Abstract
Rothia spp. are aerobic, gram-positive cocci belonging to the family Micrococcaceae, and are a part of the normal microbial flora of the human oropharynx and upper respiratory tract. We present the first case of the prosthetic valve endocarditis with cerebral hemorrhage caused by Rothia mucilaginosa in South Korea. A 65-year-old man with a prosthetic aortic valve visited the outpatient clinic with a complaint of fever. R. mucilaginosa was identified in one among four sets of blood culture bottles obtained on the on day 30 of fever onset. Brain magnetic resonance imaging (MRI) showed multiple micro-hemorrhages suggesting septic emboli in both the hemispheres, corticomedullary junctions, and cerebellum. Rothia spp. should be considered as a possible pathogen in the cases of infective endocarditis with intracranial hemorrhage.
Rothia spp. are aerobic, gram-positive cocci belonging to the family Micrococcaceae, which are part of normal flora of the human oropharynx and upper respiratory tract [1]. They are commonly associated with periodontal diseases and can cause invasive disease very rarely in healthy hosts [1]. However, Rothia spp. is increasingly recognized as an opportunistic pathogen mostly for immunocompromised patients [2]. To date, invasive infections caused by Rothia spp. have been reported for bacteremia, endocarditis, meningitis, pneumonia, skin and soft tissue infection, bone and joint infection, and endophthalmitis [1-3]. Among them, infective endocarditis was the most common infectious syndrome, and complications such as brain abscess, intracranial hemorrhage, and mycotic aneurysm could be accompanied by Rothia endocarditis [4]. Invasive infections by Rothia spp. have been rarely reported in South Korea [3,5].
Here, we report a case of prosthetic valve endocarditis with cerebral hemorrhage caused by Rothia mucilaginosa. To the best of our knowledge, this is the first case of endocarditis complicated with intracranial hemorrhage by Rothia spp. in South Korea.
A 65-year-old man presented to an outpatient clinic with persistent fever for a month and newly onset severe headache. The patient underwent aortic valvuloplasty owing to infective endocarditis with aortic stenosis 28 and 22 years ago, respectively. He had admitted to the hospital for three times with prosthetic valve endocarditis during the previous 11 years and the last episode was placed a year ago. The causative pathogens of each episode were Enterococcus avium, Enterococcus faecalis, and Streptococcus gallolyticus, respectively. Besides, he had diabetes mellitus, hypertension, dyslipidemia, and alcoholic liver cirrhosis as underlying diseases.
On admission, his vital signs were as follows: body temperature, 37.7 ºC; pulse rate, 72/min; respiration rate, 20/min; and blood pressure, 120/80 mmHg. Petechiae were observed on his trunk but no Janeway lesions or Osler’s nodes existed. There was no conjunctival hemorrhage and Roth spot was not detected on ophthalmic examination. Cardiac murmur was not heard on aortic, pulmonary, tricuspid, and mitral area. In addition, neurological deficit including localizing and lateralizing sign was not observed in both upper and lower extremities.
The initial laboratory examination revealed a white blood cell count of 6,600 cells/mm3, hemoglobin of 8.2 g/dL, platelet count of 105×103 cells/mm3 and C-reactive protein (CRP) level of 5.86 mg/dL. Electrocardiography showed atrial fibrillation with controlled ventricular response and some ventricular premature complexes. Chest and abdomen computed tomography (CT) scans were performed for differential diagnosis of fever, and there was no abnormal finding suggesting infectious syndrome.
