Abstract
Background: This study aimed to evaluate the diagnostic performance of the STANDARD F Influenza A/B FIA test (SD Biosensor Inc., Korea) for the rapid detection of influenza A virus in comparison with the Sofia Influenza A+B FIA (Quidel Corp., USA) and SD BIOLINE Influenza Ag A/B/A(H1N1) (Standard Diagnostic, Inc., Korea) tests. Methods: A total of 227 non-duplicated nasopharyngeal aspirates submitted for real-time RT-PCR analysis were included in the study. We used the three commercial tests in remnant samples from routine assays, according to the manufacturer’s instructions. We analyzed the diagnostic performance, including sensitivity and specificity, of the three tests. Results: Real-time RT-PCR analysis showed that 67 (29.5%) samples were positive and 160 (70.5%) were negative for influenza A virus, and that all the specimens were negative for influenza B. The overall sensitivity and specificity for influenza A virus detection were 50.7% and 100% for the STANDARD F, 50.7% and 100% for the Sofia, and 29.9% and 100% for the SD BIOLINE tests, respectively. The STANDARD F and SD BIOLINE tests showed negative results for influenza B virus in all specimens, whereas the Sofia test showed two false-positive results. Conclusion: The STANDARD F Influenza A/B test showed a good diagnostic performance and may be useful for the rapid diagnosis of influenza A.
Influenza is an acute respiratory illness caused by influenza A or B virus that is marked by a high fever. It is common for it to spread in the community, with outbreaks occurring mainly during the winter. According to a report by the World Health Organization (WHO), annual epidemics of influenza cause about 3 to 5 million cases of severe illness annually and about 250,000 to 500,000 deaths [1].
The rapid and accurate diagnosis of influenza viruses is important for institution of appropriate treatment and for the prevention of spread in the community [2]. Viral culture is the gold standard method; however, it is time consuming and demanding [3]. On the other hand, the molecular methods, which are appealing because of their high sensitivity and specificity, require expensive equipment with reagents and specially trained technicians [4,5]. Many enzyme immunochromatographic assays (EIAs) have been introduced for the detection of influenza A virus [6]. These are simple to use and produce rapid results. A fluorescence-based immunochromatography assay (FIA) has been adopted to overcome the low sensitivity of the standard immunoassay [7]. Recently, STANDARD F Influenza A/B FIA (SD Biosensor, Inc., Suwon, Korea), a newly developed FIA, was introduced to detect both influenza A and B viruses.
In this study, we evaluated the performance of the STANDARD F Influenza A/B FIA for the detection of influenza A virus by comparison with Sofia Influenza A+B FIA (Quidel Corp., San Diego, CA, USA) and SD BIOLINE Influenza Ag A/B/A(H1N1)(Standard Diagnostic, Inc., Suwon, Korea).
A total of 227 non-duplicated nasopharyngeal aspirates submitted for LG Advansure RV Real-time PCR (LG Life Sciences, Seoul, Korea) between December 2016 and March 2017 were included in this study. We calculated the number of specimens considering the sensitivity and specificity of other commercial antigen kits. The specimens were stored at -70°C until use. We performed the three commercial rapid antigen assays (STANDARD F, Sofia, and SD BIOLINE) with the remnants of routine assay specimens and performed them according to the manufacturer’s recommendations.
Briefly, all specimens were prepared at room temperature. After a sample extraction step, the mixture was pipetted onto the test cassette and incubated. The results of STANDARD F and Sofia were displayed on the screen by the analyzer provided with the kits. For SD BIOLINE, results were determined by the presence of a visible purple line by two technicians.
We analyzed the sensitivity, specificity, positive predictive value, and negative predictive value in relation to the real-time RT-PCR results. This study was approved by the Institutional Review Board (IRB) of Inje University Busan Paik Hospital (IRB No.17-0048).
Of the 227 respiratory specimens, 29.5% (n = 67) were positive for influenza A virus by real-time RT-PCR. All specimens showed negative results for influenza B virus using that test.
