Abstract
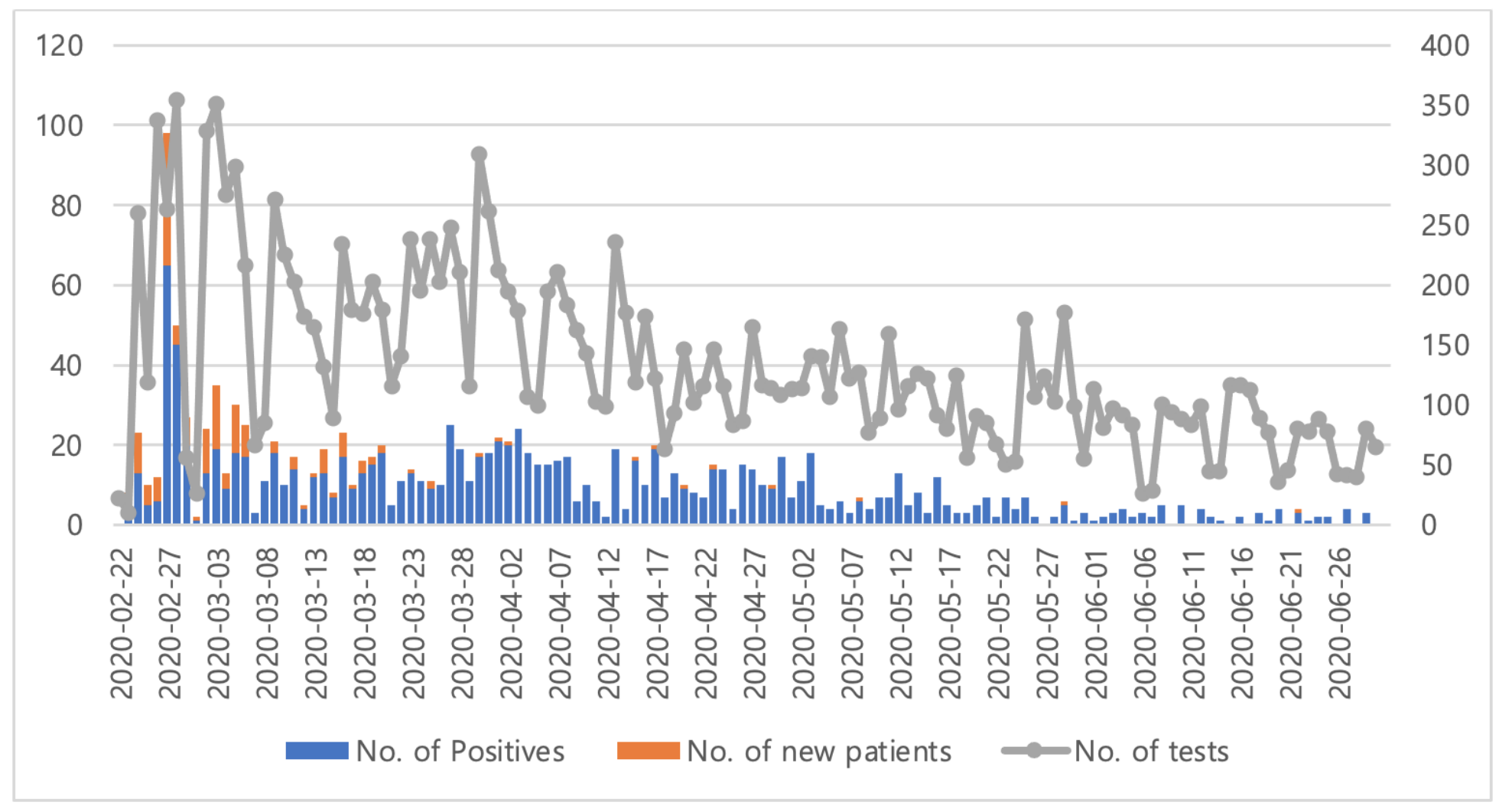
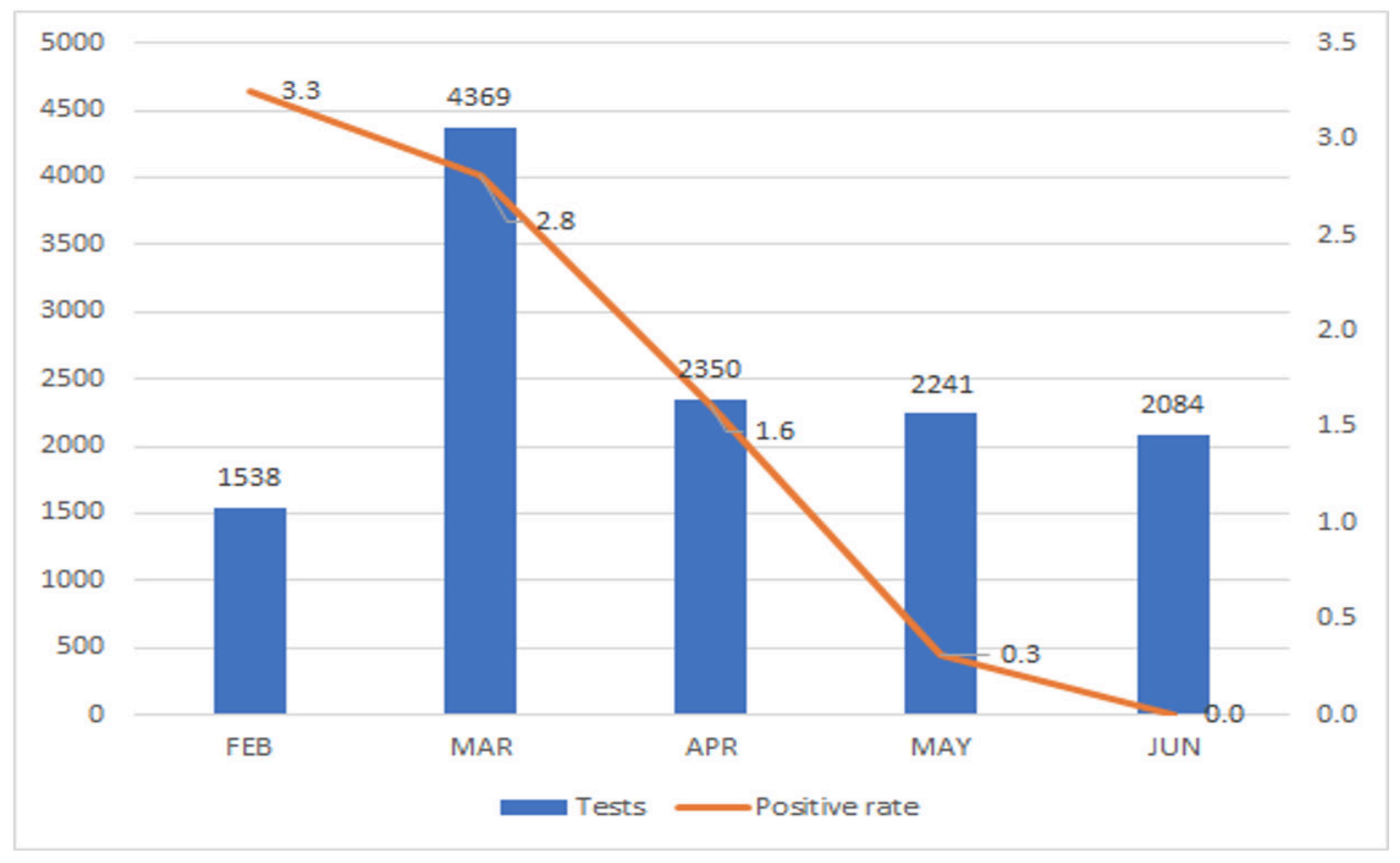
The first case of coronavirus disease 2019 (COVID-19) in Korea was reported in January 2020. As the secondary transmissions accelerated within the country, the government revised the outbreak alert for COVID-19 from attention to caution. Mid-February, when a massive outbreak was reported from a church in Daegu, our institution initiated testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). More than 300 laboratory tests were performed within the first 2 months, before the number of cases began to decline. Here, we describe our experience of 4 months at the department of Laboratory Medicine, Keimyung University Dongsan Hospital, located in Daegu, where a massive COVID-19 outbreak occurred.
