Abstract
Background: The rapid antimicrobial susceptibility testing (AST) performed on urine samples would guide the adequate choice of antibiotics for obtaining better treatment outcomes in patients. Our study aimed to evaluate the performance of the modified-EUCAST (European Committee on Antimicrobial Susceptibility Testing) rapid direct AST on urine samples. Methods:From <2,000 urine samples, a total of 128 urine samples containing bacterial counts of ≥2 × 104 CFU/mL with a uniform bacterial shape were initially included based on flow cytometry (Sysmex UF-1000i, Japan) and Gram staining, respectively. A total of 103 samples showing the presence of Enterobacteriaceae were finally selected in this study. The urine samples were directly inoculated on Mueller-Hinton agar, which was used in the current EUCAST rapid direct AST on blood samples. The size of the growth inhibition zones around antimicrobial disks was measured using a digital scanner (BIOMIC vision analyzer, Giles scientific, USA) and further confirmed by visualization with naked eyes after incubation for 4, 6, and 8 hours. The AST interpretations were compared to those of the conventional VITEK 2 AST system (bioMérieux, France) and the discrepancies between both tests were confirmed with the E-test. Results: The antibiotics, namely ampicillin, cefazolin, aztreonam, ceftazidime, cefotaxime, cefoxitin, cefepime, gentamicin, ciprofloxacin, and cotrimoxazole showed excellent correlations with modified-EUCAST rapid direct test and conventional ASTs with >0.75 weighted kappa values. The categorical agreement of the rapid direct AST was 1,442 (93.3%), with 76 (4.9%) minor error, 9 (0.6%) major error and 18 (1.2%) very major error, implicating the reliability of this method for clinical application. Conclusion: Performing the modified-EUCAST rapid direct AST on urine samples can predict reliable AST results within 8 hours. The rapid direct AST can help the physicians to initiate adequate antimicrobial treatment for urinary tract infections.
Urinary tract infections (UTIs) are a medical threat with the high morbidity of 150 million people worldwide a year [1]. Among the bacteria and fungi causing UTIs, Enterobacteriaceae is the most common [2]. Especially, Escherichia coli cause 80% of the uncomplicated UTI [3]. UTIs like other infections are treated with broad-spectrum antibiotics which consequently lead to an increase of resistance. Hence, the rapid antimicrobial susceptibility testing (AST) would increase the adequate choice of antibiotics for the better outcome of the patients [4]. Disk diffusion methods and commercial broth micro dilution kits are widely used for antimicrobial susceptibility testing in many clinical settings [5]. To ensure accurate and timely antimicrobial therapy we need rapid AST methods as it takes 2 to 3 days from the sample collection to the AST results by using the conventional methods [6].
To speed up the AST results, many methods have been developed such as Vitek Classic (bioMérieux, Marcy LE`toile, France), the more automated VITEK 2 (bioMérieux) and Microscan Walkaway (Dade-Behring Microscan, Sacramento, CA, USA), and Phoenix system (BD Diagnostic Systems, Sparks, MD, USA) [7]. Despite the introduction of these methods, many laboratories in low-income settings cannot use them due to their high cost [8]. Currently, European Committee on Antimicrobial Susceptibility Testing (EUCAST) published a guideline for rapid AST using disk diffusion methods in blood culture. But, neither EUCAST nor CLSI (Clinical and Laboratory Standards Institute) has approved the guideline for rapid AST in urine, yet. Hence, we aimed to expand the EUCAST rapid AST from blood to urine culture.
Urine culture method is totally different with blood culture. For urine culture, semi-quantitation for bacterial pathogen using calibrated loop is recommended. The most commonly used criterion to define significant bacteriuria is ≥105 colony forming unit/mL of urine. Compared to this, blood culture needs more sensitive methodology using enrichment broth. In each broth bottles, 5-10 mL of blood was inoculated to recover the bacterial pathogen.
Many papers on reducing the turnaround time of antimicrobial susceptibility testing have been published [9], however, limited studies have been done on short incubation time using disk diffusion directly on urine samples. Our study aimed to expand the sample scope and to compare the performance of EUCAST rapid direct disk diffusion AST on urine samples within 8 hr incubation with the conventional AST method.
