Abstract
Probiotics are used to restore and maintain the healthy intestinal microflora. Although Saccharomyces cerevisiae (SC) is considered as a non-pathogenic yeast, administration of SC as a probiotic is associated with a rare cause of fungemia in immunocompromised patients with central venous catheter insertion. We encountered a case of SC fungemia in a premature infant who presented with respiratory distress syndrome and had undergone central venous catheterization.
Go to : 
Probiotics are live microbes that beneficially affect the host by normalization of the intestinal microenvironment [1,2]. Saccharomyces cerevisiae (SC) is an ubiquitous yeast used in the production of beer, wine, and baked goods and it is frequently used as a probiotic to normalize gut microbiota after administration of antibiotics [3]. Although SC is usually nonpathogenic, SC can cause the fungemia in the immuno-compromised patients who have central venous catheter [4]. The overall mortality in a large review on SC fungemia was 28% [5].
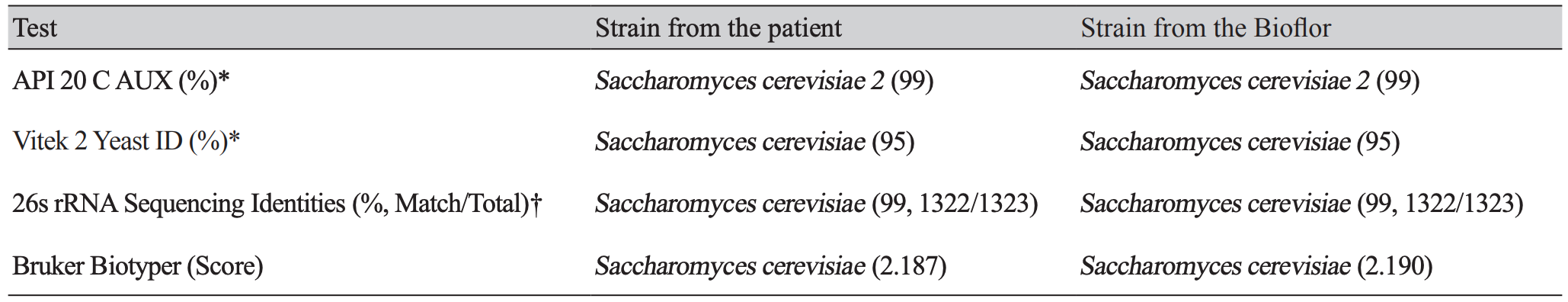
We experienced one case of SC fungemia in a premature female infant (29+3 weeks, 1.56 kg) with respiratory distress syndrome (RDS) after intake of Bioflor-250 Powder (Lyophilized Saccharomyces cerevisiae Hansen CBS 5926 282.5 mg, Gunil Pharmaceutical Co., Seoul, South Korea) under ampicillin and clarithromycin treatment. She presented with decreased activity, chest retraction and dyspnea. Initial complete blood count showed hemoglobin 15.9 g/dL, leukocyte count 10.7×109/L (neutrophils, 23%, lymphocytes 71%) and platelet count 295 x109/L. Blood culture showed non-specific findings. On the 35th hospital day, Bioflor-250 powder was administered orally, two times a day for 15 days. From the 45th hospital day, isolates from two serial blood cultures were identified as yeast in the blood agar plate and Sabouraud dextrose II agar. Microscopic examination of the strain revealed two spores in the Gram stain. The Sabouraud dextrose II agar revealed a slightly yellow, mucoid colony morphology (Fig. 1). The strain was identified as SC (95% probability) using Vitek 2 yeast 32C identification card (YST ID; bioMérieux, Durham, NC, USA). The isolate was also identified as SC (99% identical, 1322/1323) using 26s rRNA sequencing (Macrogen Inc., Seoul, Korea). On hospital day 50, the patients central venous catheter was removed and she started fluconazole therapy for 14 days. The removed catheter tip was cultured but no microorganism was grown. On hospital day 64, fluconazole therapy was stopped. On hospital day 81, the patient had completely recovered and discharged. The same Bioflor-250 powder that was administered to the patient was incubated on Sabouraud dextrose II agar for two days at 35°C. The isolates were identified as SC by using the ID 32C-system (bioMériux, USA). The isolate was identified as SC (99% identical, 1322/1323) using 26s rRNA sequencing (Macrogen Inc., Korea) (Table 1).
