Abstract
Background/Aims
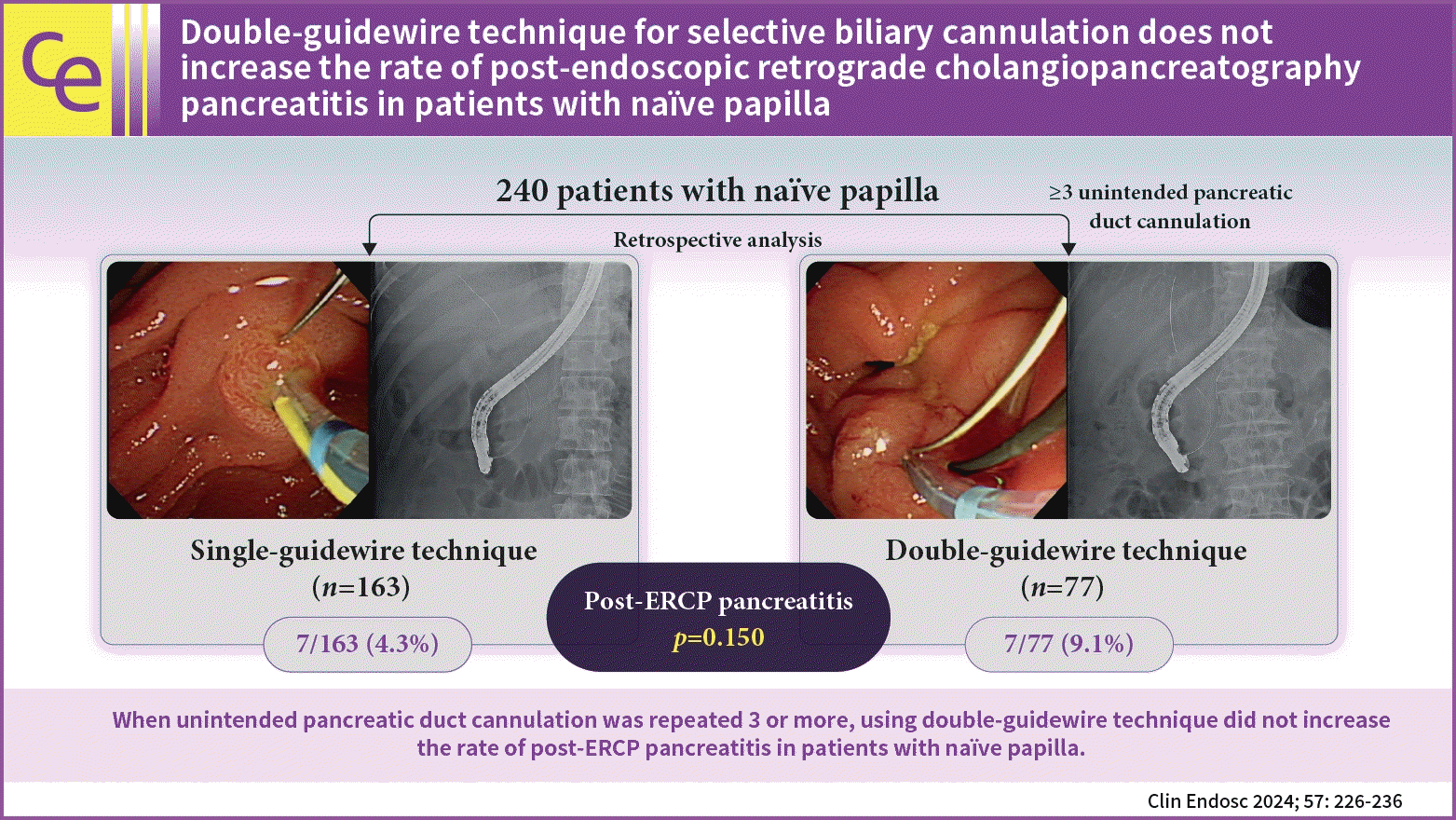
This study aimed to compare the safety of the double-guidewire technique (DGT) with that of the conventional single-guidewire technique (SGT) in real-world situations.
Methods
A total of 240 patients with naïve papilla who underwent endoscopic retrograde cholangiopancreatography (ERCP) at Daegu Catholic University Medical Center between January 2021 and December 2021 were included. The primary outcome was the rate of post-ERCP pancreatitis (PEP) in the SGT and DGT groups.
Results
A total of 163 patients (67.9%) belonged to the SGT group, and 77 (32.1%) belonged to the DGT group. The rates of successful biliary cannulation were 95.7% and 83.1% in the SGT and DGT groups, respectively (p=0.002). In the study group, PEP occurred in 14 patients (5.8%). The PEP rates were not significantly different between the SGT and DGT groups (4.3% vs. 9.1%, p=0.150). In the multivariate analysis, the age of <50 years (odds ratio [OR], 9.305; 95% confidence interval [CI], 1.367–63.358; p=0.023) and hyperlipidemia (OR, 7.384; 95% CI, 1.103–49.424; p=0.039) were significant risk factors for PEP in the DGT group.