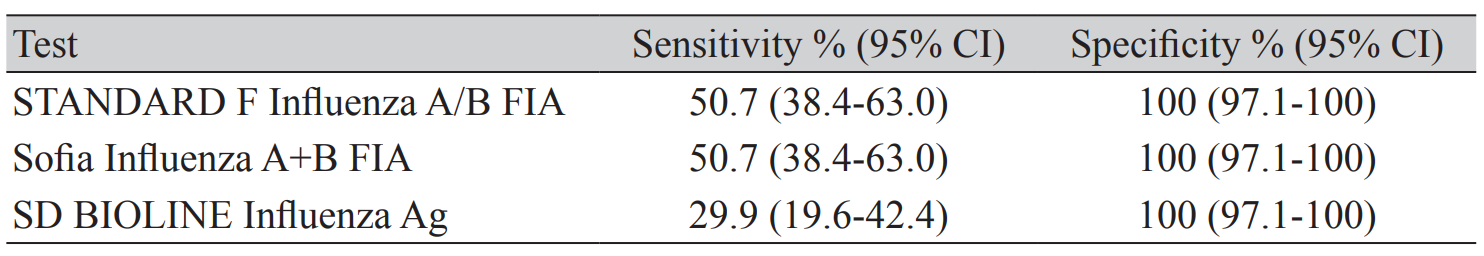
Among the 67 influenza-A positive specimens, STANDARD F, Sofia, and SD BIOLINE showed positive results in 34, 34, and 20 specimens, respectively. The diagnostic performance of the three assays is shown in Table 1. The sensitivity of STANDARD F, Sofia, and SD BIOLINE was 50.7%, 50.7% and 29.9%, respectively. The specificity of all three assays was 100%.
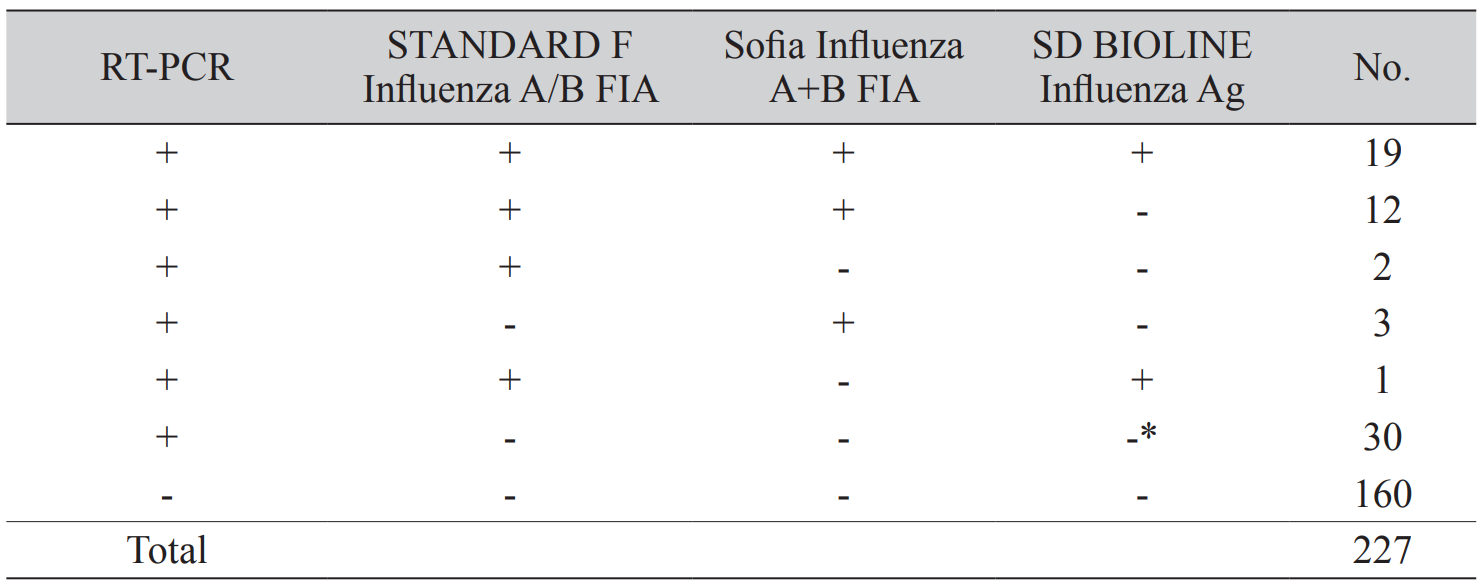
We describe the concordance of the assays according to the results of the real-time reverse transcription-polymerase chain reaction (RT-PCR) (Table 2). Among the 67 positive results by real-time RT-PCR, 19 were positive in all three rapid assays. Thirteen and five specimens had positive results in two assays and only one assay, respectively. Among these, most specimens were positive in both STANDARD F and Sofia (n = 12) assays. An invalid result was shown in one specimen by SD BIOLINE, and this sample was negative in the other two assays.