Histologically thought to be nonpathogenic, SC is emerging fungal pathogen that causes thrush, vulvovaginitis, empyema and fungemia. Exposure to probiotics may contribute to the ability of the organism to colonize and infect human hosts, and important risk factors for fungemia include immunosuppression, intensive care unit stay, total parenteral nutrition, and central venous catheters [3]. SC is a teleomorph yeast showing ascospores after 2-5 days of incubation on Fowells acetate agar or V8A (V8 Agar) medium at room temperature [3]. In routine laboratory, SC can be identified using biochemical characteristics such as those demonstrated on the carbohydrate assimilation test, and assimilation of raffinose by SC is noteworthy [3]. SC is usually susceptible to commonly used antifungal agents like liposomal amphotericin B [6]. In this case, the patient responded well to antifungal therapy.
Although probiotics with SC are usually safe, there are chance of systemic infection in premature infants with feeding intolerance, intestinal dysfunction, or immunosuppression due to cancer, HIV infection, use of corticosteroid, neutropenia, organ transplantation, burns and heart surgery [7,8].
To date, several studies have reported SC fungemia related to use of probiotics [9-11]. In premature care, probiotics can be used for prevention of necrotizing enteritis, functional gastrointestinal disorder and feeding problem. But as prior reports, in immunocompromised patients–including premature infants with central venous catheter-, there are chances for SC to cause systemic infection[4,12,13]. This case suggests that further evaluation of proper indication for probiotic administration to premature baby is needed.
Go to : 
REFERENCES
1. Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989:365-78.
2. Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, et al. Demonstration of safety of probiotics-a review. Int J Food Microbiol 1998:93-106.
3. Carroll KC, Pfaller MA, et al. eds. Manual of Clinical Microbiology. 12th ed. American Society for Microbiology; 2019.
4. Hennequin C, Kauffmann-Lacroix C, Jobert A, Viard JP, Ricour C, Jacquemin JL, et al. Possible role of catheters in Saccharomyces boulardii fungemia. Eur J Clin Microbiol Infect Dis 2000:16-20.
5. Munoz P, Bouza E, Cuenca-Estrella M, Eiros JM, Perez MJ, Sanchez-Somolinos M, et al. Saccharomyces cerevisiae fungmia:an emrging infectious disease. Clin Infect Dis 2005:1625-34.
6. Pérez-Cantero A, Thomson P, Paredes K, Guarro J, Capilla J. Antifungal susceptibility of Saccharomyces cerevisiae and therapy in a murine model of disseminated infection. Rev Iberoam Micol 2019;36:37-40.
7. Lee HY, Kim JH, Kim SJ, Lee HJ, Kwon JC, Park YJ, et al. Two cases of Saccaharomyces cerevisiae fungemia in patients with hematologic malignancies. Infect Chemother 2012:477-80.
8. Roy U, Jessani LG, Rudramurthy SM, Gopalakrishnan R, Dutta S, Chandrashish C, et al. Seven cases of Saccharomyces fungaemia related to use of probiotics. Mycoses 2017:375-80.
9. Kim CS. Saccharomyces cerevisiae sepsis after treatment of Saccharomyces boulardii probiotics in premature infants. Korean J Perinatol 2011:52-6.
10. Reid G, Jass J, Sebulsky T, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev 2003:658-72.
11. Lee SJ, Cho SJ, Park EA. Effects of probiotics on enteric flora and feeding tolerance in preterm infants. Neonatology 2007:174-9.
12. Romanio MR, Augusto Coraine LA, Maielo VP, Abramczyc ML, Souza RLD, Oliveira NF. Saccharomyces cerevisiae fungemia in a pediatric patient after treatment with probiotics. Rev Paul Pediatr 2017;35:361-4.
13. Atıcı S, Soysala A, Ceritb KK, Yılmazc Ş, Aksuc B, Kıyanb G, et al. Catheter-related Saccharomyces cerevisiae fungemia following Saccharomyces boulardii probiotic treatment: in a child in intensive care unit and review of the literature. Med Mycol Case Rep 2017;15:33-5.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download