Abstract
Background/Aims
Endoscopic ultrasonography-guided gastrojejunostomy is a minimally invasive method for the management of gastric outlet obstruction. Conventionally, a lumen-apposing metal stent (LAMS) is used to create an anastomosis. However, LAMS is expensive and not widely available. In this report, we described a tubular fully covered self-expandable metallic stent (T-FCSEMS) for this purpose.
Methods
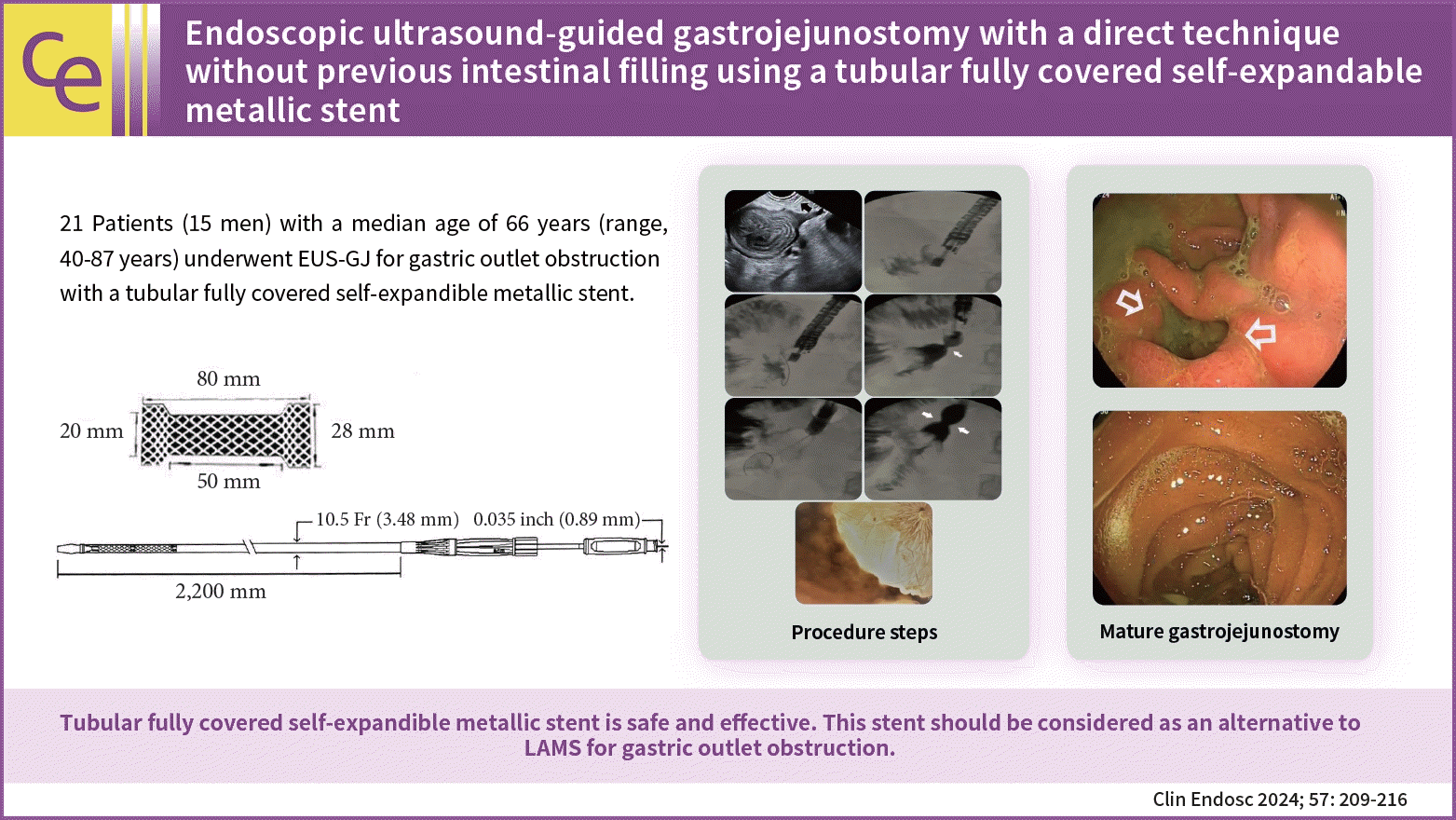
Twenty-one patients (15 men [71.4%]; median age, 66 years; range, 40–87 years) were included in this study. A total of 19 malignant (12 pancreatic, 6 gastric, and 1 metastatic rectal cancer) and 2 benign cases were observed. The proximal jejunum was punctured with a 19 G needle. The stomach and jejunum walls were dilated with a 6 F cystotome, and a 20×80 mm polytetrafluoroethylene T-FCSEMS (Hilzo) was deployed. Oral feeding was initiated after 12 to 18 hours and solid foods after 48 hours.