Successful endoscopic retrograde cholangiopancreatography (ERCP) requires deep cannulation of the common bile duct (CBD) and/or pancreatic duct (PD).1 However, selective biliary cannulation reportedly fails in up to 18% of all cases, although it is less than 5% at a high-volume center.1-3
The double-guidewire technique (DGT) is an advanced technique used when conventional cannulation fails.1 Since its introduction in 1998, it has been widely used for difficult biliary cannulation (DBC).4 It consists of leaving a guidewire in the main PD while attempting to cannulate the CBD using an additional guidewire.1 By stabilizing the papilla and allowing the axis to align with the guidewire, it is expected to lead to clear improvement in the selective biliary cannulation.5 However, a meta-analysis showed that using DGT increased the risk of post-ERCP pancreatitis (PEP) without providing any superiority in achieving biliary cannulation compared with other techniques in DBC.6 One reason for this may be that DBC was defined as prolonged cannulation time.6,7 In other words, DGT was often used after repeated and prolonged attempts at cannulation. However, a recent randomized controlled trial by Laquière et al.8 reported that the early use of DGT improved the rate of biliary cannulation without increasing the PEP rate, in which randomization was performed when at least one unintended PD cannulation was performed.
In the real world, however, DGT is not attempted immediately after an unintended PD cannulation; the timing of conversion from the single-guidewire technique (SGT) to DGT, which is usually a case of repeated unintended PD cannulation, is determined at the discretion of endoscopists. Therefore, it remains inconclusive whether DGT increases the PEP rate compared with the SGT in the real world. In addition, the risk factors associated with PEP in the DGT have not been evaluated well.6,7,9
Therefore, we aimed to investigate whether DGT increases the risk of PEP compared with SGT in patients with naïve papilla in real-world situations. Furthermore, we analyzed the risk factors associated with PEP in patients with DGT.
A total of 657 patients who underwent ERCP at Daegu Catholic University Medical Center between January 2021 and December 2021 were eligible (Fig. 1). All patients were at least 18 years old and had naïve papilla. Patients with surgically altered anatomy, including Billroth I or II gastrectomies and liver transplantation, were also included. The exclusion criteria were as follows: history of previous ERCP, acute pancreatitis, failure of duodenoscope insertion, ERCP for pancreatic intervention, over threefold increase in the serum amylase levels before ERCP, and cannulation through a choledochoduodenal fistula.
This was a retrospective single-center study. The following data were collected by reviewing the medical records: demographics, laboratory findings, comorbidities, and endoscopic findings. The primary outcome was the PEP rate in the SGT and DGT groups. The secondary outcomes were risk factors associated with PEP.
If the patients had a fluoroscopic image with a guidewire in both the PD and CBD, they were classified into the DGT group. Patients without such images were assigned to the SGT group. This was verified using endoscopic images and reports. Whether to switch to DGT or continue with SGT was at the discretion of the endoscopist. A baseline image was obtained when the duodenoscope was inserted into the second portion of the duodenum. The procedure, PD cannulation, and CBD cannulation times were calculated based on the time at which the fluoroscopic images were taken. Procedure time was defined as the time from the baseline image to the last image of the procedure. PD cannulation time was defined as the time from the baseline image to the image obtained with a guidewire in the PD. CBD cannulation time was defined as the time between the baseline image and the image obtained using a guidewire in the CBD. The success rate was defined as the rate of successful selective biliary cannulation. Comorbidities including hypertension, diabetes mellitus, hyperlipidemia, and ischemic heart disease were identified from the patients’ medical records. PEP was defined and graded according to international consensus criteria.10
All endoscopic procedures were performed by two experts (J.H. and H.G.K.) with 17 and >20 years of ERCP experience, respectively, at a large-volume center where more than 800 cases of ERCP were performed annually. The trainees did not participate in any of the procedures. The procedures were performed using a side-viewing duodenoscope (JF-260V or TJF-260V; Olympus) under conscious sedation with midazolam and pethidine. Selective bile duct cannulation was initially attempted using the SGT (Fig. 2). A standard sphincterotome (Autotome RX; Boston Scientific) preloaded with a straight 0.035-inch hydrophilic-tipped guidewire (Jagwire; Boston Scientific) was used. DGT was attempted to achieve deep biliary cannulation if there were three or more unintended accesses to the PD (Fig. 3), as is routinely performed in our practice. If PD cannulation was not possible, infundibulotomy was performed, or the procedure was discontinued according to the discretion of the endoscopist. When DGT was attempted, the papilla was fixed using the first guidewire left deep in the PD, and CBD cannulation was attempted using another guidewire. A sphincterotome with a preloaded second guidewire was passed through the same working channel as that of the first guidewire. Then, at the discretion of the endoscopist, DGT was continuously attempted, or advanced techniques, such as transpancreatic sphincterotomy (TPS), infundibulotomy, or PD-assisted techniques, were applied. A prophylactic PD stent (5 Fr, 3 cm, Geenen; Cook Medical) was placed at the discretion of the endoscopist. Rectal nonsteroidal anti-inflammatory drugs were not used because they are not available in Korea. All patients were hydrated with normal saline ranging from 1.0 to 2.0 mL/kg/h before, during, and after the procedures. The volume and nature of the intravenous infusion were changed at the discretion of the treating physician the morning after the procedure.