Specimen collection
The study was carried out in the clinical microbiology laboratory of Severance Hospital from August to November 2019. Urine samples analysis was done by flow cytometry (Sysmex UF-1000i, TOA Medical Electronics, Kobe, Japan) followed by Gram stain. Samples were included in the study only if it contained ≥ 20,000 bacteria/ml plus the presence of monobacterial Gram-negatives on microscopy. This criteria of 2 × 104 CFU/mL was from the internal validation to have a visible bacterial growth after 8 hours incubation. In detail, using the E. coli ATCC25922 strain, we prepared different dilutions (106, 105, 104CFU/mL) using
culture negative urine. Spiked samples were cultured to check how well the growth will be shown. By using the samples showing sufficient growth, the cut off value from Sysmex UF-1000i was set for positive sample to be included in our study.
Modified-EUCAST rapid direct AST and species identification
128 urine samples fulfilling the criteria were preselected. Modified-EUCAST rapid direct ASTs were performed immediately or kept at 4°C for no longer than 24 hours before testing depending on the reception time. The identification of all isolates included in our study was performed by using MALDI-TOF MS system, ASTA Micro IDSys (ASTA Inc., Suwon, Korea) according to the manufacturer's recommendation.
For the quality control, E. coli ATCC25922 strain was mixed in sterile urine samples. They were diluted 100-,1000- fold to achieve the starting concentrations of 106, 105 CFU/mL, and were inoculated on the Mueller-Hinton agar (MHA) plate using a spreader. Antimicrobial disks, ampicillin, cefazolin, ertapenem, cefepime, cefotaxime, ceftazidime, cefoxitin, aztreonam, trimethoprim-sulfamethoxazole, amikacin, gentamicin, piperacillin-tazobactam, tigecycline, ciprofloxacin, imipenem (Becton, Dickinson and Company, Sparks, MD, USA), were applied and pressed firmly onto the agar surface with sterile forceps. The 15 antibiotics were selected since they were in VITEK 2 AST cards for Enterobacteriaceae in the clinical microbiology laboratory.
The sample volume, suggested by EUCAST for direct rapid AST in blood [10], was depending on plates size. Hence, 130 μL and 350 μL of the sample were taken and spread by using cotton swab on the 90 mmand 150 mm-diameter MHA, respectively. The plates were incubated at 35±1°C and read on the same day after 8 hours of incubation. The images of inhibition zones were taken and recorded using a digital scanner (BIOMIC vision analyzer, Giles scientific, DC, USA) and confirmed by naked eyes. The interpretations of AST results were performed following the CLSI breakpoints. However, in tigecycline, we used EUCAST breakpoint since there is no CLSI breakpoint for Enterobacteriaceae.
Conventional urine culture and AST
The urine sample was inoculated on both blood agar and MacConkey agar plates. Plates were incubated at 35°C overnight. The colonies on agar plate were counted and identified using ASTA Micro IDSys. A standardized 0.5 McFarland suspension was prepared and N224 VITEK 2 cards (bioMérieux, Durham, NC, USA) for AST were inoculated following the manufacturer recommendation. Interpretation of AST was performed following the criteria of the CLSI [11].
Comparison of modified-EUCAST rapid direct methods and conventional AST
Results from both methods were compared by measuring the agreement kappa with 95% confidence interval analyzed by R studio version 3.3.3. Weighted kappa ranging from 0.75 is classified as excellent, 0.40 to 0.75 as fair to good, and below 0.40 as poor according to Fleiss [12]. The MIC discrepancies showing major and very major errors were further confirmed by E-test (bioMérieux SA, Marcy-L'E'toile, France)with samples kept in freezer -70°C. Susceptibility results obtained by E-test were used as the golden standard.
