INTRODUCTION
Hypersensitivity pneumonitis (HP) is a lung disease caused by inhaling antigenic substances, leading to inflammation in the bronchioles and alveoli.
1 The disease can manifest as acute, subacute, or chronic depending on factors such as the antigen exposure level or concentration and the individual’s immune response. In European studies, the estimated prevalence rates of HP range from 4% to 13% of all reported cases of interstitial lung disease (ILD), with a rate of 7% in Denmark.
2 A recent study in the United States reported 1-year prevalence rates ranging from 1.67 to 2.71 per 100,000 persons and 1-year cumulative incidence rates ranging from 1.28 to 1.94 per 100,000 persons.
3
To address the challenges in diagnosing HP, the American Thoracic Society (ATS), the Japanese Respiratory Society (JRS), and the Latin American Thoracic Society (ALAT) proposed diagnostic criteria in 2020,
4 with an elevated CD8/CD4 ratio in bronchoalveolar lavage (BAL) lymphocytes being a distinguishing factor. However, diagnosis can be challenging due to inconclusive results, and invasive lung biopsy is considered a last resort when there are unclear indications from bronchoscopy use in HP diagnosis.
The management of HP involves avoiding exposure to the causative antigen and pharmacotherapy using corticosteroids or other second-line immunosuppressants.
5 Although corticosteroids are commonly used as the first-line treatment, their long-term benefits may be limited. Immunosuppressive agents have shown favorable effects on the pulmonary function of patients with chronic HP.
6
Despite the global impact of HP, comprehensive data on its incidence, diagnosis, and treatment outcomes are still lacking. Consequently, this study aimed to address these gaps by analyzing data from the Health Insurance Review and Assessment Service (HIRA) in South Korea between 2011 and 2021.
RESULTS
This study identified a total of 8,678 HP incident cases (
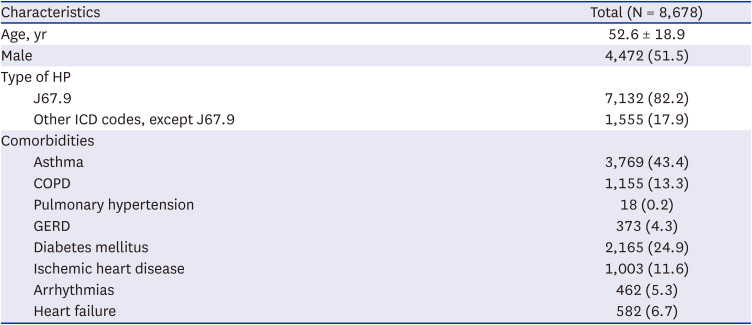
Fig. 1). The mean age of incident HP patients was 52.6 ± 18.9 years. Most patients (82.2%) were diagnosed with HP using the ICD-10-CM code J67.9, indicating HP due to unspecified organic dust. Among the identified patients, 43.4%, 13.3%, 0.2%, and 4.3% of patients had asthma, COPD, pulmonary hypertension, and GERD, respectively (
Table 1).
Fig. 1
Identification of cases of hypersensitivity pneumonitis from the Health Insurance Review and Assessment Service database.
HP = hypersensitivity pneumonitis.
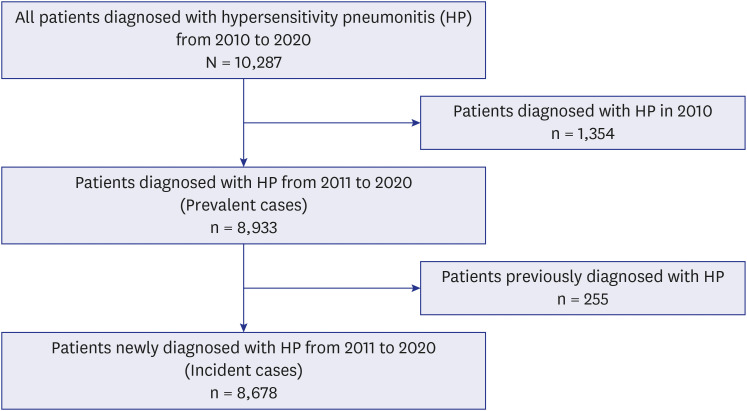