Statistical analyses were performed using IBM SPSS Statistics for Windows ver. 26.0 (IBP Corp.). The chi-square test or Fisher exact test was used to compare categorical variables. Continuous variables were described as medians with interquartile ranges, and the Mann-Whitney U-test was used. Multivariate logistic regression was used to adjust various factors affecting PEP, including age, sex, comorbidity, and procedure-related factors. Factors with a p-value of <0.200 in the univariate analysis were included in the multivariate analysis. The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). The designated level of statistical significance was p<0.05 (two-tailed).
Of the 240 patients who underwent ERCP, 163 (67.9%) belonged to the SGT group, and 77 (32.1%) belonged to the DGT group. The clinical features of patients according to the cannulation technique are shown in Table 1. The median age of the patients was 71 years. A total of 142 (59.2%) patients were male. There were no significant differences in age, sex, body mass index (BMI), or comorbidities between the two groups. Hypertension was present in 101 patients (42.1%), diabetes mellitus in 61 (25.4%), and hyperlipidemia in 37 (15.4%). PEP occurred in 14 patients, of whom 13 had mild PEP and one had moderate PEP. Although patients in the DGT group had higher amylase (103 vs. 64 U/L, p=0.016) and lipase (104 vs. 45 U/L, p=0.017) levels than those in the SGT group, there was no significant difference in the PEP rate (9.1% vs. 4.3%, p=0.150). The most common indication for ERCP was biliary stone disease (n=143, 59.6%), followed by malignant obstruction (n=60, 25.0%) and benign stricture (n=12, 5.0%). The distribution of etiologies differed between the two groups, with patients in the DGT group having a higher proportion of malignant obstruction (35.1% vs. 20.2%, p=0.009). Sixteen patients (6.7%) had a surgically altered anatomy, and the proportion was not significantly different between the two groups (8.0% vs. 3.9%, p=0.282). The proportion of patients with periampullary diverticulum was higher in the SGT group than in the DGT group (34.4% vs. 20.8%, p=0.035). The procedure time was longer in the DGT group (884 vs. 541 seconds, p<0.001), and the proportion of patients who required >5 minutes for CBD cannulation was higher (77.9% vs. 15.3%, p<0.001). The cannulation success rate was higher in the SGT group than in the DGT group (95.7% vs. 83.1%, p=0.002). Infundibulotomy was performed in seven patients (2.9%), and endoscopic sphincterotomy and endoscopic papillary balloon dilatation were performed in 142 (59.2%) and 25 (10.4%) patients, respectively. TPS and prophylactic PD stent insertion were performed only in the DGT group (36.4% and 31.2%, respectively).
The clinical features of the patients according to the occurrence of PEP are shown in Table 2. The patients with PEP were younger than those without PEP (69 vs. 71 years, p<0.001). They also had a higher rate of hyperlipidemia (42.9% vs. 13.7%, p=0.011). There were no significant differences in sex, BMI, indications for ERCP, or additional techniques between the two groups. The success rate of selective CBD cannulation was >90% in both groups. The procedure time was not significantly different between the two groups; however, patients in the DGT group showed a longer CBD cannulation time (390 vs. 168 seconds, p=0.010).
The risk factors associated with PEP in all patients, including SGT and DGT, are shown in Table 3. In the univariate analysis, the age of <50 years (OR, 3.530; 95% CI, 1.025–12.165; p=0.046), a history of hyperlipidemia (OR, 4.718; 95% CI, 1.533–14.521; p=0.007), a history of diabetes mellitus (OR, 3.185; 95% CI, 1.069–9.486; p=0.037), and CBD cannulation time of >5 minutes (OR, 3.553; 95% CI, 1.150–10.970; p=0.028) were significant factors. In the multivariate analysis, female sex (OR, 3.693; 95% CI, 1.054–12.942, p=0.041), age of <50 years (OR, 8.676; 95% CI, 1.969–38.240; p=0.004), history of hyperlipidemia (OR, 5.913; 95% CI, 1.635–21.392; p=0.007), and CBD cannulation time of >5 minutes (OR, 4.062; 95% CI, 1.208–13.658; p=0.023) were significant factors.
A subgroup analysis was performed for the DGT group to analyze the risk factors for PEP. The results are summarized in Table 4. In the univariate analysis, the age of <50 years (OR, 5.812; 95% CI, 1.096–30.822; p=0.039) was the only significant factor. In contrast, in the multivariate analysis, the age of <50 years (OR, 9.305; 95% CI, 1.367–63.368; p=0.023) and a history of hyperlipidemia (OR, 7.384; 95% CI, 1.103–49.424; p=0.039) were significant risk factors for PEP. Cannulation time and prophylactic PD stent insertion were not significant factors.