REFERENCES
1. Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci 2020;35:e61.
2. Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis 2020;39:1011-9.
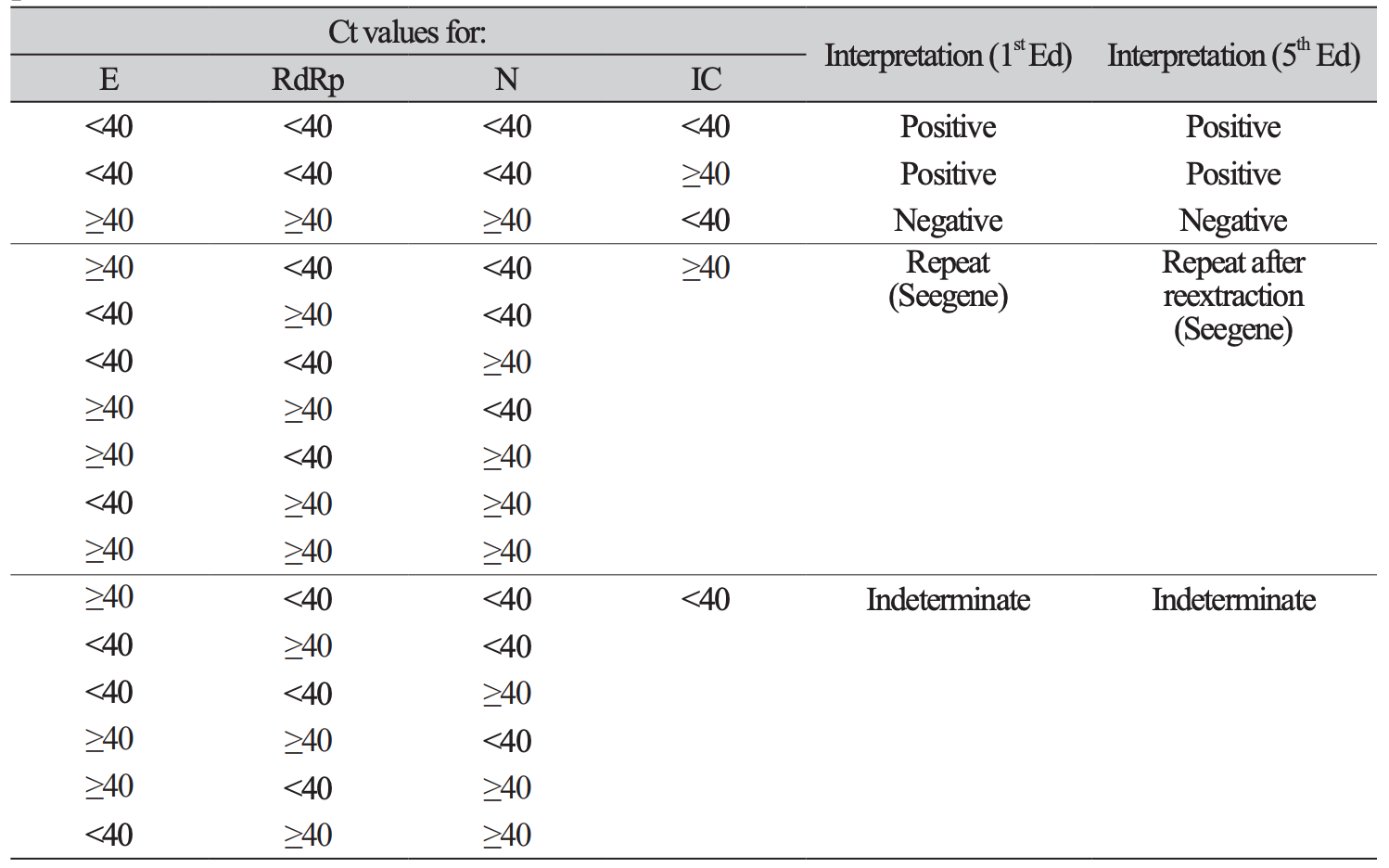
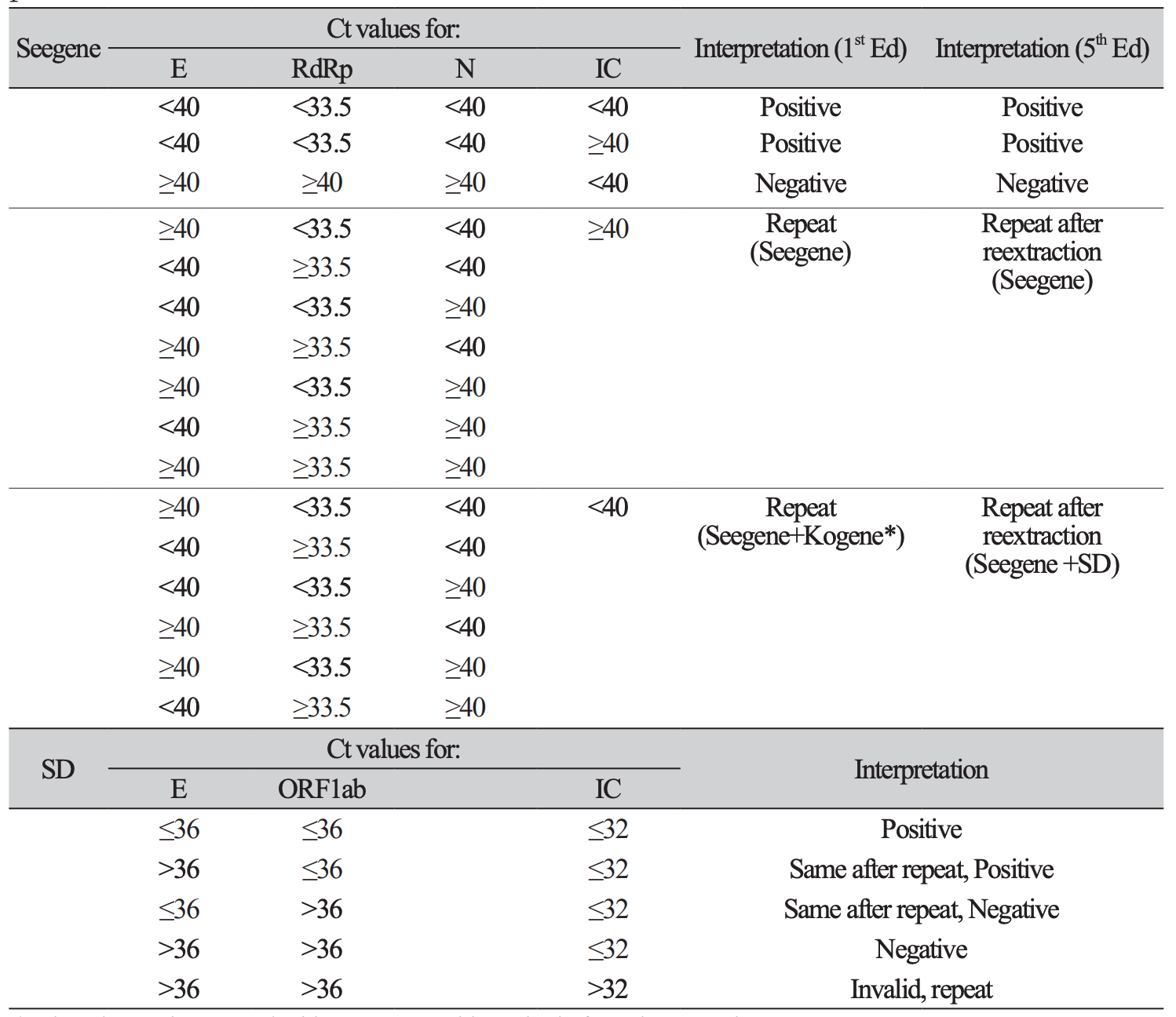
3. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med 2020;40:351-60.
4. Sung H, Roh KH, Hong KH, Seong MW, Ryoo N, Kim HS, et al. COVID-19 molecular testing in Korea: practical essentials and answers from experts based on experiences of emergency use authorization assays. Ann Lab Med 2020;40:439-47.