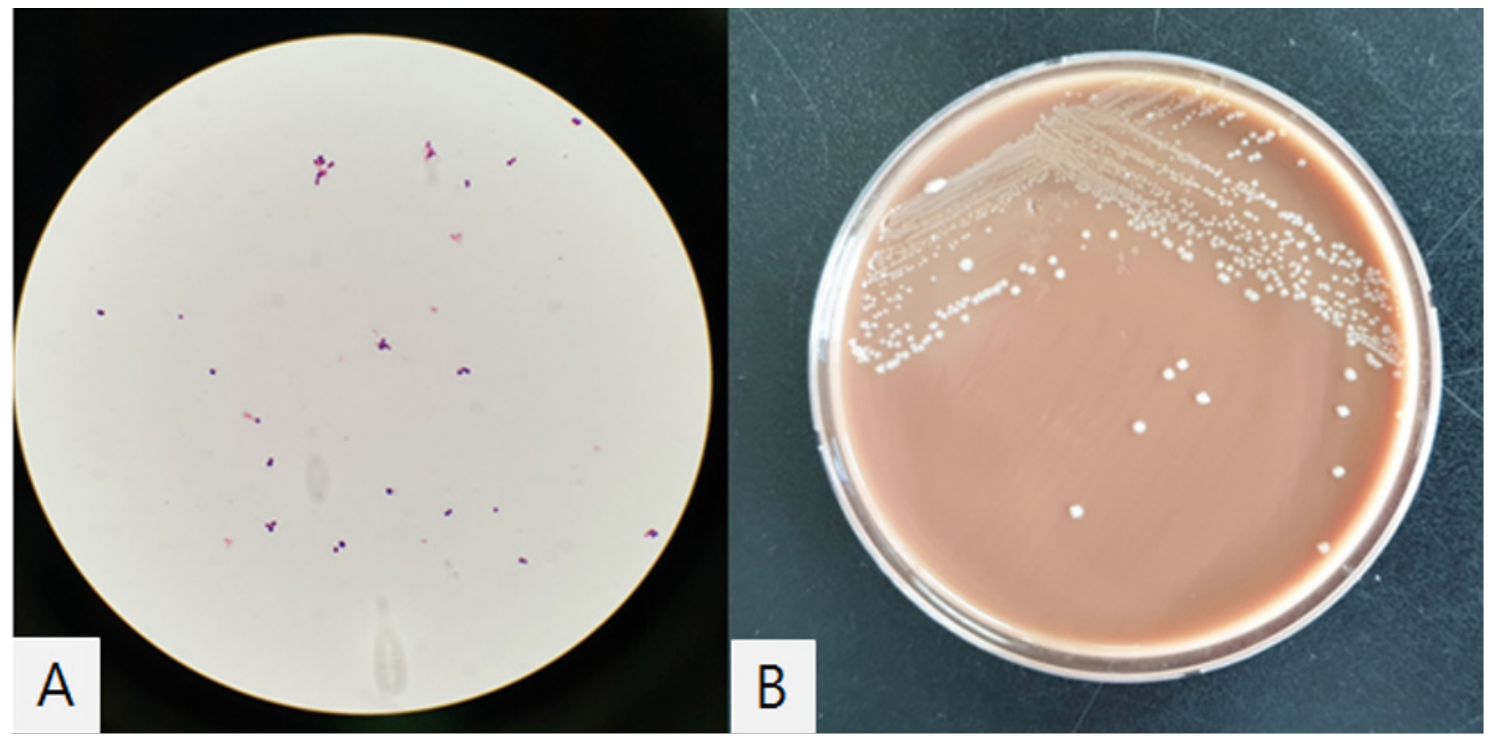
On transesophageal echocardiography (TEE), a 1.2 cm sized linear hypermobile structure was found at left ventricular outflow tract. Taking together, these findings with the positive result of blood cultures obtained at the 30 day of fever indicated a diagnosis of prosthetic valve endocarditis; Rothia mucilaginosa was reported from one blood culture bottle among 4 sets of blood culture samples which were collected in BacT/ALERT Aerobic and Anaerobic standard bottles (bioMérieux, Durham, NC, USA) through an automated blood culture system (BacT/ALERT 3D; bioMérieux, Durham, NC, USA). Gram-stained smears of cultured media revealed gram-positive cocci in small irregular clusters. The colonies on chocolate agar were whitish to grey in color, non-hemolytic, smooth and mucoid (Fig. 1). The colonies did not grow on the blood agar plate. 16S rRNA sequencing confirmed the species as R. mucilaginosa with 99% of identity. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Billerica, MA, USA) identified the strain as R. mucilaginosa (highest score = 2.092).
At the day of admission, brain magnetic resonance imaging (MRI) was taken for evaluation of headache, which showed multiple microhemorrhages suggesting septic emboli on both hemisphere, corticomedullary junction area and cerebellum.
We started vancomycin, rifampin, and ceftriaxone as an empirical therapy accordingly and the clinical response to the antibiotic therapy was good; his body temperature became normalized and CRP level decreased.
Since no Clinical and Laboratory Standards Institute (CLSI) protocols exist for R. mucilaginosa, we performed antimicrobial susceptibility test using CLSI criteria for Staphylococci (M100-S21), as described previously [6]. The result of antimicrobial susceptibility test was reported on hospital day 7 (Table 1), and we changed antibiotic regimen to ampicillin and rifampin.