DGT is one of the advanced techniques reserved for use in patients with DBC.1 According to the European Society of Gastrointestinal Endoscopy guidelines,1 DBC is defined by the presence of one or more of the following: more than five contacts with the papilla, more than 5 minutes spent attempting to cannulate following visualization of the papilla, and more than one unintended PD cannulation or opacification. In this study, the number of cannulation attempts was unknown. The number of unintended PD cannulations in the SGT group was unknown. Therefore, the procedure and CBD cannulation times were used as parameters to evaluate DBC. The DGT group had a longer procedure time than the SGT group (884 vs. 541 seconds, p<0.001), and a higher proportion of patients had CBD cannulation times exceeding 5 minutes (77.9% vs. 15.3%, p<0.001). This was probably because the DGT (Fig. 3) is a more complex procedure than the SGT (Fig. 2). Additionally, the success rate was lower in the DGT group than in the SGT group (83.1% vs. 95.7%, p<0.001). This can be attributed to the fact that the DGT group had a higher number of cases of DBC, as we preferred SGT in all patients. It was also related to a higher proportion of malignancy, which is a major factor associated with DBC,11 in the DGT group (35.1% vs. 20.2%, p=0.009).
PEP is one of the most common and severe complications of ERCP.12-14 The incidence of PEP ranges from 0.4% to 1.5% in diagnostic ERCP and 1.6% to 5.4% in therapeutic ERCP.15 It is often clinically mild or moderate in severity; however, in approximately 10% of cases, it is severe and potentially fatal.16 A combination of chemical, thermal, mechanic, hydrostatic, enzymatic, allergic, and microbiological factors, which leads to a cascade of events resulting in factors intracellular activation of pancreatic proteolytic enzymes, autodigestion, and the release of inflammatory cytokines, is involved in the development of PEP.15 Among the pathogenic factors of PEP, cannulation-induced trauma plays an important role17; there is a concern that DGT increases the risk of PEP because of irritation and/or injury to the PD from leaving the guidewire deep in place for prolonged times during the procedure.6
However, in this study, the PEP rate was not significantly different between the DGT and SGT groups (9.1% vs. 4.3%, p=0.150), although the proportion of patients with a threefold increase in the serum amylase levels was higher in the DGT group (23.4% vs. 8.0%, p=0.001). There are two possible explanations for this finding. First, frequent cannulation attempts may cause more injury to the PD than retaining a guidewire in the PD. To our knowledge, there have been two large prospective studies comparing the DGT with the conventional SGT. Maeda et al.5 reported that the DGT was superior to the SGT in achieving CBD cannulation and no PEP occurred in either group. On the contrary, Herreros de Tejada et al.18 reported that DGT may be associated with a higher risk of PEP without any superiority in achieving CBD cannulation. Moreover, the median numbers of attempts required for each group were nine in the DGT group and seven in the SGT group, respectively.18 In both studies, DBC was defined as unsuccessful cannulation within 10 and 5 minutes, respectively.5,18 In this study, however, conversion to DGT was attempted in cases of three or more unintended PD cannulations. Second, appropriate rescue techniques, including infundibulotomy, TPS, and PD stent-assisted cannulation, were used at the discretion of the endoscopists. Yang et al.19 reported that PD stent-assisted cannulation showed a lower PEP rate than DGT albeit not statistically significant (3.3% vs. 10.3%, p=0.077). Moreover, a recent meta-analysis reported that early needle-knife technique and TPS were superior to other interventions in decreasing the PEP rates and should be considered in patients with DBC.9
The risk factors for PEP are well established. In this study, the significant risk factors for PEP were female sex, CBD cannulation time of >5 minutes, age of <50 years, and a history of hyperlipidemia. Younger age, female sex, and prolonged procedure time are well-known risk factors for PEP.20 Interestingly, a history of hyperlipidemia was a significant risk factor for PEP in this study. Hyperlipidemia includes imbalance of the cholesterol level, hypertriglyceridemia, and mixed hyperlipidemia, in which both cholesterol and triglyceride levels are elevated.21 Hypertriglyceridemia is well-established cause of acute pancreatitis; although the pathogenesis is not clear, it is thought to result from toxic injury by excessive free fatty acid from hydrolysis of triglyceride.22 Sbeit and Khoury23 reported that fatty pancreas, which is related to metabolic syndrome, is associated with acute pancreatitis; the proposed pathogenesis is underlying low-grade inflammation induced by pancreatic steatosis.
Several studies also reported relationship between a high serum low-density lipoprotein cholesterol (LDL-C) level and severe acute pancreatitis.24-26 One possible explanation is that oxidative stress resulting from chronic elevation of the serum LDL-C level plays an important role in pancreatic inflammation.24,26 Thus, it may be possible that hyperlipidemia aggravates pancreatic inflammation induced by cannulation injury. However, we could not obtain a detailed history of hyperlipidemia, including lipid profile and medication history, because this was a retrospective study. Therefore, future prospective studies are required to evaluate the association between hyperlipidemia and PEP.
