spectrum β-lactamase-producing strains of Salmonella enterica serovar London. J Clin Microbiol 2005;43:3519-21.
36. Tamang MD, Nam HM, Kim A, Lee HS, Kim TS, Kim MJ, et al. Prevalence and mechanisms of quinolone resistance among selected nontyphoid Salmonella isolated from food animals and humans in Korea. Foodborne Pathog Dis 2011;8:1199-206.
37. Oh JY, Yu HS, Kim SK, Seol SY, Cho DT, Lee JC. Changes in patterns of antimicrobial susceptibility and integron carriage among Shigella sonnei isolates from Southwestern Korea during epidemic periods. J Clin Microbiol 2003;41:421-3.
38. European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2010. EFSA J 2012;10:2598.
39. Zaidi MB, McDermott PF, Campos FD, Chim R, Leon M, Vazquez G, et al. Antimicrobialresistant Campylobacter in the food chain in Mexico. Foodborne Pathog Dis 2012;9:841-7. .
40. Serichantalergs O, Pootong P, Dalsgaard A, Bodhidatta L, Guerry P, Tribble DR, et al. PFGE, Lior serotype, and antimicrobial resistance patterns among Campylobacter jejuni isolated from travelers and US military personnel with acute diarrhea in Thailand, 1998-2003. Gut Pathogens 2010;2:15.