Table 1
Baseline characteristics of patients with incident HP, 2011–2020
|
Characteristics |
Total (N = 8,678) |
|
Age, yr |
52.6 ± 18.9 |
|
Male |
4,472 (51.5) |
|
Type of HP |
|
|
J67.9 |
7,132 (82.2) |
|
Other ICD codes, except J67.9 |
1,555 (17.9) |
|
Comorbidities |
|
|
Asthma |
3,769 (43.4) |
|
COPD |
1,155 (13.3) |
|
Pulmonary hypertension |
18 (0.2) |
|
GERD |
373 (4.3) |
|
Diabetes mellitus |
2,165 (24.9) |
|
Ischemic heart disease |
1,003 (11.6) |
|
Arrhythmias |
462 (5.3) |
|
Heart failure |
582 (6.7) |

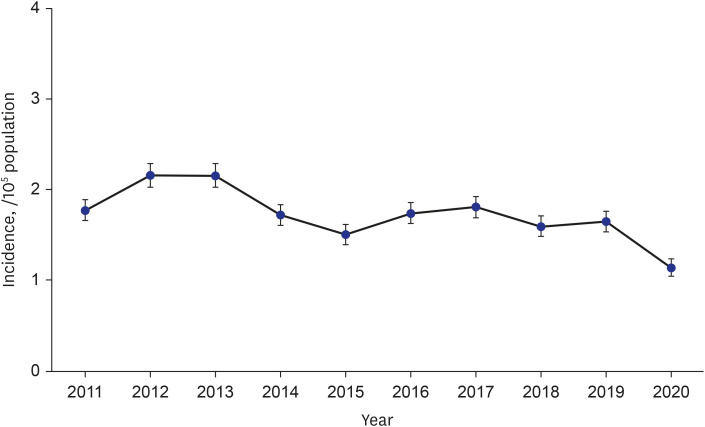
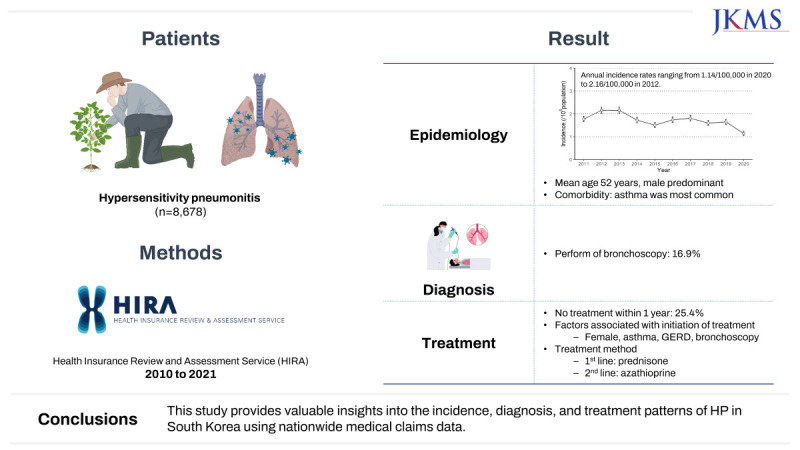
The age- and sex-adjusted annual incidence HP rates varied from 1.14/100,000 (95% CI, 1.05–1.24) in 2020 to 2.16/100,000 (95% CI, 2.03–2.29) in 2012 (
Fig. 2,
Supplementary Table 1).
Fig. 2
Annual trend of the incidence of hypersensitivity pneumonitis in Korea, 2011–2020.