We conducted a subgroup analysis to analyze the risk factors for PEP in the DGT group. In subgroup analysis, cannulation time and female sex were not significant risk factors for PEP. Female sex is a well-known risk factor for PEP; however, the underlying mechanism remains unknown.27 Sphincter of Oddi dysfunction, which occurs primarily in women, and DBC are hypothesized to be contributing factors to PEP.27,28 Given that both risk factors are associated with DBC, it can be speculated that the guidewire placed in the PD may prevent additional injury to the PD during cannulation attempts. In addition, in the subgroup analysis, PD stent placement was not associated with PEP. This is probably because, at the discretion of the endoscopist, the PD stent was inserted in patients who were likely to develop PEP.
This study has some limitations. First, this was a retrospective single-center study. Therefore, there was selection bias. Moreover, it is difficult to generalize the results of this study. Second, the DGT was used at the discretion of the endoscopist instead of for a specific indication. Advanced techniques other than the DGT were sequentially used in the same manner. Consequently, there were differences in the additional techniques used, including TPS and prophylactic PD stent, which could potentially affect the occurrence of PEP between the two groups. However, our aim was to determine whether the use of the DGT increases PEP compared with the SGT in real-world scenarios. Therefore, the sequential transition to these advanced techniques reflects actual clinical practices. Third, the confirmation of hyperlipidemia in our study was based solely on patients’ medical records. Therefore, there is a possibility of misdiagnosis and variation in the current lipid profiles among patients. Furthermore, the protective effect of statins could not be verified. Fourth, because the procedures were performed by endoscopists with >20 years of experience, it may be difficult to expect the same results from an inexperienced endoscopist.
The strength of this study is that we compared the DGT with the SGT in real-world situations. The conversion to DGT was determined at the discretion of the endoscopist. This time point was neither immediately after the first unintended PD cannulation nor delayed until the definition of DBC was met. In addition, we included patients with a surgically altered anatomy for whom DGT was found to be useful.5
In conclusion, when unintended PD cannulation was repeated three or more times, DGT did not increase the PEP rate in patients with naïve papilla. Therefore, early conversion to DGT should be considered if unintended PD cannulation occurs. A history of hyperlipidemia and age of <50 years were significant risk factors for PEP in the DGT group.
Notes
REFERENCES
1. Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016; 48:657–683.
2. Tse F, Yuan Y, Moayyedi P, et al. Guidewire-assisted cannulation of the common bile duct for the prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Cochrane Database Syst Rev. 2012; 12:CD009662.
3. Williams EJ, Taylor S, Fairclough P, et al. Are we meeting the standards set for endoscopy?: results of a large-scale prospective survey of endoscopic retrograde cholangio-pancreatograph practice. Gut. 2007; 56:821–829.
4. Dumonceau JM, Devière J, Cremer M. A new method of achieving deep cannulation of the common bile duct during endoscopic retrograde cholangiopancreatography. Endoscopy. 1998; 30:S80.
5. Maeda S, Hayashi H, Hosokawa O, et al. Prospective randomized pilot trial of selective biliary cannulation using pancreatic guide-wire placement. Endoscopy. 2003; 35:721–724.
6. Tse F, Yuan Y, Moayyedi P, et al. Double-guidewire technique in difficult biliary cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2017; 49:15–26.
7. Takenaka M, Kudo M. Usefulness of the double-guidewire technique for endoscopic procedures in the field of biliary and pancreatic diseases. Clin Endosc. 2022; 55:605–614.
8. Laquière A, Privat J, Jacques J, et al. Early double-guidewire versus repeated single-guidewire technique to facilitate selective bile duct cannulation: a randomized controlled trial. Endoscopy. 2022; 54:120–127.
9. Facciorusso A, Ramai D, Gkolfakis P, et al. Comparative efficacy of different methods for difficult biliary cannulation in ERCP: systematic review and network meta-analysis. Gastrointest Endosc. 2022; 95:60–71.
10. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991; 37:383–393.
11. Fugazza A, Troncone E, Amato A, et al. Difficult biliary cannulation in patients with distal malignant biliary obstruction: an underestimated problem? Dig Liver Dis. 2022; 54:529–536.
12. Lee ES, Kim H. The prevention and management of post-ERCP pancreatitis. Korean J Pancreas Biliary Tract. 2016; 21:68–75.
13. Wu CC, Lim SJ, Khor CJ. Endoscopic retrograde cholangiopancreatography-related complications: risk stratification, prevention, and management. Clin Endosc. 2023; 56:433–445.
14. Sadeghi A, Jafari-Moghaddam R, Ataei S, et al. Role of vitamin C and rectal indomethacin in preventing and alleviating post-endoscopic retrograde cholangiopancreatography pancreatitis: a clinical study. Clin Endosc. 2023; 56:214–220.
15. Arata S, Takada T, Hirata K, et al. Post-ERCP pancreatitis. J Hepatobiliary Pancreat Sci. 2010; 17:70–78.
16. Dumonceau JM, Andriulli A, Deviere J, et al. European Society of Gastrointestinal Endoscopy (ESGE) guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy. 2010; 42:503–515.