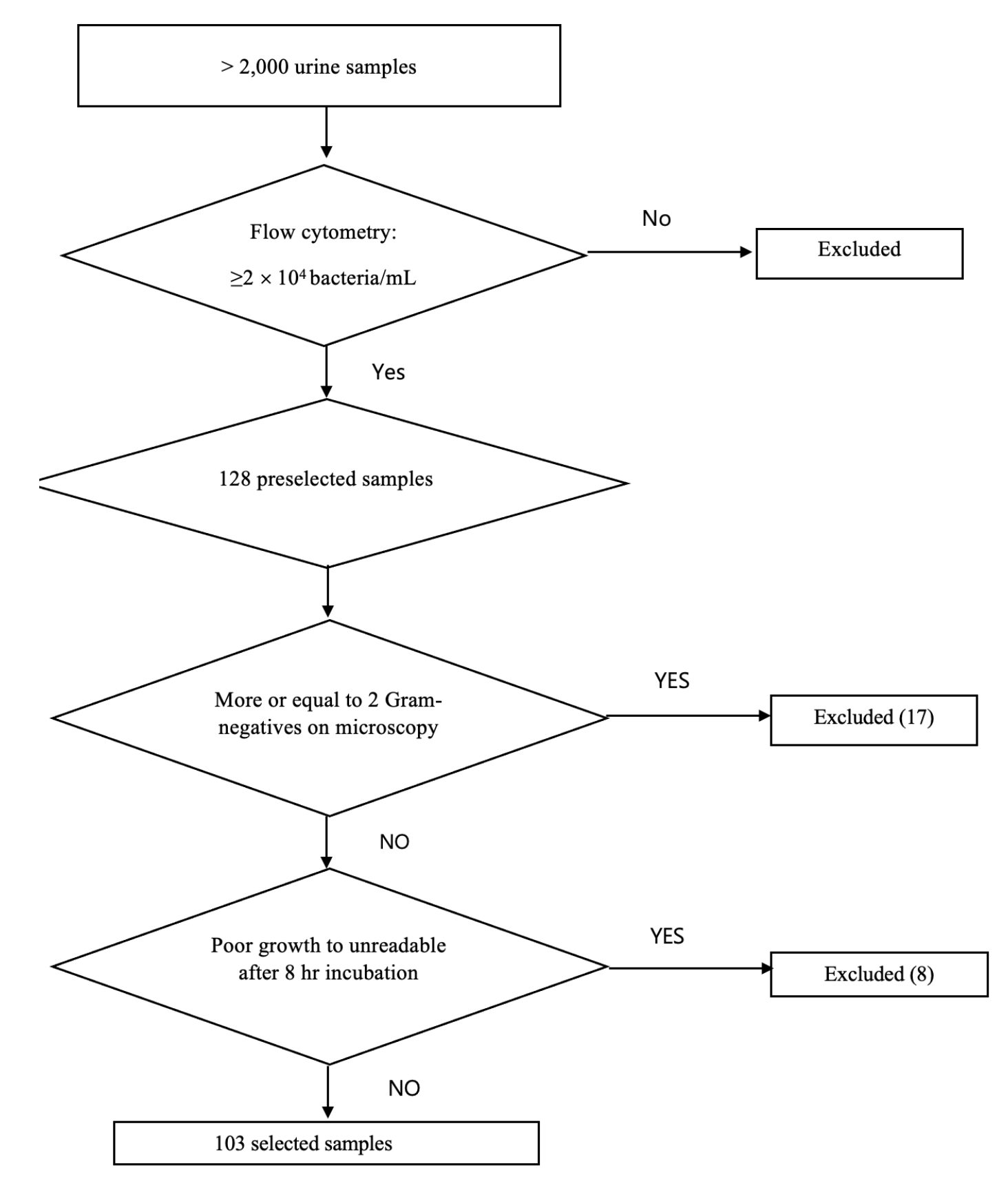
We have screened >2,000 urine samples by using flow cytometry and gram stain during the study period. 128 urine samples were preselected, and 103 samples grown with Enterobacteriaceae were selected (Fig. 1). They were distributed as 68 E. coli (66%), 17 Klebsiella pneumoniae (16.5%), 5 Citrobacter freundii (4.9%), 2 Citrobacter braakii (1.9%), 2 Enterobacter cloacae (1.9%), 2 Klebsiella ocytica (1.9%), 2 Proteus vulgaris (1.9%), 2 Klebsiella aerogenes (1.9%), 1 Raoultella ornithinolytica (1%), 1 Proteus mirabilis (1%), 1 Morganella morganii (1%). Concerning 25 samples excluded, 17 had more than one bacterium grown on the plate and 8 exhibited poor growth after 8 hours to be unreadable.