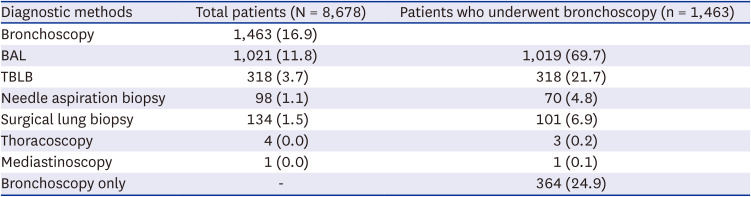
Among the 8,678 patients included in the study, 16.9%, 11.8%, and 3.7% of patients underwent bronchoscopy, BAL, and transbronchial lung biopsy (TBLB), respectively. In addition, 1.1% of patients underwent needle aspiration biopsy, and 1.5% of patients underwent surgical lung biopsy (
Table 2). Of the 1,463 patients who underwent bronchoscopy, almost 70% underwent BAL, and around 21% underwent TBLB (
Table 2).
Table 2
Diagnostic methods for patients with incident hypersensitivity pneumonitis
|
Diagnostic methods |
Total patients (N = 8,678) |
Patients who underwent bronchoscopy (n = 1,463) |
|
Bronchoscopy |
1,463 (16.9) |
|
|
BAL |
1,021 (11.8) |
1,019 (69.7) |
|
TBLB |
318 (3.7) |
318 (21.7) |
|
Needle aspiration biopsy |
98 (1.1) |
70 (4.8) |
|
Surgical lung biopsy |
134 (1.5) |
101 (6.9) |
|
Thoracoscopy |
4 (0.0) |
3 (0.2) |
|
Mediastinoscopy |
1 (0.0) |
1 (0.1) |
|
Bronchoscopy only |
- |
364 (24.9) |

Within 1 year after diagnosis with HP, 2,212 patients (25.4%) did not receive any treatment. A total of 6,207 patients received steroids alone, and 257 (2.96%) patients received both steroids and second-line immunosuppressants; only two patients received second-line immunosuppressants alone (
Table 3).
Table 3
Analysis of patients who initiated treatment within 1 year after diagnosis with incident hypersensitivity pneumonitis

|
Variables |
Total patients (N = 8,678) |
|
Steroids alone |
6,207 (71.5) |
|
Steroids and second-line immunosuppressants |
257 (2.96) |
|
Second-line immunosuppressants alone |
2 (0.14) |
|
No treatment |
2,212 (25.4) |

Prednisone was the most commonly prescribed steroid, followed by methylprednisolone and dexamethasone (
Table 4). Among the 6,207 patients who received only steroids, the average daily dose (± standard deviation) was equivalent to 25.89 mg of prednisone (± 50.87 mg/day); among those who received both steroids and second-line immunosuppressants, it was 29.83 mg (± 42.90 mg/day). The mean steroid dose for the 2,498 users who used steroids for 7 days or less was 39.05 mg, whereas the mean dose for the 3,709 users who used steroids for more than 7 days was 31.71 mg. The mean steroid dose for the 251 subjects who used a combination of steroids for more than 7 days along with a second-line immunosuppressant was 40.99 mg (
Supplementary Table 2). The mean treatment duration was 43.40 ± 80.65 days for steroids alone and 279.98 ± 151.11 days for the combined treatment (
Table 5).
Table 4
Medication use in incident cases of hypersensitivity pneumonitis during the initial treatment: Type of systemic steroid
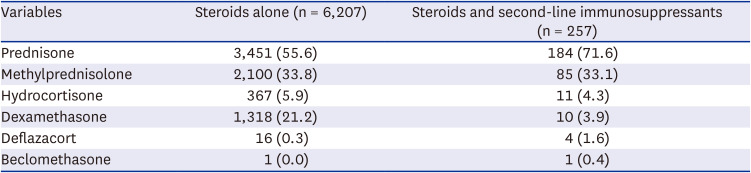
|
Variables |
Steroids alone (n = 6,207) |
Steroids and second-line immunosuppressants (n = 257) |
|
Prednisone |
3,451 (55.6) |
184 (71.6) |
|
Methylprednisolone |
2,100 (33.8) |
85 (33.1) |
|
Hydrocortisone |
367 (5.9) |
11 (4.3) |
|
Dexamethasone |
1,318 (21.2) |
10 (3.9) |
|
Deflazacort |
16 (0.3) |
4 (1.6) |
|
Beclomethasone |
1 (0.0) |
1 (0.4) |

Table 5
Medication use in incident cases of hypersensitivity pneumonitis during the initial treatment: Analysis of the steroid dosage and duration of patients with hypersensitivity pneumonitis
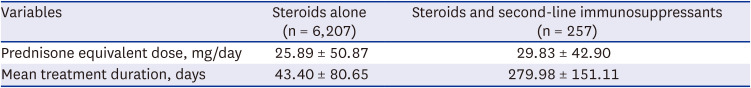
|
Variables |
Steroids alone (n = 6,207) |
Steroids and second-line immunosuppressants (n = 257) |
|
Prednisone equivalent dose, mg/day |
25.89 ± 50.87 |
29.83 ± 42.90 |
|
Mean treatment duration, days |
43.40 ± 80.65 |
279.98 ± 151.11 |