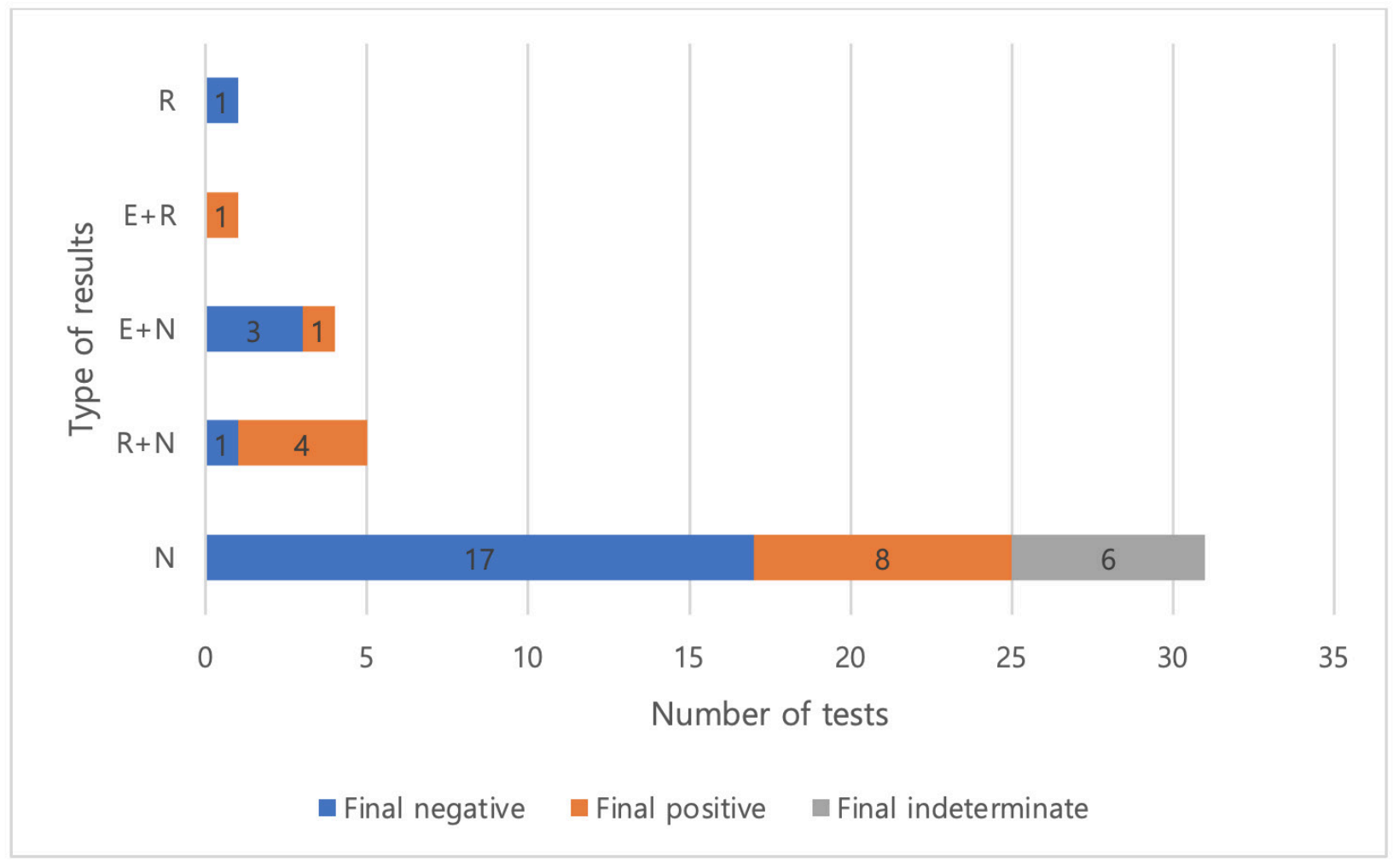
Fig. 1.
Distribution of "Indeterminate" results according to three specific genes, separately or in combination.Abbreviations: R, RNA-dependent RNA polymerase gene; E, envelope gene; N, nucleocapsid gene.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download