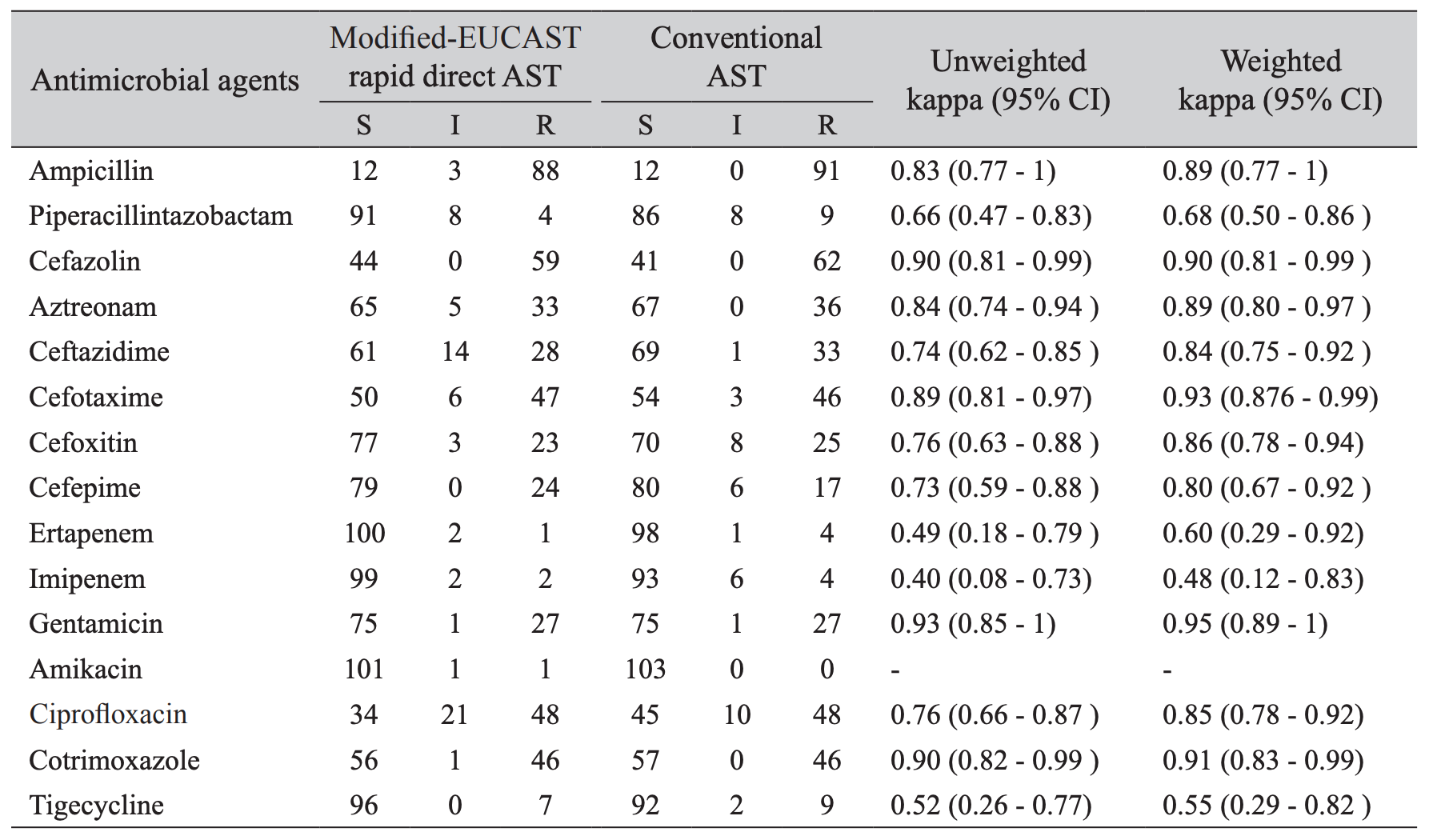
This criteria of 2 × 104 CFU/mL in Sysmex UF-1000i was from the internal validation. Direct antimicrobial susceptibility testing on urine form bacteriuria samples (≥ 2 × 104 bacteria/mL) showed that the most effective agents were amikacin (98.1%), ertapenem (97.1%), imipenem (96.1%), tigecycline (93.2%) and piperacillin-tazobactam (88.3%) (Table 1). The unweighted kappa scores of 1,545 antibioticsisolates combinations of modified-EUCAST rapid direct and conventional AST methods ranged from 0.40 to 0.93. The weighted kappa values were from 0.55 to 0.95. Ampicillin, cefazolin, aztreonam, ceftazidime, cefotaxime, cefoxitin, cefepime, gentamicin, ciprofloxacin, cotrimoxazole had excellent correlations between modified-EUCAST rapid direct and conventional ASTs with >0.75 weighted kappa value. Others including piperacillin-tazobactam, ertapenem, imipenem, and tigecycline, showed a fair to good agreement, i.e., 0.68, 0.60, 0.48, and 0.55, respectively.