Among the 259 patients who received second-line immunosuppressants with or without steroids within 1 year of HP diagnosis, the most commonly used medications were azathioprine (AZA), mycophenolate mofetil (MMF), cyclosporine (CsA), leflunomide (LEF), and rituximab (RTX). For approximately 90% of patients who received immunosuppressants other than rituximab, long-term treatment for 28 days or more was required. CsA had the shortest mean treatment duration at 130.82 ± 136.55 days, whereas MMF had the longest mean treatment duration at 257.19 ± 134.47 days (
Table 6). Additionally, the number of rituximab doses was three (IQR, 1–6), and the dosing interval was 24.5 days (IQR, 21–29.25).
Table 6
Medication use in incident cases of hypersensitivity pneumonitis during the initial treatment: Analysis of the second-line immunosuppressant treatment duration of patients with hypersensitivity pneumonitis (N = 259)

|
Variables |
AZA |
MMF |
CsA |
LEF |
RTXa
|
|
Number of patients |
126 (48.6) |
74 (28.6) |
50 (19.3) |
18 (6.9) |
19 (7.3) |
|
Use for ≥ 28 days |
113 (89.68) |
71 (95.95) |
36 (89.29) |
17 (94.44) |
14 (73.68) |
|
Treatment duration, days |
188.71 ± 150.79 |
257.19 ± 134.47 |
130.82 ± 136.55 |
251.22 ± 111.33 |
101.68 ± 62.06 |

A comparison of patients diagnosed with HP showed that treatment initiation within 1 year of diagnosis was influenced by several factors (
Table 7). Multivariate analysis indicated that the male sex was associated with a lower likelihood of treatment initiation (adjusted odds ratio [aOR], 0.80; 95% CI, 0.73–0.88), whereas asthma (aOR, 1.52; 95% CI, 1.37–1.68), GERD (aOR, 1.46; 95% CI, 1.10–1.92), and bronchoscopy use as a diagnostic method (aOR, 2.15; 95% CI, 1.84–2.52) were associated with a higher likelihood of treatment initiation (
Fig. 3 and
Supplementary Table 3).
Fig. 3
Multivariate logistic regression analysis of factors associated with the treatment initiation of patients with hypersensitivity pneumonitis.
COPD = chronic obstructive pulmonary disease, GERD = gastroesophageal reflux disease, aOR = adjusted odds ratio, CI = confidence interval.
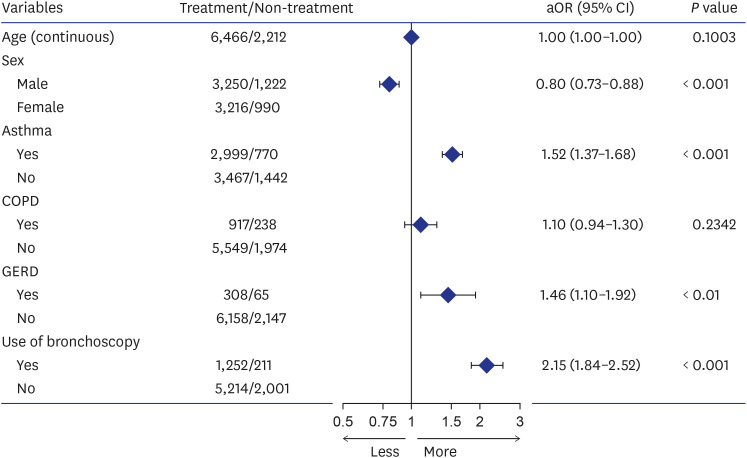