Results
The median procedure time was 33 minutes (range, 23–55 minutes). After two weeks, 19 patients tolerated oral feeding. In patients with malignancy, the median survival time was 118 days (range, 41–194 days). No serious complications or deaths occurred. All patients with malignancy tolerated oral food intake until they expired.
Gastric outlet obstruction (GOO) occurs in at least one-fifth of patients with pancreatic head carcinoma.1 GOO is also found in gastric, duodenal, papillary, and biliary malignancies.2 Duodenal ulcer, groove, chronic pancreatitis, and annular pancreas are the most common benign etiologies.3 In unresectable malignancies, the conventional approach is to insert a duodenal stent endoscopically. However, migration and stent occlusion rates are high, and reintervention is required in most patients.4
Endoscopic ultrasonography-guided gastrojejunostomy (EUS-GJ) is a novel procedure. Several techniques, such as prototype double-balloon catheter assistance and continuous perfusion of the duodenum with a nasobiliary drainage catheter, have been previously described.5-7 Anastomosis is performed using a lumen-apposing metallic stent (LAMS).8 Some of these stents have an electrocautery ring at the tip. The size used for the GJ is usually 15-20 to 30 mm. However, LAMS is considerably more expensive than a tubular fully covered self-expandable metallic stent (T-FCSEMS). Importantly, they may occasionally cause pressure necrosis of the gut wall, resulting in bleeding. Furthermore, the use of LAMS requires special training.
In this pilot study, we evaluated the use of T-FCSEMS in 21 patients who underwent EUS-GJ using a direct technique without previous fluid filling.
Twenty-one patients (15 men) with a median age of 66 years (range, 40–87 years) who underwent EUS-GJ for GOO with T-FCSEMS, from January 2018 to December 2022, were included. The technical and clinical outcomes were assessed retrospectively.
The procedures were performed with the patient supine under general anesthesia. Briefly, we identified the most proximal jejunal segment, just distal to the fourth segment of the duodenum, from the greater curvature of the stomach. The endoscope was kept straight with a 90° clockwise rotation, and the knobs were in a neutral position. The advantages are as follows. First, the Treitz ligament fixes this portion. Second, this portion has a downward course that facilitates the insertion of the guide wire into the deep jejunum, which can make stent deployment easier. The point was reached at approximately 50 cm (range, 43–55 cm) from the teeth. After confirming that the identified segment was the jejunum and not the transverse colon, we injected glucagon to decrease peristalsis. In cases of suspicion, we first punctured to ensure that the segment was the jejunum, and then, injected glucagon. During this time, rapid transit can be observed in the jejunum, while the transverse colon remains stagnant. This is useful for differentiating the two segments. We used a 19 G needle (Microtech Company) for injection. Before inserting the needle, we slightly withdrew the stylet to sharpen the needle, and then, inserted as little of the inner sheath as possible to avoid excessive force on the stomach wall. Subsequently, we inserted with a quick and forceful push, while tolerating overshoot (if this occurred, we slowly withdrew the needle from the bowel). Subsequently, we injected a 50:50 contrast mixture (diluted with saline) stained with a few drops of methylene blue using a 10 mL syringe. In cases where we observed filling of the jejunum with rapid contractions, we exchanged the stylet with a 0.035-inch guidewire (Microtech Company). We did not try to fill the jejunum with a substantial amount of fluid. We inserted a guidewire deep into the jejunum. Notably, its soft distal part can easily become stuck in the jejunum, and a forceful push may result in an increased distance between the stomach and duodenum, which can occasionally dislodge the needle and guidewire. We waited for several seconds for the wire to move distally using peristaltic waves. Sometimes, the guidewire may be proximal. In these cases, we would change the endoscope and needle positions to manipulate the guidewire. If the guidewire cannot be redirected distally, the stent may be deployed in this direction. Peristaltic waves usually turn it distally. Excessive manipulation may increase the risk of dislodging the guidewire, and hesitancy may result in failure. After securing the guidewire in the jejunum, we exchanged the 19 G needle for a 6 F cystotome (Endoflex) and dilated the gastric and jejunal walls with a pure-cut electrocautery current. The 6 F cystotome is secured deep in the jejunum. If we needed to ensure the correct position of the cystotome, we would withdraw the guidewire and inject contrast to check the location of the cystotome. Subsequently, the guidewire is reinserted and the 6 F cystotome is exchanged with a 20×80 mm T-FCSEMS with 28 mm proximal and distal flanges (Fig. 1).