We confirmed all 160 negative specimens were negative by all three rapid antigen assays. All 227 specimens were negative for influenza B virus by real-time RT-PCR. We found that the influenza B virus was not detected in a total of 227 specimens in STANDARD F and SD BIOLINE; however, Sofia showed false-positive results in two specimens.
The rapid antigen assays based on EIA have been adopted for the diagnosis of influenza infection [8]. They are commonly used as a point-of-care test because they are simple to perform and provide results within 15 minutes. Several rapid antigen assays such as BinaxNOW Influenza A&B (Binax, Inc., Portland, ME, USA), QuickVue Influenza A+B (Quidel Corporation, CA, USA), SD BIOLINE, and BD Directigen EZ Flu A+B (Becton Dickinson and Company, Sparks, MD, USA) are used in many laboratories [9]. However, despite the convenience, this method has a critical limitation of low sensitivity, although specificity is high. The Centers for Disease Control and Prevention (CDC) published sensitivities for BinaxNOW Influenza A&B, Directigen EZ Flu A+B, and QuickVue Influenza A+B compared with RT-PCR were 40%, 49%, and 69%, respectively [10]. Also, Uyeki et al. reported an unacceptably low sensitivity of the QuickVue Influenza A+B Test, 27%, compared with RT-PCR [11].
To overcome this limitation, a rapid antigen assay based on FIA has been introduced. The Sofia Influenza A+B test enhances the sensitivity by using immunofluorescence and a digital detection system. The previous report showed that the sensitivity of the Sofia was as high as 78% compared with RT-PCR for influenza A virus [12]. In another study, the sensitivity and specificity compared with RT- PCR were 72.4% and 98.3%, respectively [13]. Much like the Sofia, STANDARD F Influenza A/B FIA was developed for the rapid detection of influenza virus based on FIA.
In our study, the sensitivity of the STANDARD F, Sofia, and SD BIOLINE for influenza A virus was 50.7%, 50.7%, and 29.9%, respectively. We confirmed that the sensitivity of STANDARD F was similar to that of Sofia, whereas SD BIOLINE showed low sensitivity compared with the other two FIAs. So, we can conclude that the sensitivity of FIA is twice that of EIA. In this study, the overall sensitivity was lower than in previous reports, although the specificity was as high as 100%. We preserved the specimens after real-time RT-PCR until the employment of the three rapid antigen assays. We assume that this delay in assay is the reason for the low sensitivity of FIA compared with the previous reports.
We reviewed the cycle threshold (Ct) value of positive specimens of influenza A virus. The mean Ct value of real-time RT-PCR for STANDARD F, Sofia, and SD BIOLINE were 7.08 (n = 34; range 1.96–12.85), 7.89 (n = 34; range 1.96–22.88), and 5.60 (n = 20; range 1.96–8.82). We found that Ct values of positive specimens in STANDARD F were similar to those of Sofia, expect one (Ct value 22.88). In addition, the specimens revealing positive results in SD BIOLINE had significantly lower Ct values than those of STANDARD F and Sofia. These results are similar to those in the previous report [14].
We did not include influenza B virus-positive specimens, so we cannot evaluate the efficacy in detecting that virus. However, there were two false-positive results with the Sofia. We noted similar results in other reports [15,16]. Remarkably, Dunn et al. reported that Sofia generated 55 false-positive influenza B virus results among 188 samples that were negative on real-time RT-PCR [16]. We should keep in mind the possibility of false-positive results for influenza B virus when using Sofia.