Table 7
Characteristics of patients with incident hypersensitivity pneumonitis according to treatment initiation within 1 year of diagnosis
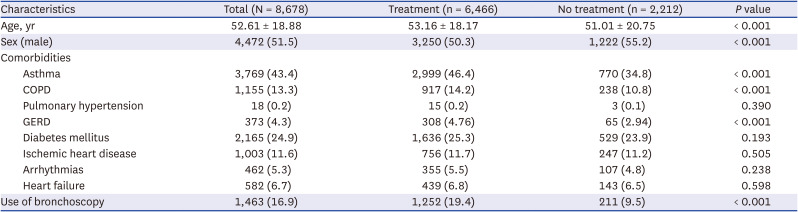
|
Characteristics |
Total (N = 8,678) |
Treatment (n = 6,466) |
No treatment (n = 2,212) |
P value |
|
Age, yr |
52.61 ± 18.88 |
53.16 ± 18.17 |
51.01 ± 20.75 |
< 0.001 |
|
Sex (male) |
4,472 (51.5) |
3,250 (50.3) |
1,222 (55.2) |
< 0.001 |
|
Comorbidities |
|
|
|
|
|
Asthma |
3,769 (43.4) |
2,999 (46.4) |
770 (34.8) |
< 0.001 |
|
COPD |
1,155 (13.3) |
917 (14.2) |
238 (10.8) |
< 0.001 |
|
Pulmonary hypertension |
18 (0.2) |
15 (0.2) |
3 (0.1) |
0.390 |
|
GERD |
373 (4.3) |
308 (4.76) |
65 (2.94) |
< 0.001 |
|
Diabetes mellitus |
2,165 (24.9) |
1,636 (25.3) |
529 (23.9) |
0.193 |
|
Ischemic heart disease |
1,003 (11.6) |
756 (11.7) |
247 (11.2) |
0.505 |
|
Arrhythmias |
462 (5.3) |
355 (5.5) |
107 (4.8) |
0.238 |
|
Heart failure |
582 (6.7) |
439 (6.8) |
143 (6.5) |
0.598 |
|
Use of bronchoscopy |
1,463 (16.9) |
1,252 (19.4) |
211 (9.5) |
< 0.001 |