If we failed to insert the stent directly, we further dilated the tract with a 4 mm dilation balloon (Boston Scientific Co.). This was required in 6 of the 21 patients. Subsequently, the stent was easily introduced into the jejunum. After one-third to half of the T-FCSEMS was opened in the jejunum, we pulled the stent to bring the intestinal loop closer to the stomach wall, and deployed completely under radiological guidance and, where possible, endoscopic control. We attempted to keep the middle of the stent at the gastrojejunal anastomosis site and the proximal flanges inside the scope, which obviates the need for endoscopic control. Finally, we dilated the stent with a 15 mm dilatation balloon (Microtech Company). We did this for 20 of the 21 patients (except for the first case, in whom we used a 20 mm balloon). The procedure is shown in Figure 2.
Oral feeding was withheld for 12 to 18 hours. If no complications were observed, the patients were allowed to start taking liquids, followed by solid foods within 48 hours.
Table 1 summarizes the relevant patient data. Twelve patients were diagnosed with pancreatic carcinoma (9 metastatic and 3 locally advanced), 6 had gastric carcinoma with peritoneal invasion, and one had metastatic colon cancer. Two procedures were performed for the benign cases: (1) stricture of the Roux-n-Y anastomosis site after pancreaticoduodenectomy due to side-branch intraductal papillary mucinous neoplasia (IPMN) that was subsequently confirmed as having low-grade dysplasia, and (2) duodenal stricture due to peptic ulcer resistance to endoscopic dilation with long-term stenting. The patient had hepatitis B-related cirrhosis with portal hypertension, collaterals, and esophageal varices. The case was discussed at a multidisciplinary meeting and the EUS-guided procedure was preferred over surgery.
A total of 8 out of 21 patients had tense ascites drained before the EUS-guided procedure. Three other patients had mild ascites that we did not attempt to drain. For patients with tense ascites before the procedure, repeat paracentesis if necessary to avoid stent migration (this did not occur in any patient). Moreover, three patients had previously deployed malfunctioning enteric stents (one stent-in-stent).
The median procedure time was 33 minutes (range, 23–55 minutes), excluding anesthesia induction and recovery time. One, two, and three or more punctures were required in 13, 5, and 3 patients, respectively. In two of the patients, the guidewire was dislodged while exchanging the 19 G needle for the 6 F cystotome, and the stomach wall was punctured. We immediately withdrew the cystotome and repeated the procedure and no adverse event occurred. In one patient with ascites, we accidentally punctured the transverse colon and noticed the issue after puncturing with a 6 F cystotome. For this patient, the cystotome was withdrawn and the jejunum was punctured on the second attempt. Again, no adverse events occurred (Fig. 3).
After the procedure, a nasogastric tube (NG) was placed. Computed tomography (CT) without contrast medium was performed if perforation was suspected (did not occur in any patient). Otherwise, CT examination with oral contrast was performed the following day. The NG tube was withdrawn, and a liquid diet was started. Fourteen patients (66.7%) tolerated the liquid diet well. In contrast, moderate bloating with vomiting was observed in seven patients. For these seven patients, we administered metoclopramide before meals thrice a day to improve tolerance. However, in one patient with gastric carcinoma and peritonitis carcinomatosa, bloating was aggravated within 48 hours, and postoperative ileus developed. Repeat CT with oral-IV contrast revealed a distal ileal obstruction, possibly due to peritonitis carcinomatosa. A surgical ileostomy was performed, and the patient was discharged four days later on a solid diet.
