Abstract
SARS-CoV-2 antibody assay is a test that checks whether an antibody against the SARS- CoV-2 virus has been formed in the blood after SARS-CoV-2 infection or vaccination. SARS- CoV-2 antibody is detected 1–2 weeks after infection, and antibodies are produced in more than 90% of infected patients. The duration for the formation of antibodies differs by individual and by type of antibody. In the case of IgG, it is at least several months or longer, and the relationship between antibodies and immunity is being studied. As test methods, enzyme-linked immunosorbent assay (ELISA), chemiluminescence immunoassay (CIA), immunochromatographic assay, and neutralizing antibody assay have been developed and used. The target antibody to be detected differs depending on the type of recombinant antigen and the type of secondary antibody in reagents. Many kinds of commercialized SARS-CoV-2 antibody assays are currently being developed, and the S (spike) protein, N (nucleocapsid) protein, S1 or RBD (receptor binding domain) part of the S protein, and a mixture of these antigens are used as recombinant antigens of reagents. IgG, IgM, IgA, or total immunoglobulin antibodies in patients’ blood that react with these reagent antigens are detected. In this review, the types and performance of SARS-CoV-2 antibody tests and the guidelines for COVID-19 antibody tests published domestically and abroad were investigated.
REFERENCES
1. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13.
2. Mousavizadeh L and Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect 2021;54:159-163.
3. Infantino M, Damiani A, Gobbi FL, Grossi V, Lari B, Macchia D, et al. Serological assays for SARS-CoV-2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J 2020;22:203-10.
4. Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci 2020;6:591-605.
5. Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2020;6:CD013652.
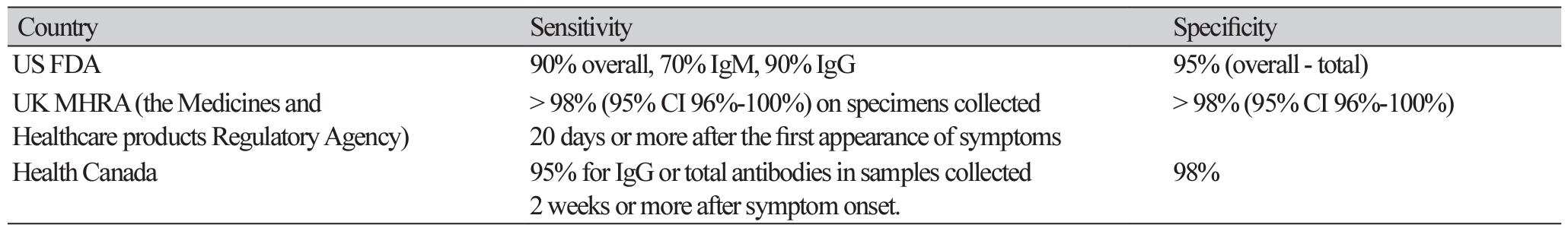
6. Post N, Eddy D, Huntley C, van Schalkwyk MCI, Shrotri M, Leeman D, et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 2020;15:e0244126.
7. Centers for Disease Control and Prevention (CDC). Interim guidelines for COVID-19 antibody testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines. html [Online] (last visited on 30 March 2021).
8. Centers for Disease Control and Prevention (CDC). EUA authorized serology test performance. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-useauthorizations-medical-devices/eua-authorized-serology-test-performance [Online] (last visited on 30 March 2021).
9. Government of Canada. COVID-19 serological testing devices: notice on sensitivity and specificity values. https://www.canada.ca/en/health-canada/services/drugs-health-products/ covid19-industry/medical-devices/testing/serological/notice-sensitivity-specificity-values.html [Online] (last visited on 30 March 2021).
10. COVID-19 laboratory response task force of Korean Society for Laboratory Medicine. Guidelines for the laboratory diagnosis of COVID-19 in Korea, 4th ed. Bureau of Infectious Disease Diagnosis Control of Korea Disease Control and Prevention Agency; 2020.
11. Infectious Diseases Society of America (IDSA). IDSA guidelines on the diagnosis of COVID-19: serologic testing. https://www.idsociety.org/practice-guideline/covid-19-guidelineserology/ [Online] (last visited on 30 March 2021).
12. World Health Organization (WHO). Diagnostic testing for SARS-CoV-2: interim guidance, 11 September 2020. https://apps.who.int/iris/handle/10665/334254 [Online] (last visited on 31 March 2021).
13. De Gruyter. IFCC Interim guidelines on serological testing of antibodies against SARS-CoV-2. https://www.degruyter.com/document/doi/10.1515/cclm-2020-1413/html [Online] (last visited on 30 March 2021).
14. Bailey D, Konforte D, Barakauskas VE, Yip PM, Kulasingam V, Hassan MAE, et al. Canadian society of clinical chemists (CSCC) interim consensus guidance for testing and reporting of SARS-CoV-2 serology. Clin Biochem 2020;86:1-7.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download