DISCUSSION
This nationwide study in South Korea analyzed medical claims data to gain valuable insights into the incidence, diagnostic approaches, and treatment patterns of HP. This was the first analysis of its kind in the country, and it provided unique perspectives within the East Asian context by examining patient characteristics, comorbidities, medication use, and factors associated with treatment initiation.
In a study conducted in the United States, patients were selected based on specific criteria, including at least 6 months of continuous enrollment and no prior claims for HP.
3 In contrast, a study conducted in England excluded patients with an HP diagnosis within 1 year after the start date.
12 This study defined incident cases as patients with new claims for HP, excluding those with claims in the previous year.
The incidence of HP in this study ranged from 1.14/100,000 to 2.16/100,000 between 2011 and 2020. In a study conducted in the United States, the 1-year prevalence of HP between 2004 and 2013 was between 1.67 and 2.71 per 100,000 persons.
3 In Denmark, the incidence of HP varied from 0.89 in 2004 to 1.53 in 2005, with an average incidence rate of 1.16 per 100,000 persons during the study period.
2 These findings showed that the HP incidence rates in South Korea are similar to those in western countries, demonstrating the global burden of this respiratory condition.
The average age of HP onset was around 52 years old, consistent with the finding of a previous study conducted in the United States.
3 However, the sex distribution of HP patients differed across countries. In the United States, HP showed a higher prevalence among females,
3 whereas in Denmark, it was more prevalent among males.
2 In South Korea, the prevalence of HP was nearly equal between males and females.
In addition, this study identified asthma as a common comorbidity among HP patients. Although HP and asthma have different origins and causal agents,
13 they can present similar symptoms, such as cough and shortness of breath, making it challenging to distinguish between them based solely on clinical presentation. GERD is commonly observed as a comorbidity in patients with IPF.
14 However, the relationship between HP and GERD remains unclear. According to a previous study, the prevalence of GERD was 4.3%, and in a tertiary center in Germany, 24% of patients diagnosed with chronic HP also had GERD.
9 Notably, a previous national population study reported that the prevalence of doctor-diagnosed GERD ranged from 4.6% to 7.3% between 2005 and 2008 in South Korea.
15 In this study, the prevalence of GERD in HP patients was lower than that reported in previous studies.
Bronchoscopy, specifically BAL, is the primary diagnostic method used in differential diagnosis to rule out other potential causes.
416 However, bronchoscopy use was limited in this study, with only a small percentage (16.9%) of patients undergoing the procedure. Among those who underwent bronchoscopy, BAL was the most commonly performed procedure. Additionally, TBLB was performed for only 3.7% of patients. Improving the accuracy of diagnosis is necessary to enhance the identification and management of HP cases.
Most patients received treatment with steroids alone, and 25% of patients received no treatment at all. In a previous single-center study involving 43 patients diagnosed with pathologically-confirmed HP in South Korea, 18.6% received no other treatment, including antigen avoidance, while corticosteroid treatment was administered to 79.1% of patients.
17 Prednisone was the most commonly prescribed steroid, with methylprednisolone as an alternative option. In addition, second-line immunosuppressants, such as AZA and MMF, were commonly prescribed in this study.
18 In previous studies, both AZA and MMF demonstrated effectiveness in improving the lung function of patients with chronic HP.
181920 AZA or MMF may be highly effective in maintaining long-term immunosuppression, which could be particularly beneficial for patients with HP.
A combination of steroids and second-line immunosuppressants has demonstrated a steroid-sparing effect in some studies.
2122 However, contrary to prior findings, this study observed a higher mean steroid dosage in the group receiving combination therapy compared to the group receiving steroids alone. We also conducted an additional analysis based on the duration of steroid administration (7 days or less and more than 7 days). The mean steroid dose used for more than 7 days was lower than that of individuals using steroids for 7 days or less. This might mean that the steroid-only users tapered the steroid over the treatment duration or received a high dose of steroids within 7 days. However, users of combined therapy, which involved more than 7 days using steroids with a second-line immunosuppressant, had a higher steroid dose with a longer treatment duration than those in the steroid-only user group. This study suggested that steroid therapy was maintained over an extended period and additive immunosuppressant therapy was administered in South Korea.
Treatment initiation for HP was found to be associated with the female sex, comorbidities such as asthma and GERD, and individuals who underwent bronchoscopy. HP diagnosis can be challenging because its manifestation with bronchial hyperresponsiveness resembles asthma symptoms.
23 Population-based case-control studies have shown that GERD is more frequently observed in non-IPF ILD patients than in patients with IPF, indicating its potential role as a contributor to lung fibrosis development.
24 Our study is the first to demonstrate an association between GERD and treatment initiation for HP. However, the pathophysiology between GERD and HP has not been established, and further research is needed to explore this association. Current guidelines for HP diagnosis highlight the importance of bronchoscopy; however, this study found that only 16.9% of patients underwent bronchoscopy. Nevertheless, the use of bronchoscopy as a diagnostic tool was associated with treatment initiation. Therefore, more comprehensive diagnostic testing is required with a multidisciplinary approach for better diagnosis and management of HP.
This study had several limitations. First, the reliance on ICD-10 codes in the health insurance claims data to identify HP cases may have led to underreporting. However, the validity of the diagnostic code data was relatively high, as demonstrated by the number of patients who received treatment. Second, the lack of detailed clinical information, such as environmental exposures and pulmonary function tests, may have limited the ability to identify factors contributing to HP development. Third, this study did not consider the classification of HP based on three categories: acute, subacute, or chronic.
25 Recent guidelines categorize patients as having non-fibrotic HP or fibrotic HP
4; however, this information was not available in the claims data used for this study. Lastly, although the importance of a multidisciplinary approach in diagnosing HP was acknowledged, the process was not identified in this claims-based study.
In summary, this nationwide study in South Korea analyzed medical claims data to investigate the incidence, diagnosis, and management of HP. A total of 8,678 HP incident cases were identified between 2011 and 2020, with annual incidence rates ranging from 1.14 to 2.16 per 100,000 persons. Diagnostic methods such as bronchoscopy were commonly used. Moreover, treatment patterns primarily involved the use of steroids, particularly prednisone, while second-line immunosuppressants were less frequently prescribed, with variations in the medication type and treatment duration. Treatment initiation was associated with the female sex, having asthma or GERD, and undergoing bronchoscopy. This study highlights the importance of improving HP diagnostic methods and management to enhance patient outcomes.













 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download