Upper gastrointestinal (GI) bleeding requiring blood transfusion occurred in two patients (3 and 9 units of blood, respectively). One patient had a pancreatic carcinoma and the other patient had a gastric carcinoma. For the patient with pancreatic carcinoma, hepaticogastrostomy was also performed in the same session, and bleeding occurred two weeks after the procedure. However, an upper GI endoscopy at 24 hours after transfusion revealed no active bleeding, and further bleeding did not occur. The patient with gastric carcinoma was admitted to the hospital, and the gastroscopy revealed active bleeding from the tumor away from the GJ site. The bleeding was stopped by pulverizing the blood stopper. Technical success was achieved in all patients, and clinical success was observed in 19/21 (90.5%) patients. Patients with gastric carcinoma fared worse. The adverse events are presented in Table 2.
Two weeks after the procedure, 3 and 16 patients tolerated semi-solid and solid diets, respectively. Two patients with gastric carcinoma tolerated only liquids, and experienced frequent bouts of bloating and vomiting. These were considered clinical failures. In patients with malignancy, the median survival time was 118 days (range, 41–194 days). For the two benign cases, the patient operated on for IPMN with a migrated stent was alive after 14 months, and the other patient tolerated oral feeding perfectly after removing the stent after three weeks. All patients with malignancy demonstrated initial success and tolerated oral intake until their death.
EUS-GJ is an emerging technique for the management of GOO that was previously used to be managed with duodenal stenting (DS) and surgical gastroenterostomy.9 However, the former is frequently complicated by stent migration and tumor ingrowth, which was observed in three of our patients, the insertion of a guidewire through the stricture is occasionally impossible, as in two of our patients. In contrast, surgical gastroenterostomy demonstrates better results but is a high-cost palliative intervention with considerable morbidity, especially when used for patients with severely limited lifespans. Furthermore, it may be obstructed or occluded, as observed in two of our patients.
Although the EUS-GJ technique was first described more than a decade ago, it is not widely used. There may be several reasons for the limited use: most endoscopists are accustomed to DS, which is easier and considered safer; the number of endoscopists trained in interventional EUS is limited; performing an endoscopic transperitoneal intervention is fearsome for endoscopists due to the risk of peritonitis, which can be catastrophic; and the last but not the least, LAMS are expensive, and endoscopists may be unfamiliar with its use.
In the beginning, prototype double-balloon catheters were used for EUS-GJ.10 Their use was cumbersome because it required passing a guidewire through the pylorus, withdrawing the scope, and putting a double-balloon over the guidewire into the jejunum. Given that the wire is commonly looped in the stomach, an overtube is required. Placing the balloon at the desired location was also difficult. Furthermore, during dilation with a 6 F cystotome over the guidewire, the cystotome was attached to the balloon because of the heat generated in one of our previous cases. Finally, the procedure time was extremely long, usually lasting hours. Due to these issues, we switched to placing a nasogastric catheter first, changed the scope, and punctured the jejunum guided by methylene blue-infused saline or saline-diluted contrast. In addition to the long procedure time, the large amounts of fluids obscured the field and caused difficulties in tracing the guidewire. Furthermore, the perfused fluids did not remain in the desired location because of the peristaltic waves. Stabilizing the jejunum to a standstill with spasmolytics and glucagon was impossible. The final step required the LAMS, which costs several times more that of an enteric stent.
We finally decided to perform the procedure directly without infusing fluids beforehand. We put in a fully covered intestinal stent with large flanges and achieved perfect results. Previous reviews have reported that in attempting to puncture a non-distended intestinal loop, it may be pushed aside, rendering the puncture difficult.6 This was different from our experience. The reasons for the difference may be as follows. First, we punctured the jejunum just distal to the ligament of Treitz, which was relatively fixed in this area. Second, we kept the scope in a straight position and punctured with a forceful rapid push, tolerating any overshoot. In a previous study, the authors reported that they failed to place the LAMS over the wire in their first two cases because the jejunum was pushed aside.11 We had a different experience with T-FCSEMS. We placed the T-FCSEMS after dilation with a 6 F cystotome in 15 patients. Subsequent dilation with a 4 mm balloon was needed in six patients. Given that the tip of the intestinal stent is thinner than that of the LAMS, it facilitated a smooth passage over the guidewire. Our method may be criticized, as intestinal stents are not designed for this purpose and may potentially migrate easily. However, migration occurred in only one of our patients, for whom we performed dilation with a 20 mm balloon. The stent migrated spontaneously into the jejunum and defecated within two weeks. Endoscopy revealed a fully mature gastrojejunal anastomosis (Fig. 4). In subsequent procedures, we used a 15 mm dilatation balloon and did not encounter any further spontaneous migration. Given that our study was a pilot research, extensive data are required to determine the safety of T-FCSEMS, especially concerning its potential migration. However, the T-FCSEMS we used had 20×28 mm flanges, thus spontaneous migration was not expected as in-procedure dilations over 15 mm were not performed. The size of the balloon used for in-procedure dilation, and the necessity for dilation after stent removal, are issues yet to be fully resolved. Bleeding occurred in two patients but it was not directly related to the procedure. Bleeding in one of the patients occurred two weeks later and stopped spontaneously. Endoscopy did not reveal any source. In the other patient, bleeding originated from a gastric malignancy away from the anastomosis site and was managed endoscopically (Table 2).