41. Igimi S, Okada Y, Ishiwa A, Yamasaki M, Morisaki N, Kubo Y, et al. Antimicrobial resistance of Campylobacter: prevalence and trends in Japan. Food Addit Contam 2008;25:1080-3.
42. Kim YJ, Park KH, Park DA, Park J, Bang BW, Lee SS, et al. Guideline for the antibiotic use in acute gastroenteritis. Infect Chemother 2019;51:217-43.
Table 2
Number of patients with nontyphoidal Salmonella and Campylobacter spp. isolation by month in 2011-2019
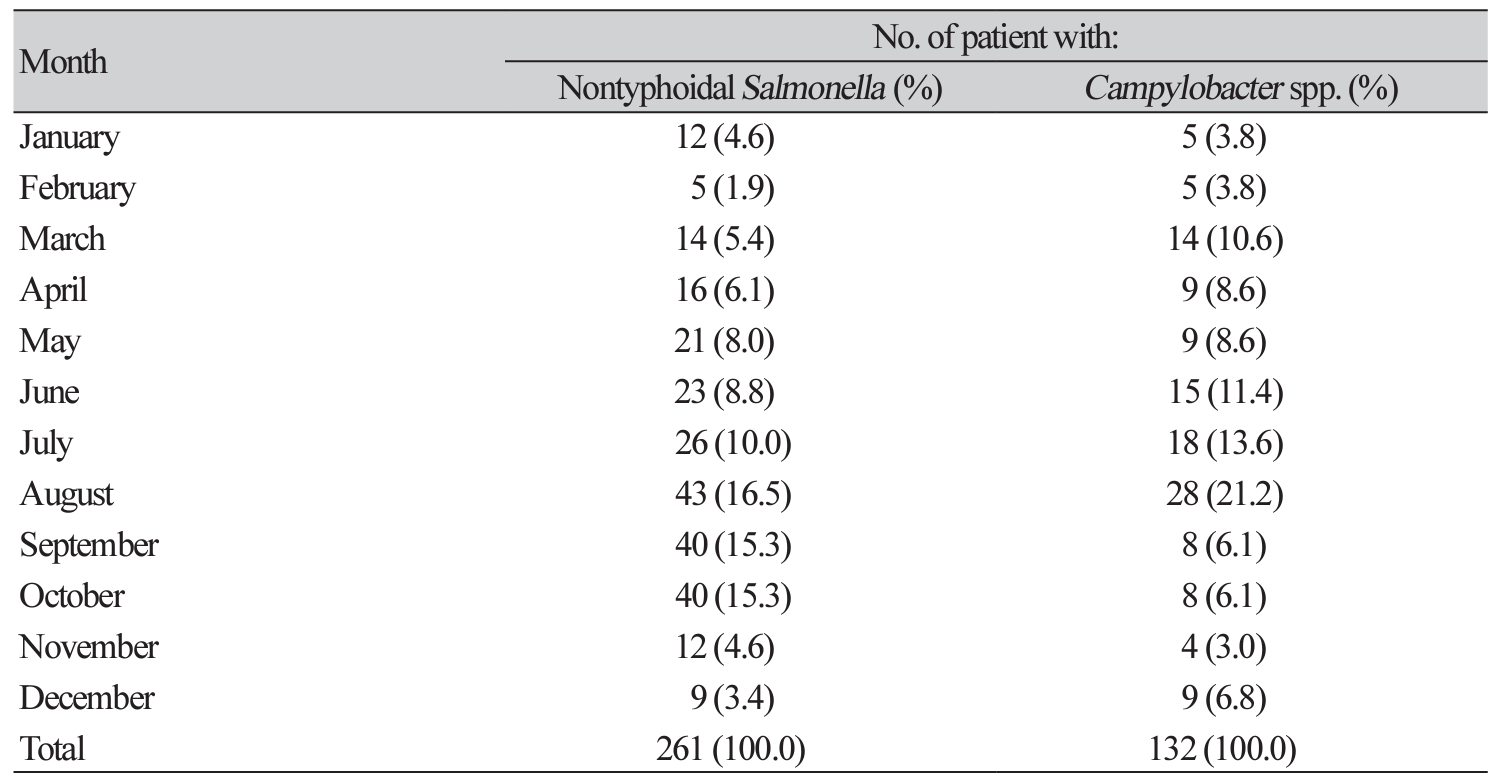
Table 4
Comparison of trends in antimicrobial susceptibility of nontyphoidal Salmonella
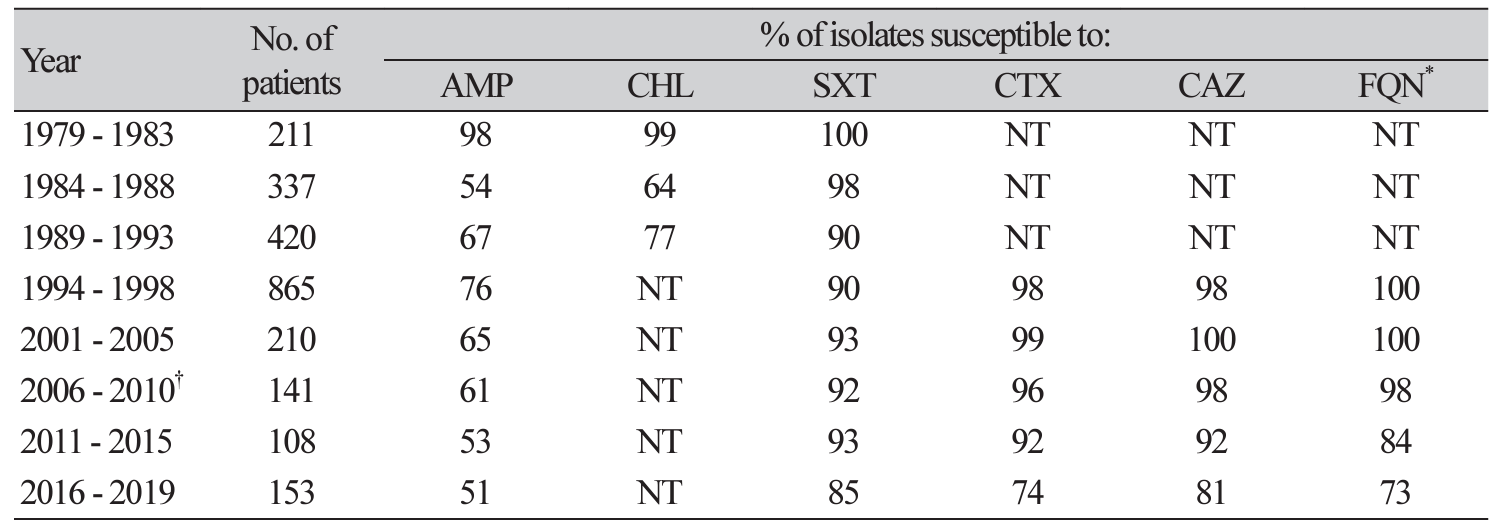
*FQN: ofloxacin (1994-1996), levofloxacin (1997-1998), levofloxacin (2001-2010) and levofloxacin (2011-2019).†The data from 1969-2010 were adapted from reference [7].Abbreviation: AMP, ampicillin; CHL, chloramphenicol; SXT, sulfamethoxazole-trimethoprim; CTX, cefotaxime; CAZ, ceftazidime; FQN, fluoroquinolone; NT, not tested.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download