On hospital day 11, the patient presented gait disturbance with aggravation of headache. Follow-up brain CT and magnetic resonance angiography were performed: newly developed intracranial hemorrhage at left temporal lobe and head of caudate nucleus was observed without vascular malformation such as mycotic aneurysm. Dysphagia and proximal muscle weakness on upper extremities were aggravated gradually and increased size of intracranial hemorrhage was found at follow up brain CT on hospital day 24. After changing antibiotics from ampicillin to vancomycin again, his symptoms were stabilized and there was no progression in neurologic sign.
However, on hospital day 56, the patient’s mental status became stuporous and he was transferred to intensive care unit. Follow-up brain CT showed newly developed intracranial hemorrhage at left parietal region, and intraventricular hemorrhage in both lateral ventricles. The antibiotic regimen was maintained with vancomycin and rifampin and his neurologic symptoms and signs showed waxing and waning. On hospital day 67, he was transferred to general ward and finally was transferred to another hospital for his family's request on hospital day 75. Right side motor weakness remained as a sequela to the patient.
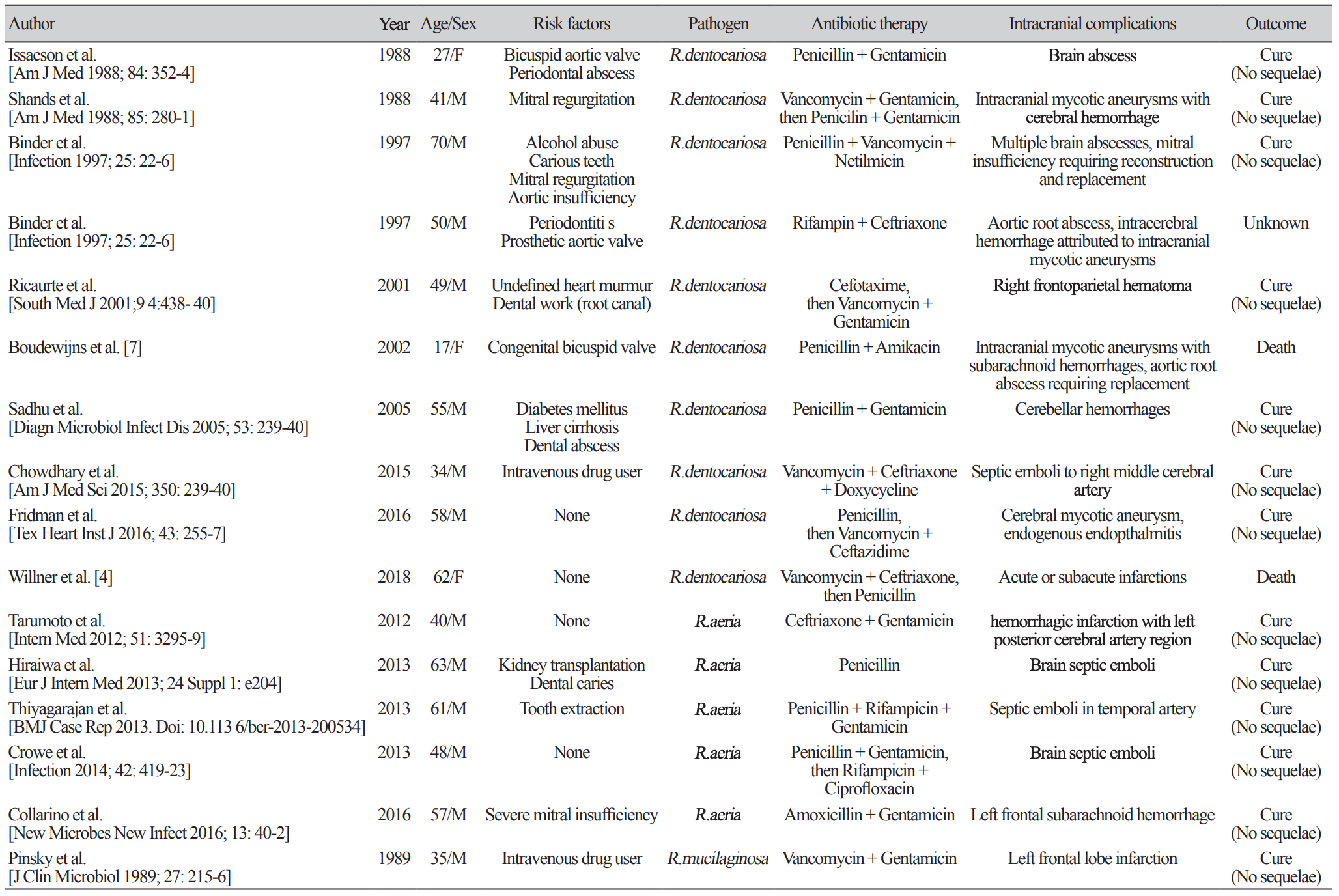
Since the first case report of infective endocarditis caused by Rothia spp., a total of 39 cases of Rothia spp. associated infective endocarditis have been reported. The majority of identified pathogens were Rothia dentocariosa (66.7%, 26/39), followed by Rothia aeria (17.9%, 7/39) and R. mucilaginosa (15.4%, 6/39). One of the most important underlying conditions for acquisition of Rothia spp. associated invasive diseases is dental or periodontal diseases [1]. Immunocompromised conditions such as hematological malignancy, severe neutropenia, diabetes mellitus, alcoholism, chronic liver disease and HIV infection are known to be another important risk factor [1].