The stent we used had two main advantages. First, it was deployed deep in the jejunum, and we did not experience any difficulties inserting it into the jejunum when we applied it to an area just distal to the Treitz ligament because of the thin stent tip. Second, given that its flanges are large, after deployment into the distal part of the intestine, we pulled and anchored the opened part of the stent to the jejunal wall, and subsequent deployment was very secure. Based on our experience with a patient in whom we punctured the colon with a 6 F cystotome, even if maldeployment was noticed after the distal part of the stent was opened, no significant complications would develop if the opened part was reinserted into the catheter and withdrawn.
In several meta-analyses that evaluated the overall outcome of EUS-GJ, the overall technical success rate was between 90% and 100%, and the clinical success rate was between 80% and 90%.12 These results were comparable to those of surgical GJ and DS. However, the complication rate was lower than that of surgical GJ and the reintervention rate of DS.13,14
The reported overall adverse event rate associated with LAMS is 7% to 27%. The surgical reintervention rate was 13% to 33%, the mortality rate was 2% to 5%, and the maldeployment rate was 10%.4 Most of the higher rates of adverse events came from centers with relatively low case volumes and/or endoscopists with limited experience. We speculate that the use of enteric stents rather than LAMS will decrease the maldeployment and complication rates. As the stent we used was easily deployed after dilation with a 6 F cystotome and most endoscopists are already adept at its use for other indications, they will be able to deploy the T-FCSEMS more confidently than LAMS. Finally, with experience and use, maldeployment will likely become less frequent. In our previous experiences with other techniques, we observed that a fistula can form within one week. Therefore, even if a stent migrates after this period, it will not mitigate the success of the procedure.
In conclusion, we described a novel method of EUS-GJ using the T-FCSEMS rather than the LAMS, without prior filling of the intestine. A multicenter study comparing this novel technique with existing methods is warranted.
Notes
Author Contributions
Conceptualization: HŞ; Data curation: İHK, HŞ; Formal analysis: İHK, HŞ; Investigation: İHK; Methodology: HŞ; Project administration: İHK; Resources: GS, SK; Supervision: HŞ, ATİ; Validation: İHK, KK, ATİ; Visualization: GS, SK, KK, ATİ; Writing–original draft: İHK; Writing–review & editing: all authors.
Supplementary Material
Supplementary Video 1.
Step by step our method of gastrojejunostomy (https://doi.org/10.5946/ce-2023-022.v1).
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2023.022.
REFERENCES
1. Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010; 71:490–499.
2. Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005; 61:421–426.
3. ASGE Standards of Practice Committee, Fukami N, Anderson MA, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc. 2011; 74:13–21.
4. Krishnamoorthi R, Bomman S, Benias P, et al. Efficacy and safety of endoscopic duodenal stent versus endoscopic or surgical gastrojejunostomy to treat malignant gastric outlet obstruction: systematic review and meta-analysis. Endosc Int Open. 2022; 10:E874–E897.
5. Rimbas M, Larghi A, Costamagna G. Endoscopic ultrasound-guided gastroenterostomy: are we ready for prime time? Endosc Ultrasound. 2017; 6:235–240.
6. Irani S, Baron TH, Itoi T, et al. Endoscopic gastroenterostomy: techniques and review. Curr Opin Gastroenterol. 2017; 33:320–329.
7. Tonozuka R, Tsuchiya T, Mukai S, et al. Endoscopic ultrasonography-guided gastroenterostomy techniques for treatment of malignant gastric outlet obstruction. Clin Endosc. 2020; 53:510–518.