17. Tryliskyy Y, Bryce GJ. Post-ERCP pancreatitis: pathophysiology, early identification and risk stratification. Adv Clin Exp Med. 2018; 27:149–154.
18. Herreros de Tejada A, Calleja JL, Díaz G, et al. Double-guidewire technique for difficult bile duct cannulation: a multicenter randomized, controlled trial. Gastrointest Endosc. 2009; 70:700–709.
19. Yang MJ, Hwang JC, Yoo BM, et al. Wire-guided cannulation over a pancreatic stent versus double guidewire technique in patients with difficult biliary cannulation. BMC Gastroenterol. 2015; 15:150.
20. Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline: updated June 2014. Endoscopy. 2014; 46:799–815.
21. Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care. 2017; 23(9 Suppl):S139–S148.
22. Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008; 14:4558–4561.
23. Sbeit W, Khoury T. Fatty pancreas represents a risk factor for acute pancreatitis: a pilot study. Pancreas. 2021; 50:990–993.
24. Hong W, Zimmer V, Basharat Z, et al. Association of total cholesterol with severe acute pancreatitis: a U-shaped relationship. Clin Nutr. 2020; 39:250–257.
25. Socea B, Bolocan A, Bratu OG, et al. Hypercholesterolemia, as a predictor factor of severe acute pancreatitis. Mod Med. 2018; 25:219–222.
26. Hong W, Zimmer V, Stock S, et al. Relationship between low-density lipoprotein cholesterol and severe acute pancreatitis (“the lipid paradox”). Ther Clin Risk Manag. 2018; 14:981–989.
27. Vihervaara H, Salminen P, Hurme S, et al. Female gender and post-ERCP pancreatitis: is the association caused by difficult cannulation? Scand J Gastroenterol. 2011; 46:1498–1502.
28. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001; 54:425–434.
Fig. 1.
Flowchart of the study population. ERCP, endoscopic retrograde cholangiopancreatography; SGT, single-guidewire technique; DGT, double-guidewire technique.
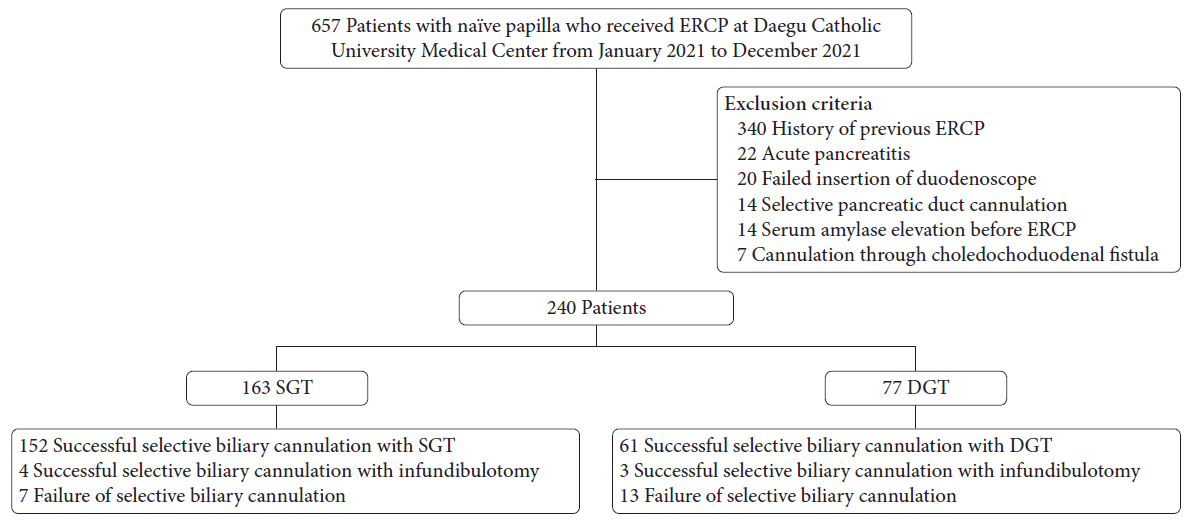
Fig. 2.
Endoscopic and radiologic images of the single-guidewire technique. (A) Selective biliary cannulation is attempted using a sphincterotome. (B) Successful biliary cannulation is achieved (arrowhead).
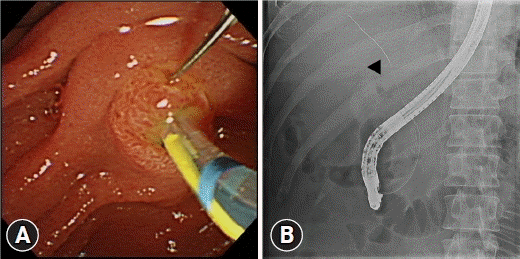
Fig. 3.