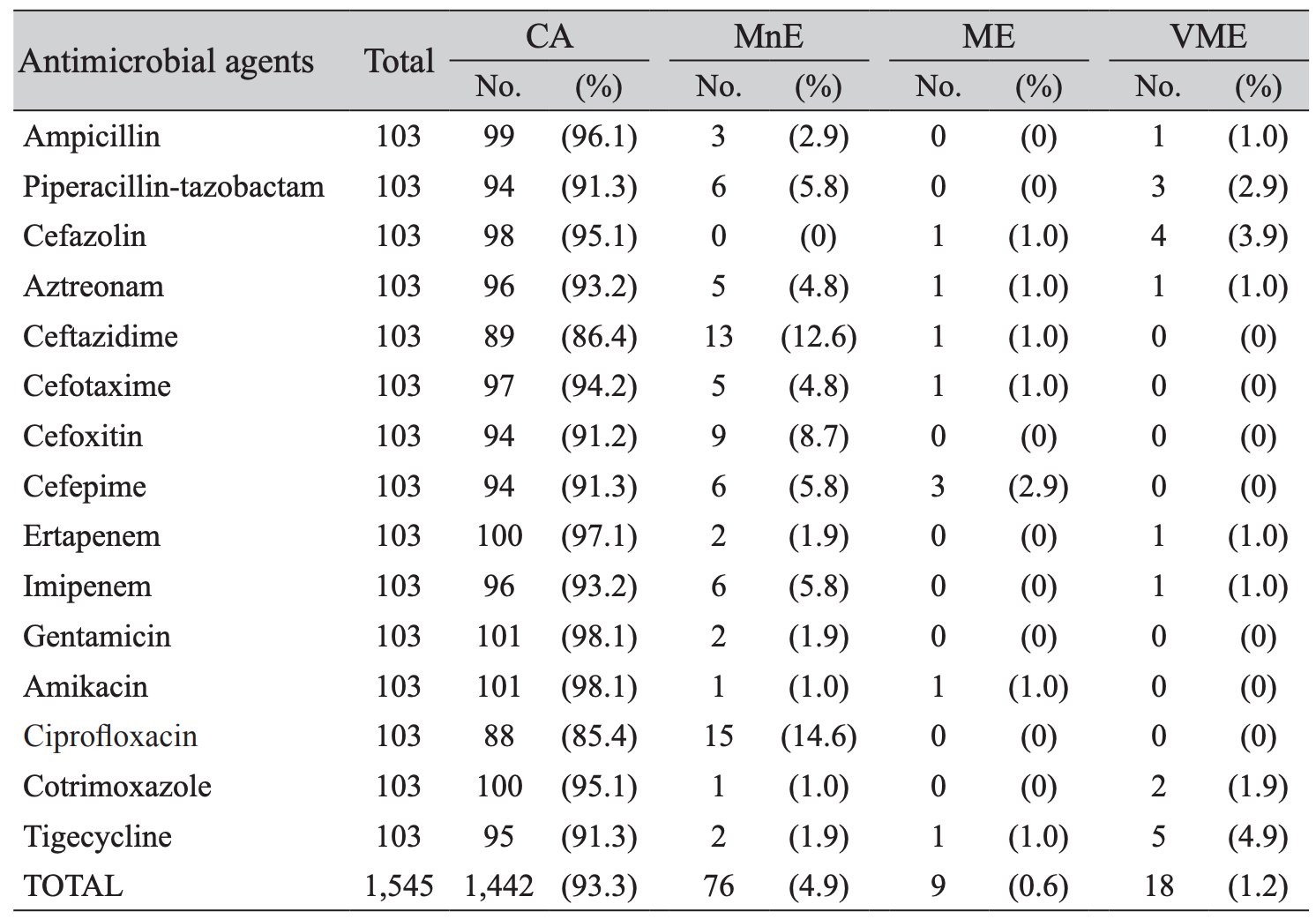
The overall categorical agreement of the modified-EUCAST rapid direct and the standard ASTs by using additional E-tests was 93.2%, with 4.9% minor errors, 0.6% major error and 1.2% very major errors (Table 2). Concerning major errors observed, 3 (2.9%) were in cefepime. Out of 18 very major errors observed, 5 (4.9%) were shown in tigecycline, 4 (3.9%) in cefazolin, 3 (2.9%) in piperacillin-tazobactam, and 2 (1.9%) in cotrimoxazole.