8. Duan B, Guo J, Ge N, et al. Preliminary use of a double-flanged, fully covered, self-expandable, metal stent with cautery in endoscopic ultrasound-guided gastroenterostomy. Endoscopy. 2018; 50:E29–E31.
9. Khashab MA, Kumbhari V, Grimm IS, et al. EUS-guided gastroenterostomy: the first U.S. clinical experience (with video). Gastrointest Endosc. 2015; 82:932–938.
10. Chen YI, Kunda R, Storm AC, et al. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointest Endosc. 2018; 87:1215–1221.
11. Ge PS, Young JY, Dong W, et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019; 33:3404–3411.
12. Iqbal U, Khara HS, Hu Y, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: a systematic review and meta-analysis. Endosc Ultrasound. 2020; 9:16–23.
13. Kouanda A, Binmoeller K, Hamerski C, et al. Endoscopic ultrasound-guided gastroenterostomy versus open surgical gastrojejunostomy: clinical outcomes and cost effectiveness analysis. Surg Endosc. 2021; 35:7058–7067.
14. Fan W, Tan S, Wang J, et al. Clinical outcomes of endoscopic ultrasound-guided gastroenterostomy for gastric outlet obstruction: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol. 2022; 31:159–167.
Fig. 1.
A 20×80 mm polytetrafluoroethylene covered tubular self-expandable metallic stent (from Manufacturer’s Catalogue with permission; Hilzo).
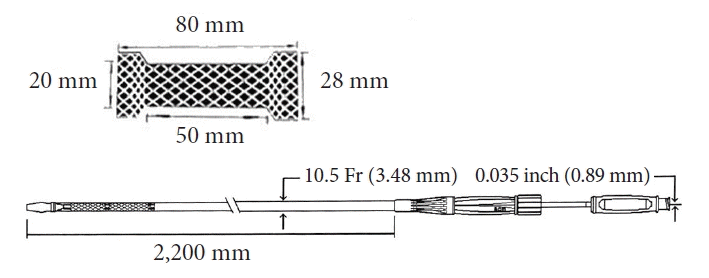
Fig. 2.
(A) Puncture of the proximal jejenum with a 19 G needle (arrow). (B) Contrast medium injection. (C) Guidewire placement and the insertion of the 6 Fr cystotome over the guidewire. (D) Deployment of the tubular fully covered self-expandable metallic stent (T-FCSEMS). (E) Balloon diltation of the T-FCSEMS. (F) Contrast medium injection control after the dilatation of the T-FCSEMS. (G) Endoscopic view of the fully deployed stent.
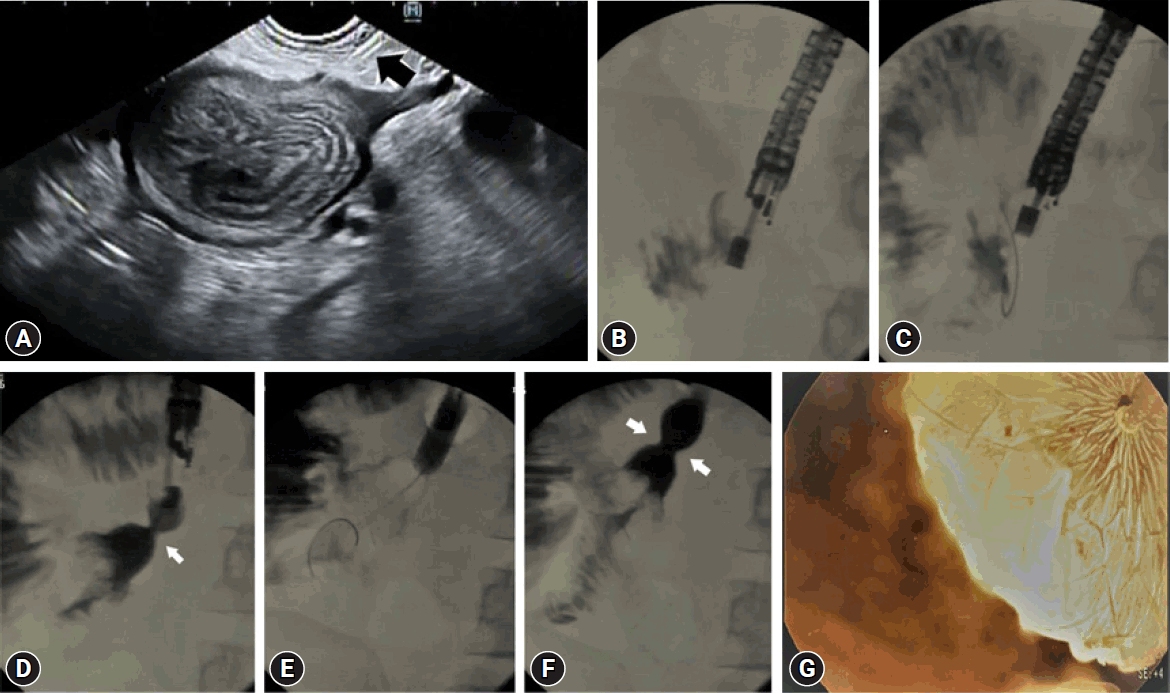
Fig. 3.