Endoscopic and radiologic images of the double-guidewire technique. (A) The papilla is fixed by a guidewire, and selective biliary cannulation is attempted using a sphincterotome. (B) A guidewire is placed in the pancreatic duct (arrow), and successful biliary cannulation is achieved (arrowhead).
Table 1.
Clinical features of the patients according to cannulation technique
| Variable | Total (n=240) | SGT (n=163) | DGT (n=77) | p-value |
|---|---|---|---|---|
| Age (yr) | 71 (60–80) | 71 (60–80) | 71 (58–80) | 0.609 |
| Male | 142 (59.2) | 93 (57.1) | 49 (63.6) | 0.399 |
| Body mass index (kg/m2) | 23.6 (21.4–25.4) | 23.5 (21.4–25.6) | 23.8 (21.2–25.4) | 0.703 |
| Comorbidity | ||||
| Hypertension | 101 (42.1) | 70 (42.9) | 31 (40.3) | 0.780 |
| Diabetes mellitus | 61 (25.4) | 44 (27.0) | 17 (22.1) | 0.433 |
| Hyperlipidemia | 37 (15.4) | 24 (14.7) | 13 (16.9) | 0.703 |
| Ischemic heart disease | 18 (7.5) | 12 (7.4) | 6 (7.8) | 1.000 |
| Amylase (U/L) | 74 (47–141) | 64 (43–113) | 103 (68–290) | 0.016 |
| Lipase (U/L) | 57 (33–143) | 45 (29–89) | 104 (53–640) | 0.017 |
| Amylase >3×UNL | 31 (12.9) | 13 (8.0) | 18 (23.4) | 0.001 |
| Post-ERCP pancreatitis | 14 (5.8) | 7 (4.3) | 7 (9.1) | 0.150 |
| Mild | 13 (92.9) | 6 (85.7) | 7 (100.0) | |
| Moderate | 1 (7.1) | 1 (14.3) | 0 (0) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
| Etiology | 0.009 | |||
| Stone | 143 (59.6) | 108 (66.3) | 35 (45.5) | |
| Malignant | 60 (25.0) | 33 (20.2) | 27 (35.1) | |
| Benign stricture | 12 (5.0) | 9 (5.5) | 3 (3.9) | |
| Othersa) | 25 (10.4) | 13 (8.0) | 12 (15.6) | |
| Surgically altered anatomy | 16 (6.7) | 13 (8.0) | 3 (3.9) | 0.282 |
| Liver transplantation | 10 (4.2) | 8 (4.9) | 2 (2.6) | |
| Billroth I | 3 (1.3) | 2 (1.2) | 1 (1.3) | |
| Billroth II | 3 (1.3) | 3 (1.8) | 0 (0) | |
| Periampullary diverticulum | 72 (30.0) | 56 (34.4) | 16 (20.8) | 0.035 |
| Procedure time (sec) | 617 (421–958) | 541 (350–807) | 884 (653–1,142) | <0.001 |
| CBD cannulation time >5 min | 85 (35.4) | 25 (15.3) | 60 (77.9) | <0.001 |
| Success rate | 220 (91.7) | 156 (95.7) | 64 (83.1) | 0.002 |
| Additional procedures | ||||
| Infundibulotomy | 7 (2.9) | 4 (2.5) | 3 (3.9) | 0.683 |
| Transpancreatic sphincterotomy | 28 (11.7) | 0 (0) | 28 (36.4) | <0.001 |
| Prophylactic PD stent | 24 (10.0) | 0 (0) | 24 (31.2) | <0.001 |
| Endoscopic sphincterotomy | 142 (59.2) | 97 (59.5) | 45 (58.4) | 0.889 |
| Endoscopic papillary balloon dilatation | 25 (10.4) | 20 (12.3) | 5 (6.5) | 0.186 |
Table 2.