Among the reported infective endocarditis cases due to Rothia spp., 41% (16/39) had intracranial complications, which was higher proportion compared with general infective endocarditis patients; symptomatic neurological complications occur in 15%–30% of patients with infective endocarditis and are mainly the consequence of embolism from vegetations [7]. The exact reason for the higher rate of intracranial complication in Rothia spp. associated infective endocarditis is yet to be identified.
The reported cases of infective endocarditis with intracranial complications are summarized in Table 2. The most common presentation was intracranial hemorrhage (37.5%, 6/16), followed by septic emboli (25.0%, 4/16), mycotic aneurysm (25.0%, 4/16), cerebral infarction (18.7%, 3/16), and brain abscess (12.5%, 2/16). Among them, 75% (12/16) of patients had risk factors for Rothia spp. associated invasive diseases: 7 with dental or periodontal diseases, 3 with immunocompromised condition, and 8 with cardiac valve diseases.
The optimal antimicrobial treatment of R. mucilaginosa infection has not been determined. According to the previous case reports, the organism seems to be susceptible to a variety of antibiotics targeting gram-positive pathogens such as ampicillin, rifampin, and vancomycin [3]. However, the pathogen was frequently resistant to penicillin, clindamycin and aminoglycosides as well as to trimethoprim-sulfamethoxazole and ciprofloxacin [1]. Although the antimicrobial susceptibility test showed the organism is susceptible to ampicillin, vancomycin and rifampin, rapid progression of neurologic symptoms and signs with aggravation of cerebral hemorrhage were observed in the present case after changing antibiotic regimen from vancomycin to ampicillin. Interestingly, his neurologic symptoms started to improve after changing antibiotic regimen from ampicillin to vancomycin again. Further studies are necessary to clarify this issue.
In South Korea, one case of infective endocarditis caused by R. aeria was reported in the patient taking immunosuppressive medication and the patient was successfully treated without intracranial complications [5]. To the best of our knowledge, this is the first case of infective endocarditis with cerebral hemorrhage caused by R. mucilaginosa in South Korea. Clinicians should consider Rothia spp. as a possible pathogen in the cases of infective endocarditis with intracranial complications.
ACKNOWLEDGEMENTS
The authors thank Mi-Ran Seo and Sujeong Kim for their cooperation in antimicrobial susceptibility test.
REFERENCES
1. Ramanan P, Barreto JN, Osmon DR, Tosh PK. Rothia bacteremia: a 10-year experience at mayo clinic, rochester, minnesota. J Clin Microbiol 2014;52:3184-9.
2. Maraki S and Papadakis IS. Rothia mucilaginosa pneumonia: a literature review. Infect Dis 2015;47:125-9.
3. Cho EJ, Sung H, Park SJ, Kim MN, Lee SO. Rothia mucilaginosa pneumonia diagnosed by quantitative cultures and intracellular organisms of bronchoalveolar lavage in a lymphoma patient. Ann Lab Med 2013;33:145-9.
4. Willner S, Imam Z, Hader I. Rothia dentocariosa endocarditis in an unsuspecting host: a case report and literature review. Case Rep Cardiol 2019:7464251.