(A, B) Accidental transvers colon puncture and filling with contrast. (C) Puncturing of the jejenum (clockwise from top left). The procedure was completed uneventfully.
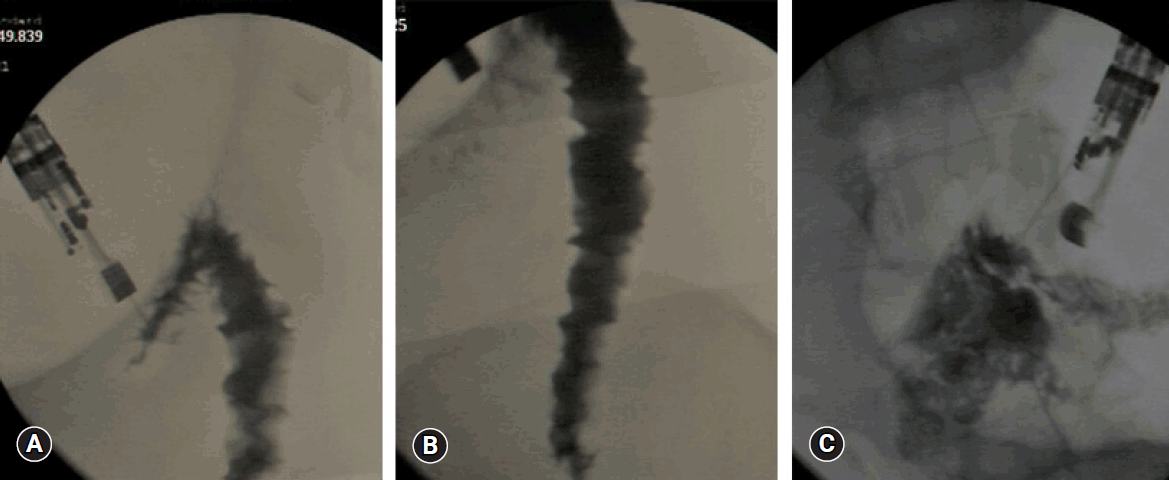
Fig. 4.
Two weeks after deployment of the fully covered self-expandible metallic stent. (A) A well-developed gastrojejunal anastomosis after migration and defecation of the stent. (B) The postanastomotic area.
Table 1.
Relevant patient characteristics
| Characteristic | Value |
|---|---|
| Number | 21 |
| Male | 15 (71.4) |
| Age (yr) | 66 (40–87) |
| Etiology | |
| Pancreas cancer | 12 (57.1) |
| Gastric cancer | 6 (28.6) |
| Metastatic cancer | 1 (4.8) |
| Benigna) | 2 (9.5) |
| GOO location and pathology | |
| Pylorus | 7 (33.3) |
| Gastric carcinoma | 6 |
| Benign etiologya) | 1 |
| Duodenum | 12 (57.1) |
| D1: pancreatic carcinoma | 3 |
| D2: pancreatic carcinoma | 8 |
| D3: metastatic colon cancer | 1 |
| Surgical gastrojejunostomy site | 2 (9.5) |
| Pancreatic carcinoma palliation | 1 |
| Pancreaticoduodenectomy due to pancreatic cystb) | 1 |
| Ascites | |
| Not present | 10 (47.6) |
| Mild | 3 (14.4) |
| Tense | 8 (38.1) |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download