Among 1,545 antimicrobial agents-strain results, 15 were discrepant between modified-EUCAST rapid direct AST and conventional AST; 7 E. coli for aztreonam, cefepime, amikacin, cotrimoxazole, and gentamicin, 1 K. pneumoniae for tigecycline, 1 M. morganii for tigecycline, and 3 Proteus spp. for tigecycline and imipenem (Table 3). However, after reanalysis the AST with E-test, the discrepancies reached 2 major and 2 minor errors in E. coli for aztreonam, cefepime, amikacin and 2 very major errors in P. vulgaris and P. mirabilis for tigecycline.
The purpose of this study was to evaluate the performance of rapid direct AST on urine compared to the standard method using VITEK 2. E. coli and K. pneumoniae were the most predominant isolates in bacteriuria with a prevalence of 66%, 16.5%, respectively. EUCAST showed that the rapid AST directly from blood culture bottles was validated only for E. coli, K. pneumonia, P. aeruginosa, S. aureus, S. pneumoniae, E. faecalis, E. faecium, and A. baumannii. In this study, we did include other Enterobacteriaceae, but not Gram-positives and glucose-non fermenters. Hence, the data in the tables represents the overall performance of modified-EUCAST in Enterobacteriaceae, which is not confined to E. coli and K. pneumoniae
Resistant rates to ampicillin, cefazolin, cefotaxime, ciprofloxacin, and cotrimoxazole were more than 40 of the tested strains, whereas piperacillin-tazobactam, ertapenem, imipenem, amikacin, and tigecycline were found to be the most active in vitro (Table 1). Our study showed excellent results compared to the conventional AST overall. Weighted kappa values of the results from the comparisons between both methods showed an excellent concordance for some antibiotics, however, others like piperacillintazobactam, ertapenem, imipenem, and tigecycline, showed a fair to good agreement (0.68, 0.60, 0.48, 0.55), respectively. Piperacillin-tazobactam or carbapenem was usually recommended for acute pyelonephritis patients with severe conditions such as severe sepsis or septic shock. Therefore, rapid direct AST can help the physicians to start adequate antimicrobial treatment. Kappa statistics of amikacin was not calculated since conventional AST categorized all samples into a single level, i.e, susceptible. Specifically, for cefazolin, cefotaxime, gentamicin, and cotrimoxazole, modified-EUCAST rapid direct AST showed over 0.9 weighted kappa values
Furthermore, the overall agreement and error rates were within the acceptable limits which gives it the credential to be adopted into routine microbiology laboratory workflow since it helps us to save more than 24 hours (Table 2). According to Jorgensen criteria, the rates of VME (very major error) and the sum of ME (major error) and MnE (minor error) should be < 3%, < 7% respectively [13]. Cefoxitin, gentamicin, and ciprofloxacin did not show neither major error nor very major error. When considering every single antibiotic, the results of ciprofloxacin, cefoxitin, cefepime, cefazolin, ceftazidime, tigecycline exceeded the acceptable limits. However, overall ME was 1.2% and the sum of ME and MnE were 5.5% in this study. These findings implicated its usefulness to report rapid AST results of many antibiotics in clinical urine samples.
The modified-EUCAST rapid direct AST is more likely to give the real image of population AST because it was performed with the whole bacterial population in the sample rather than with a single colony which was picked on the plate. Therefore, the results are likely to be clinically relevant [9]. However, the errors in the case of polymicrobial microscope findings are quite high thus it is suggested to be done in monobacterial microscopy findings only [14]. Numbers of urine samples (13.2%, 17/128) have been excluded from this study due to the mixed microscopy of ≥2 Grma-negative bacterial findings
In a study done by Perillaud et al., the direct AST compared to the conventional disk diffusion AST showed 97.9% categorical agreement, 1.5% minor errors, 0.3% major error, and 0.3% vary major error were found [15], which were quite comparable with the results of this study. Though the AST of rapid method seems not to be complete, the result generated in a relatively short period compared to the conventional method and thus can help the physician give the appropriate empirical therapy [9]. For some of the discrepancies, inhibition zones were close to clinical breakpoints, where a slight difference in mm zone of diameter would yield to a major or very major error. The agreement for each antimicrobial agent exceeds 85% in all isolates studied.