5. Kim UJ, Won EJ, Kim JE, Jang MO, Kang SJ, Jang HC, et al. Rothia aeria infective endocarditis: a first case in Korea and literature review. Ann Lab Med 2014;34:317-20.
6. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 21st ed. CLSI supplement M100. Wayne; PA: 2011.
7. Boudewijns M, Magerman K, Verhaehen J, Debrock G, Peetermans WE, Donkersloot P, et al. Rothia dentocariosa, endocarditis and mycotic aneurysms: case report and review of the literature. Clin Microbiol Infect 2003;9:222-9.
Table 1
Antibiotic susceptibility for Rothia mucilaginosa
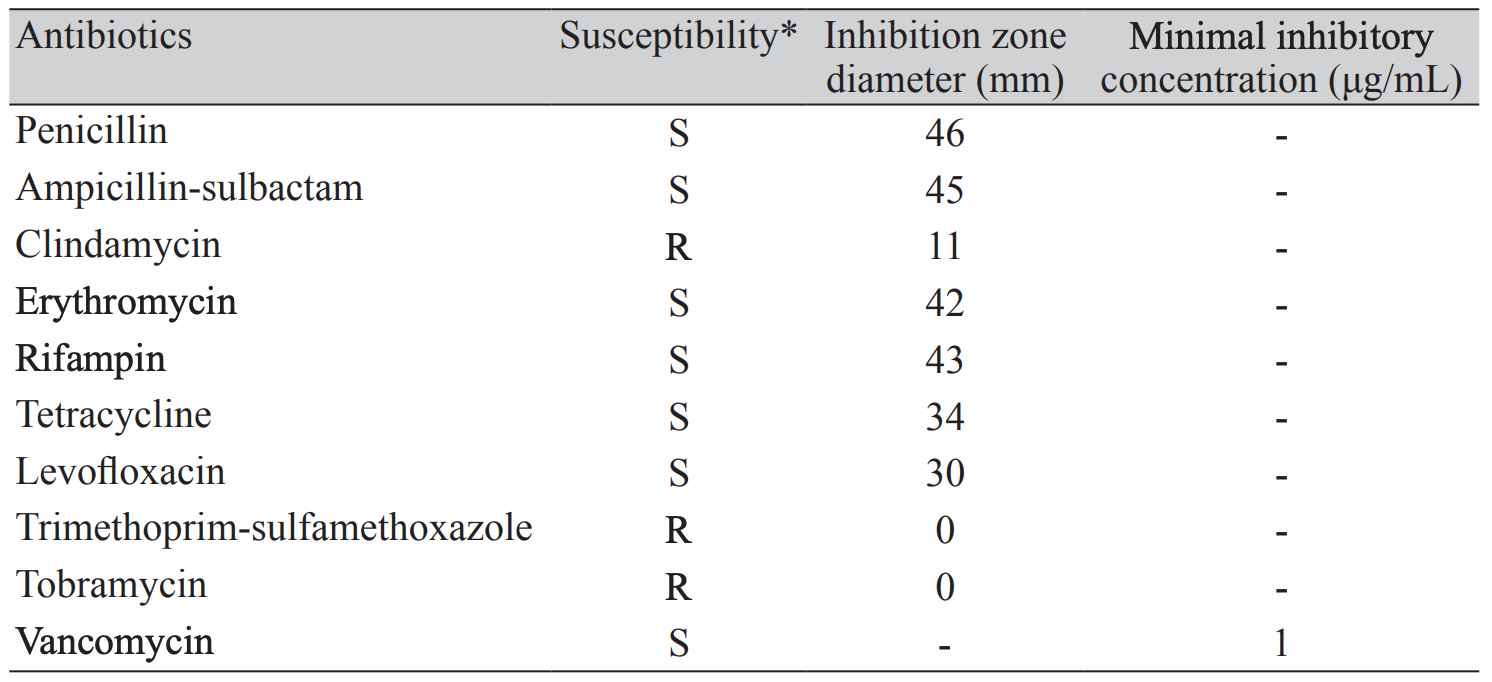
* The drug susceptibility of Rothia mucilaginosa in the present case was determined by using disk diffusion test according to the 2011 Clinical and Laboratory Standards Institute (M100-S21) criteria for Staphylococci. The vancomycin susceptibility was determined by E-test because the disk test is not available for differentiating among vancomycin-susceptible, -intermediate, and -resistant isolates of Staphylococcus spp.Abbreviations: S,susceptible; R,resistant.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download