In conclusion, we confirmed the diagnostic performance of a newly developed test, the STANDARD F Influenza A/B FIA, which has sensitivity and specificity similar to those of the Sofia Influenza A+B FIA. We suggest that STANDARD F Influenza A/B FIA may be a useful diagnostic tool for the detection of influenza A virus.
FUNDING
This work was supported by a grant from SD BIOSENSOR. The funding sources played no role in the design, data collection, analysis, interpretation of data, or writing of the manuscript.
REFERENCES
1. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 2012;87:461-76.
2. Uyeki TM. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J 2003;22:164-77.
3. Steed LL, Salmon VC, Overall JC Jr. Identification of influenza A virus by shell vial culture and two commercially available antigen detection methods. Clin Diagn Virol 1994;2:261-9.
4. Ko DH, Kim HS, Hyun J, Kim HS, Kim JS, Park KU, et al. Comparison of the luminex xTAG respiratory viral panel fast v2 assay with anyplex II RV16 detection kit and AdvanSure RV real-time RT-PCR assay for the detection of respiratory viruses. Ann Lab Med 2017;37:408-14.
5. Lee J, Lee HS, Cho YG, Choi SI, Kim DS. Evaluation of allplex respiratory panel 1/2/3 multiplex real-time PCR assays for the detection of respiratory viruses with influenza A virus subtyping. Ann Lab Med 2018;38:46-50.
6. Vemula SV, Zhao J, Liu J, Wang X, Biswas S, Hewlett I. Current approaches for diagnosis of influenza virus infections in humans. Viruses 2016;8:96.
7. Lee CK, Cho CH, Woo MK, Nyeck AE, Lim CS, Kim WJ. Evaluation of Sofia fluorescent immunoassay analyzer for influenza A/B virus. J Clin Virol 2012;55:239-43.
8. Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med 2012;156:500-11.
9. Sutter DE, Worthy SA, Hensley DM, Maranich AM, Dolan DM, Fischer GW, et al. Performance of five FDA-approved rapid antigen tests in the detection of 2009 H1N1 influenza A virus. J Med Virol 2012;84:1699-702.
10. Centers for Disease Control and Prevention. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus - United States, 2009. Morb Mortal Wkly Rep 2009;58:826-9.
11. Uyeki TM, Prasad R, Vukotich C, Stebbins S, Rinaldo CR, Ferng YH, et al. Low sensitivity of rapid diagnostic test for influenza. Clin Infect Dis 2009;48:e89-92.
12. Lewandrowski K, Tamerius J, Menegus M, Olivo PD, Lollar R, Lee-Lewandrowski E. Detection of influenza A and B viruses with the Sofia analyzer: a novel, rapid immunofluorescence-based in vitro diagnostic device. Am J Clin Pathol 2013;139:684-9.
13. Hazelton B, Nedeljkovic G, Ratnamohan VM, Dwyer DE, Kok J. Evaluation of the Sofia Influenza A+B fluorescent immunoassay for the rapid diagnosis of influenza A and B. J Med Virol 2015;87:35-8.
14. Baas C, Barr I, Fouchier R, Kelso A, Hurt A. A comparison of rapid point-of-care tests for the detection of avian influenza A (H7N9) virus, 2013. Eurosurveillance 2013;18:20487.
15. Yang JH, Huang PY, Shie SS, Yang S, Tsao KC, Wu TL, et al. Diagnostic performance of the Sofia® Influenza A+B fluorescent immunoassay in adult outpatients in Northern Taiwan. J Med Virol 2018;90:1010-8.
16. Dunn J, Obuekwe J, Baun T, Rogers J, Patel T, Snow L. Prompt detection of influenza A and B viruses using the BD Veritor System Flu A+B, Quidel® Sofia® Influenza A+B FIA, and Alere BinaxNOW® Influenza A&B compared to real-time reverse transcription-polymerase chain reaction (RT-PCR). Diagn Microbiol Infect Dis 2014;79:10-3.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download