The limitation of our study was the spectrum of test strains, because we included Enterobacteriaceae, but not other Gram-negative bacilli, such as P. aeruginosa. Relatively slow growing bacteria do not allow the reading of inhibition zone diameter before 8 hours incubation. Multiple bacterial infections with similar colony appearance would be also a huddle to this rapid AST method although it is rare, because the discrimination of different isolates on single plate would not be possible by naked eyes. Thus it can make an error in inhibition zone diameter measure.
In conclusion, despite some errors and unstandardized inoculums ize, we showed the modified-EUCAST rapid direct AST on urine would be reliable in Enterobacteriaceae. The rapid AST reports by using direct susceptibility testing in UTIs can shorten the time for the start of adequate antimicrobial treatment.
FUNDING
We gratefully acknowledge Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine. We would like to thank the clinical microbiology staff for their assistance. Our appreciations go to the clinical chemistry staff as well, for providing urine samples. This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1324); by the Research Program funded by the Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (2019-ER5403-00); by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agricultural Microbiome R&D Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
REFERENCES
1. Mohammad RN and Omer SA. Direct disk testing versus isolation and antimicrobial susceptibility testing of urine from urinary tract infection. Iran J Microbiol 2018;10:37-44.
2. Al-Naqshbandi AA, Chawsheen MA, Abdulqader HH. Prevalence and antimicrobial susceptibility of bacterial pathogens isolated from urine specimens received in rizgary hospital - Erbil. J Infect Public Health 2019;12:330-6.
3. Sundqvist M, Olafsson J, Matuschek E. EUCAST breakpoints can be used to interpret direct susceptibility testing of Enterobacteriaceae from urine samples. APMIS 2015;123:152-5.
4. Schoepp NG, Schlappi TS, Curtis MS, Butkovich SS, Miller S, Humphries RM, et al. Rapid pathogen-specific phenotypic antibiotic susceptibility testing using digital LAMP quantification in clinical samples. Sci Transl Med 2017;9:eaal3693.
5. Mishra P, Mishra K, Singh D, Ganju L, Kumar B, Singh S. Advances in rapid detection and antimicrobial susceptibility tests: a review. Def Life Sci J 2018;4:12-20.
6. Paul S, Kannan I, Duraipandian J, Premavathi RK, Shantha S. Evaluation of chromogenic agar and direct antimicrobial susceptibility testing in rapid diagnosis of acute urinary tract infection. Int J Pharm Clin Res 2015;7:333-6.
7. Eigner U, Schmid A, Wild U, Bertsch D, Fahr AM. Analysis of the comparative workflow and performance characteristics of the VITEK 2 and Phoenix systems. J Clin Microbiol 2005;43:3829-34.
8. Perillaud C, Pilmis B, Diep J, Pean de Ponfilly G, Vidal B, Couzigou C, et al. Prospective evaluation of rapid antimicrobial susceptibility testing by disk diffusion on Mueller-Hinton rapid-SIR directly on blood cultures. Diagn Microbiol Infect Dis 2019;93:14-21.
9. Coorevits L, Boelens J, Claeys G. Direct susceptibility testing by disk diffusion on clinical samples: a rapid and accurate tool for antibiotic stewardship. Eur J Clin Microbiol 2015;34:1207-12.
10. EUCAST. EUCAST rapid antimicrobial susceptibility testing (RAST) directly from positive blood culture bottles. Version 1.1, 2019.
11. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 29th ed. CLSI supplement M100. Wayne; PA: 2019.
12. Fleiss JL, Cohen J, Everitt BS. Large sample standard errors of kappa and weighted kappa. Psychol Bull 1969;72:323–7.
13. Stokkou S, Geginat G, Schlüter D, Tammer I. Direct disk diffusion test using European clinical antimicrobial susceptibility testing breakpoints provides reliable results compared with the standard method. Eur J Microbiol Immunol 2015;5:103-11.
14. Breteler KB, Rentenaar RJ, Verkaart G, Sturm PD. Performance and clinical significance of direct antimicrobial susceptibility testing on urine from hospitalized patients. Scand J Infect Dis 2011;43:771-6.
15. Perillaud-Dubois C, Pilmis B, Diep J, de Ponfilly GP, Perreau S, Ruffier d'Epenoux L, et al. Performance of rapid antimicrobial susceptibility testing by disk diffusion on MHR-SIR agar directly on urine specimens. Eur J Clin Microbiol Infect Dis 2019;38:185-9.