Clinical features of the patients with and without post-endoscopic retrograde cholangiopancreatography pancreatitis
| Variable | Total (n=240) | Without PEP (n=226) | With PEP (n=14) | p-value |
|---|---|---|---|---|
| Age (yr) | 71 (60–80) | 71 (61–80) | 69 (38–77) | <0.001 |
| Male | 142 (59.2) | 137 (60.6) | 5 (35.7) | 0.092 |
| Body mass index (kg/m2) | 23.6 (21.4–25.4) | 23.5 (21.3–25.5) | 24.5 (23.2–25.3) | 0.250 |
| Comorbidity | ||||
| Hypertension | 101 (42.1) | 95 (42.0) | 6 (42.9) | 1.000 |
| Diabetes mellitus | 61 (25.4) | 54 (23.9) | 7 (50.0) | 0.051 |
| Hyperlipidemia | 37 (15.4) | 31 (13.7) | 6 (42.9) | 0.011 |
| Ischemic heart disease | 18 (7.5) | 16 (7.1) | 2 (14.3) | 0.283 |
| Amylase (U/L) | 74 (47–141) | 71 (45–120) | 1,308 (937–1,867) | <0.001 |
| Lipase (U/L) | 57 (33–143) | 55 (33–117) | 2,763 (1,575–4,115) | <0.001 |
| Amylase >3×UNL | 31 (12.9) | 17 (7.5) | 14 (100.0) | <0.001 |
| Etiology | 0.455 | |||
| Stone | 143 (59.6) | 136 (60.2) | 7 (50.0) | |
| Malignant | 60 (25.0) | 56 (24.8) | 4 (28.6) | |
| Benign stricture | 12 (5.0) | 12 (5.3) | 0 (0.0) | |
| Othersa) | 25 (10.4) | 22 (9.7) | 3 (21.4) | |
| Surgically altered anatomy | 16 (6.7) | 16 (7.1) | 0 (0.0) | 0.607 |
| Periampullary diverticulum | 72 (30.0) | 71 (31.4) | 1 (7.1) | 0.070 |
| Procedure time (sec) | 617 (421–958) | 617 (416–955) | 708 (488–1,613) | 0.163 |
| CBD cannulation time (sec) | 190 (84–415) | 168 (77–403) | 390 (239–487) | 0.010 |
| PD cannulation time (sec)b) | 121 (78–230) | 121 (73–234) | 133 (109–195) | 0.563 |
| CBD cannulation time >5 min | 85 (35.4) | 76 (33.6) | 9 (64.3) | 0.039 |
| Success rate | 220 (91.7) | 207 (91.6) | 13 (92.9) | 1.000 |
| Additional procedures | ||||
| Double-guidewire technique | 77 (32.1) | 70 (31.0) | 7 (50.0) | 0.150 |
| Infundibulotomy | 7 (2.9) | 6 (2.7) | 1 (7.1) | 0.347 |
| Transpancreatic sphincterotomy | 28 (11.7) | 26 (11.5) | 2 (14.3) | 1.000 |
| Prophylactic PD stent | 24 (10.0) | 22 (9.7) | 2 (14.3) | 0.638 |
| Endoscopic sphincterotomy | 142 (59.2) | 133 (58.8) | 9 (64.3) | 0.785 |
| Endoscopic papillary balloon dilatation | 25 (10.4) | 24 (10.6) | 1 (7.1) | 1.000 |
Values are presented as median (interquartile range) or number (%).
PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; UNL, upper normal limit; CBD, common bile duct; PD, pancreatic duct.
Table 3.
Risk factors associated with post-ERCP pancreatitis
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Sex | ||||
| Malea) | ||||
| Female | 2.771 (0.899–8.537) | 0.076 | 3.693 (1.054–12.942) | 0.041 |
| Age <50 yr | ||||
| ≥50 yra) | ||||
| <50 yr | 3.530 (1.025–12.165) | 0.046 | 8.676 (1.969–38.240) | 0.004 |
| Double-guidewire technique | ||||
| Noa) | ||||
| Yes | 2.229 (0.753–6.595) | 0.148 | ||
| Hyperlipidemia | ||||
| Noa) | ||||
| Yes | 4.718 (1.533–14.521) | 0.007 | 5.913 (1.635–21.392) | 0.007 |
| Diabetes mellitus | ||||
| Noa) | ||||
| Yes | 3.185 (1.069–9.486) | 0.037 | ||
| Periampullary diverticulum | ||||
| Noa) | ||||
| Yes | 0.168 (0.022–1.309) | 0.089 | ||
| CBD cannulation time | ||||
| ≤5 mina) | ||||
| >5 min | 3.553 (1.150–10.970) | 0.028 | 4.062 (1.208–13.658) | 0.023 |
| Body mass index (kg/m2)b) | ||||
| ≤23.0a) | ||||
| >23.0 | 2.910 (0.790–10.713) | 0.108 | ||
Table 4.
Risk factors associated with post-ERCP pancreatitis in double-guidewire technique
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Sex | ||||
| Malea) | ||||
| Female | 1.350 (0.280-6.520) | 0.709 | ||
| Age <50 yr | ||||
| ≥50 yra) | ||||
| <50 yr | 5.812 (1.096-30.822) | 0.039 | 9.305 (1.367-63.358) | 0.023 |
| Ttranspancreatic sphincterotomy | ||||
| Noa) | ||||
| Yes | 0.677 (0.122-3.743) | 0.655 | ||
| Prophylactic PD stent | ||||
| Noa) | ||||
| Yes | 0.873 (0.157-4.853) | 0.876 | ||
| Hyperlipidemia | ||||
| Noa) | ||||
| Yes | 4.500 (0.873-23.194) | 0.072 | 7.384 (1.103-49.424) | 0.039 |
| Diabetes mellitus | ||||
| Noa) | ||||
| Yes | 3.000 (0.601-14.970) | 0.180 | ||
| CBD cannulation time | ||||
| ≤5 mina) | ||||
| >5 min | 0.682 (0.120-3.870) | 0.666 | ||
| PD cannulation time | ||||
| ≤2 mina) | ||||
| >2 min | 2.500 (0.454-13.760) | 0.292 | ||
| Body mass index (kg/m2)b) | ||||
| ≤23.0a) | ||||
| >23.0 | 4.000 (0.457-35.045) | 0.211 | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download