Table 3
Summary of discrepancies in 13 strains showing discrepant results between modified-EUCAST rapid direct and conventional ASTs
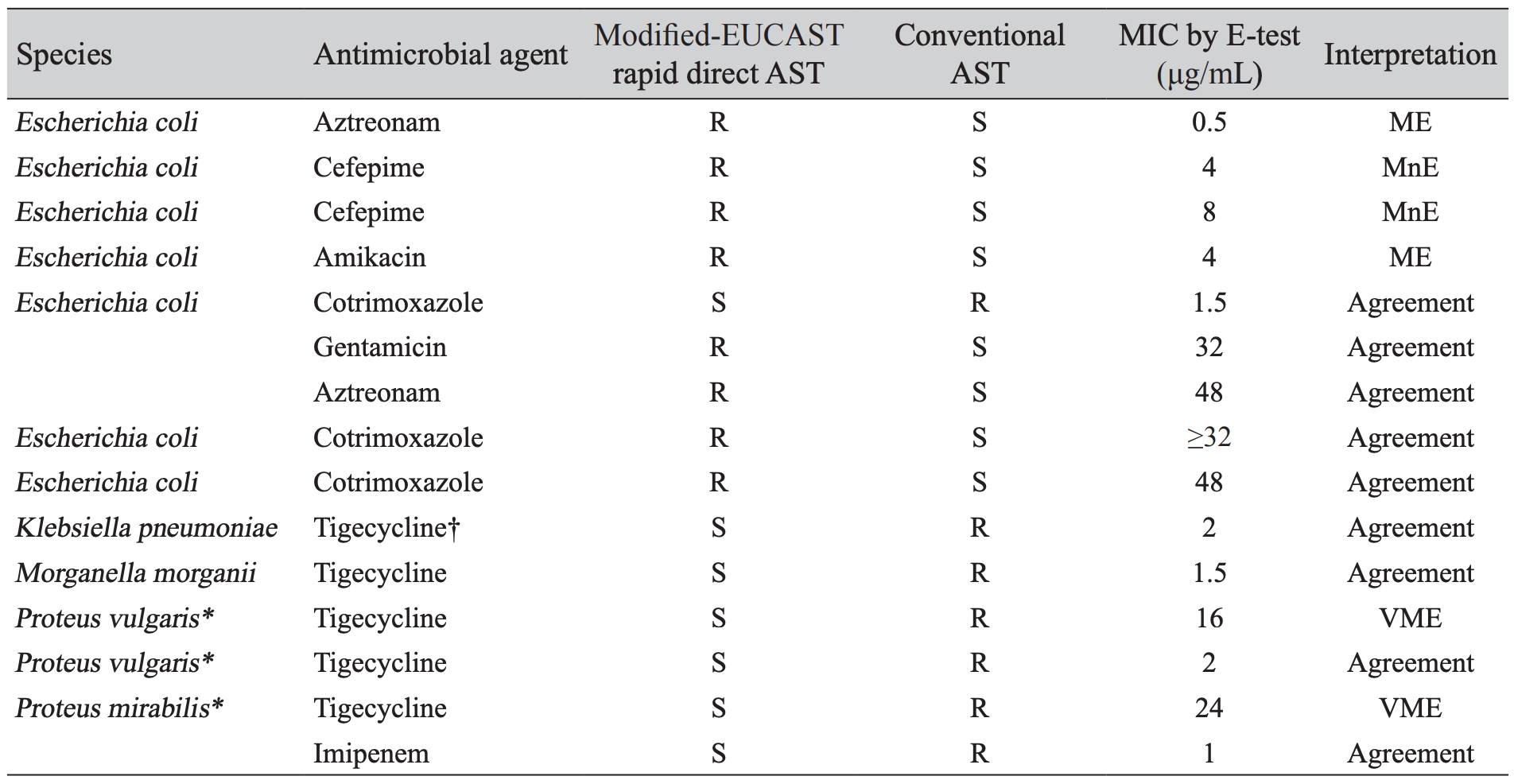
*The most very major errors were noted in tigecycline for P. vulgaris and P. mirabilis. †The FDA MIC breakpoints for susceptible (≤ 2 μg/mL), intermediate (4 μg/mL), and resistant (≥ 8 μg/mL) were used to categorize tigecycline susceptibility in Enterobacteriaceae strains.Abbreviation: EUCAST, European committee on antimicrobial susceptibility testing; AST, antimicrobial susceptibility test; MIC, minimal inhibitory concentration; R, resistant; S, susceptible; ME, major error; MnE, minor error; VME, very major error.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download